Sitagliptin-mediated preservation of endothelial progenitor cell function via augmenting autophagy enhances ischaemic angiogenesis in diabetes
Abstract
Recently, the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, a major anti-hyperglycaemic agent, has received substantial attention as a therapeutic target for cardiovascular diseases via enhancing the number of circulating endothelial progenitor cells (EPCs). However, the direct effects of sitagliptin on EPC function remain elusive. In this study, we evaluated the proangiogenic effects of sitagliptin on a diabetic hind limb ischaemia (HLI) model in vivo and on EPC culture in vitro. Treatment of db/db mice with sitagliptin (Januvia) after HLI surgery efficiently enhanced ischaemic angiogenesis and blood perfusion, which was accompanied by significant increases in circulating EPC numbers. EPCs derived from the bone marrow of normal mice were treated with high glucose to mimic diabetic hyperglycaemia. We found that high glucose treatment induced EPC apoptosis and tube formation impairment, which were significantly prevented by sitagliptin pretreatment. A mechanistic study found that high glucose treatment of EPCs induced dramatic increases in oxidative stress and apoptosis; pretreatment of EPCs with sitagliptin significantly attenuated high glucose-induced apoptosis, tube formation impairment and oxidative stress. Furthermore, we found that sitagliptin restored the basal autophagy of EPCs that was impaired by high glucose via activating the AMP-activated protein kinase/unc-51-like autophagy activating kinase 1 signalling pathway, although an autophagy inhibitor abolished the protective effects of sitagliptin on EPCs. Altogether, the results indicate that sitagliptin-induced preservation of EPC angiogenic function results in an improvement of diabetic ischaemia angiogenesis and blood perfusion, which are most likely mediated by sitagliptin-induced prevention of EPC apoptosis via augmenting autophagy.
Introduction
Diabetes is a disease that is strongly associated with both microvascular and macrovascular complications, including retinopathy, nephropathy, and neuropathy (microvascular) and ischaemic heart disease, peripheral vascular disease and cerebrovascular disease (macrovascular), resulting in organ and tissue damage in approximately one-third to one-half of people with diabetes 1, 2. Vascular complications associated with diabetes are the leading causes of morbidity and mortality for diabetic patients. Vascular complications in diabetes are associated with dysregulation of vascular remodelling and vascular growth, decreased responsiveness to ischaemic/hypoxic stimuli, impaired or abnormal neovascularization, and a lack of endothelial regeneration. Thus, there is a great need for therapeutic interventions aimed at accelerating the repair of dysfunctional endothelium and restoring blood flow in damaged organs and tissues 3.
Endothelial progenitor cells (EPCs) are found in bone marrow, peripheral blood and certain organs, such as the spleen and liver. In the event of endothelial injury or tissue ischaemia, EPCs are mobilized into the circulation from the bone marrow, home to the site of injury, differentiate into mature endothelial cells and incorporate into the endothelium, replacing apoptotic or damaged cells and mediating neovascularization. However, the number of EPCs in diabetic patients is reduced, and the function of EPCs is attenuated as a consequence of exposure to the dysmetabolic diabetic environment 4. An efficient therapeutic strategy includes mobilizing more EPCs into peripheral blood and promoting EPCs homing to ischaemic tissue to enhance angiogenesis with pharmacological agents.
Dipeptidyl peptidase 4 (DPP-4), a membrane-bound extracellular peptidase, also designated CD26, has been demonstrated to cleave cytokines and chemokines 5. Stromal-derived factor-1α (SDF-1α), one of the chemokine substrates of DPP-4[5], serves as a chemoattractant for EPCs and stem/progenitor cells and plays critical roles in EPCs and stem cell mobilization and homing 6. Previous studies have reported that inhibition of DPP-4 activity in the blood leads to an increase in the circulating number of EPCs 7-9, enhancing angiogenesis and blood flow in hind limb ischaemia (HLI) 8, 9 and protecting the heart in models of myocardial infarction 10, 11. However, the direct effects of DPP-4 inhibition on EPCs function remain elusive.
In this study, sitagliptin, a DPP-4 inhibitor that is widely used in the clinic, was used to treat HLI in db/db type 2 diabetic mice in vivo and EPCs under high glucose (HG) conditions in vitro. We aimed to evaluate (1) the therapeutic effects of sitagliptin on HLI; (2) the mobilization of EPCs induced by sitagliptin; (3) and the proangiogenic effects and mechanisms of sitagliptin on EPCs under HG conditions, which was used to mimic a dysmetabolic environment.
Materials and methods
Animals
db/db (FVB background) male mice at 2–3 months of age were used in this study. All animal procedures were approved by the Animal Policy and Welfare Committee of Wenzhou Medical University and/or the Institutional Animal Care and Use Committee of the University of Louisville, which conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication No. 85-23, revised 1996).
HLI and sitagliptin treatment
The db/+ founder mice [FVB.BKS(D)-Leprdb/+/ChuaJ, FVB background] were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained under specific pathogen-free conditions at the University of Louisville Animal Facility (Louisville, KY, USA) or the Wenzhou Medical University. The db/db mice were generated by breeding male db/+ to female db/+ mice following Jackson Laboratory's instructions. Twelve-week-old male db/db mice were equally divided into two groups (n = 16/group) to develop the HLI model as described in our previous report 12. One group was pretreated with sitagliptin (25 mg/kg, daily) for 1 week before HLI surgery and continually treated with sitagliptin for an additional 7 or 35 days after surgery via gavage administration. Another group was treated with H2O as the vehicle control. At 7 or 35 days after surgery, eight mice from each group were sacrificed to collect blood and gastrocnemius muscle samples. The HLI procedure is briefly described below: under sufficient anaesthesia with isoflurane (1–3% isoflurane in 100% oxygen at a flow rate of 1 l/min.), the hind limbs were shaved and the entire right superficial femoral artery and vein (from just below the deep femoral arteries to the popliteal artery and vein) were ligated with 6-0 silk sutures, cut and excised with an electrical coagulator (Fine Science Tools Inc., Foster City, CA, USA). The overlying skin was closed with 4-0 silk sutures.
Measurement of blood flow perfusion with a Pericam perfusion speckle imager (PSI)
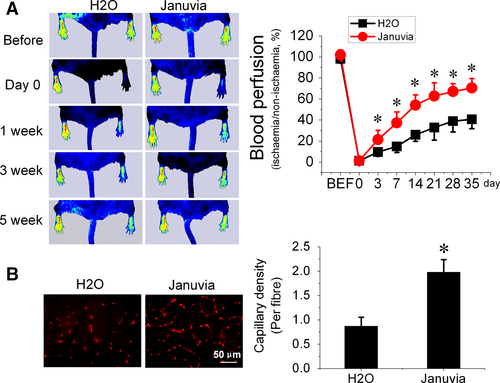
To evaluate the limb perfusion ratio [ischaemic limb (right)/normal limb (left)], real-time microcirculation imaging analysis was performed using a Pericam PSI based on laser speckle contrast analysis technology (Perimed Inc., Kings Park, NY, USA) before surgery and at day 0, 3, 7 14, 21, 28 and 35 post-surgery.
Quantification of circulating EPCs by flow cytometry
At days 3 and 7 after surgery, blood samples were collected in 0.1 mol/l EDTA-2Na-coated tubes from the tail vein. Whole blood was incubated with CD34-PE and VEGFR-2-APC antibodies 13, 14 (BD Pharmingen, San Jose, CA, USA) for 1 hr, then lysed and fixed in FACS™ lysing solution (BD Biosciences, San Jose, CA, USA) for 5 min. Flow cytometry analysis was performed with a BD FACS (BD Biosciences) to count VEGFR-2 and CD34 double-positive EPCs. Isotype control IgG (BD Pharmingen) was used to exclude false-positive cells.
Determination of SDF-1 and glucagon-like peptide-1 (GLP-1) in plasma
At day 14 after sitagliptin administration (7 days after surgery), the plasma was collected to detect the levels of SDF-1 and GLP-1. The levels of SDF-1α and GLP-1 were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits CXCL12/SDF-1α immunoassay (R&D systems, Minneapolis, MN, USA) and Glucagon Like Peptide-1 (Active) ELISA kit (Millipore, Billerica, MA, USA), respectively.
Histological assessment
The extent of angiogenesis at day 35 post-ischaemic surgery was assessed by measuring capillary density using isolectin B4 staining. Ischaemic gastrocnemius muscle tissues were fixed with 4% paraformaldehyde and embedded with paraffin. Paraffin sections (5 μm) were stained with Alexa Fluor® 594 conjugated isolectin GS-IB4 antibody (Thermo Scientific, Waltham, MA, USA) to evaluate the capillary density. The capillaries were counted in randomly selected fields for a total of 20 different fields (×40 magnification) per section and three sections per animal. The capillary density is presented as capillary number per muscle fibre.
Isolation and culture of bone marrow-derived EPCs
EPCs were isolated from the bone marrow of wild-type (WT) FVB mice and cultured according to our established methods with minor modifications 15. Briefly, bone marrow mononuclear cells (MNCs) were isolated from the femurs and tibias of the mice by density gradient centrifugation with histopaque-1083 (Sigma-Aldrich, St. Louis, MO, USA). After two washes, MNCs were plated on vitronectin-coated culture dishes (Sigma-Aldrich) and maintained in endothelial growth factor-supplemented media (EGM-2 bullet kit; Lonza, Basel, Switzerland) with 10% foetal bovine serum. Cells were cultured at 37°C with 5% CO2 in a humidified atmosphere. EGM-2 medium was replaced after the first 24 hrs and every 3 days thereafter. Cell colonies appeared after 7 days of culture and were expanded to the fourth or fifth passage for further analysis.
Characterization of bone marrow-derived MNCs
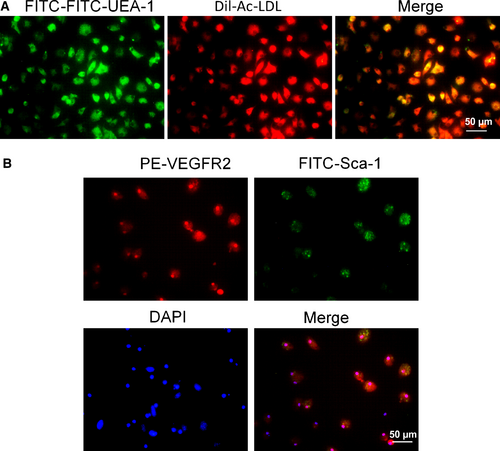
After 14 days of culture in endothelial-specific media and the removal of non-adherent MNCs, the remaining cells were characterized by uptake of Dil-Ac-LDL and ulex europaeus lectin-1 binding. For the uptake of Dil-Ac-LDL and ulex europaeus lectin-1 binding assay, cells were seeded on a vitronectin-coated 8-well μ-slide (ibidi, Martinsried, Germany) one day before the procedure, and then, the cells were incubated with 5 μg/ml acetylated DiI lipoprotein from human plasma (Dil-Ac-LDL, Thermo Fisher Scientific, Waltham, MA, USA) at 37°C for 4 hrs, followed by three washes with Dulbecco's phosphate-buffered saline (DPBS) and fixed with 4% paraformaldehyde. Then, the cells were incubated with 10 μg/ml fluorescein isothiocyanate-labelled ulex europaeus lectin-1 (FITC-UEA-1; Sigma-Aldrich) for 1 hr at room temperature. After incubation, the cells were rinsed with DPBS three times and were visualized via confocal microscopy.
To further confirm the identity of these cells, immunofluorescence staining was performed to analyse the expression of specific cell markers VEGFR2 and Sca-1. For the immunofluorescence staining, cells were seeded on a vitronectin-coated 8-well μ-slide one day before the procedure and then fixed with 4% paraformaldehyde after three washes with DPBS. The fixed cells were incubated with PE-VEGR2 and FITC-Sca-1 16, 17 antibodies (Abcam, Cambridge, MA, USA) overnight at 4°C. DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride) was used to label nuclei.
DPP-4 enzymatic activity assay
DPP-4 enzymatic activity was assayed in the culture medium of EPCs under different treatment conditions using a DPP-4 Activity Assay Kit (Sigma-Aldrich). EPCs were seeded on 12-well plates and maintained under basal culture conditions with or without HG (25 mmol/l) for 24 hrs 18 in the presence or absence of different concentrations of sitagliptin. The culture medium was collected for the DPP-4 enzymatic activity assay. Briefly, a 50 μl volume of medium was diluted with 48 μl of DPP-4 assay buffer, mixed with 2 μl substrate gly-pro-7-amino-4-methylcoumarin (AMC) and then incubated at 37°C for 30 min. The release of AMC from the substrate was measured with a fluorescence spectrophotometer at 360 nm excitation and 460 nm emission.
Apoptosis assay
EPCs were seeded on 12-well plates (1 × 105 cells/well) and maintained under basal culture conditions or with HG (25 mmol/l) for 24 hrs in the presence or absence of sitagliptin. Non-adherent cells were removed by washing with PBS. In some experiments, cells were pretreated with the autophagy inhibitor 3-methyladenine (3-MA, 5 mmol/l; Sigma-Aldrich) or the autophagy activator rapamycin (10 μmol/l; Sigma-Aldrich) for 30 min. and then continually exposed to HG for the indicated durations in the presence or absence of sitagliptin. Subsequently, adherent cells were released with 0.25% trypsin without EDTA. EPCs were collected by centrifugation and stained with an APC-conjugated Annexin V Apoptosis Detection Kit according to the manufacturer's instructions (Biolegend, San Diego, CA, USA). Apoptotic EPCs were detected by flow cytometry. Early apoptotic cells were defined as AnnexinV+/PI–.
Tube formation assay
The in vitro angiogenic capability of EPCs was determined by a Matrigel tube formation assay. Briefly, 48-well plates were coated with growth factor-reduced matrix gel (150 μl/well, BD Biosciences). EPCs (5 × 104 cells/well) in 200-μl basal culture medium or medium containing HG in the presence or absence of different concentrations of sitagliptin were incubated at 37°C with 5% CO2 for 12 hrs to form tubes. In some experiments, cells were pretreated with 3-MA (5 mmol/l) or rapamycin (10 μmol/l) for 30 min. and then continually exposed to HG for the indicated durations in the presence or absence of sitagliptin. Images of tubes in each well were acquired using an inverted microscope (Nikon Eclipse E600, Nikon, Kanagawa, Japan). The tube lengths were calculated by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Quantitative determination of oxidative stress
To detect the reactive oxygen species (ROS) levels of EPCs with HG treatment in the presence or absence of different concentrations of sitagliptin, a dihydroethidium (DHE; Molecular Probes, Eugene, OR, USA) probe was used to stain EPCs. DHE is cell permeable and able to react with superoxide to form ethidium, which in turn intercalates with DNA and produces nuclear fluorescence. EPCs were seeded on 24-well plates, treated with HG in the presence or absence of sitagliptin for 6 hrs and then incubated with 5 μmol/l DHE in PBS for 30 min. at 37°C. Nuclear DHE-positive staining indicates superoxide generation in cells. Fluorescence intensity was detected by a microplate reader (SpectraMax M3; Molecular Devices, Sunnyvale, CA, USA) under specific wave length conditions (excitation = 518 nm; fluorescence = 605 nm). Meanwhile, fluorescence images of random EPCs were captured at 20× magnification (XI 71 Olympus, Tokyo, Japan).
Autophagic signalling assay
Autophagic signalling was assayed by detecting microtubule-associated protein 1A/1B-light chain 3 (LC3) and p62 protein expression levels and LC3-bound puncta formation. For the LC3 and p62 protein expression assay, EPCs were seeded on 6-well plates (1 × 105 cells/well) and maintained under basal culture conditions with or without HG for 24 hrs in the presence or absence of sitagliptin. Non-adherent cells were removed by washing with PBS. In some experiments, cells were pretreated with AMP-activated protein kinase (AMPK) inhibitor compound C (10 μmol/l; Sigma-Aldrich) for 30 min. and then continually exposed to HG for the indicated durations in the presence or absence of sitagliptin. Then, EPCs were harvested for a Western blot assay of LC3 and p62 protein expression levels. For the LC3-bound puncta assay, EPCs were seeded on an 8-well microculture chamber (2 × 104 cells/well) and maintained under basal culture conditions with or without HG for 24 hrs in the presence or absence of sitagliptin. Non-adherent cells were removed by washing with PBS. The remaining cells were stained with a LC3B primary antibody (1:100; Novus Biologicals, Littleton, CO, USA) and an appropriate FITC-conjugated secondary antibody. LC3 puncta were observed with immunofluorescence microscopy.
Western blot assay
Western blotting was performed as described in a previous study 15. Gastrocnemius muscle tissues and harvested cells were homogenized or lysed in ice-cold RIPA lysis buffer (Santa Cruz Biotechnology, Dallas, TX, USA). The protein concentration was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). The total proteins were separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad). The membranes were blocked in tris-buffered saline with 5% non-fat milk and 0.5% bovine serum albumin for 1 hr and then incubated with primary antibody overnight at 4°C, followed by an incubation with secondary antibodies for 1 hr at room temperature after standard washing procedures. The primary antibodies against VEGF (1:10,000), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:5000) and β-actin (1:3000) were purchased from Santa Cruz Biotechnology; SDF-1 (1:2000), AMPK and p-AMPK (1:2000), mTOR and p-mTOR (1:2000), unc-51-like autophagy activating kinase 1 (ULK1) and p-ULK1 and P62 (1:2000) were purchased from Cell Signaling Technology (Danvers, MA, USA); and LCB (1:2000) was purchased from Novus Biologicals (Littleton, CO, USA). All horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology. Blots were visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) and quantified with Quantity 5.2 software (Bio-Rad).
Statistical analysis
All data are presented as the mean ± S.D. Statistical analysis was performed using Origin 7.5 (OriginLab data analysis and graphing software, Northampton, MA, USA) with one-way or two-way anova, followed by post hoc multiple comparisons with Scheffe's test. Statistical significance was considered P < 0.05.
Results
Sitagliptin enhances blood perfusion and angiogenesis in HLI in type 2 diabetic mice
To evaluate the angiogenic effect of DPP-4 inhibition in diabetes, db/db mice were pretreated with sitagliptin (Januvia) for 1 week; then, the mice were subjected to HLI surgery and continually treated with sitagliptin for an additional 5 weeks until to the end of the experiments. Blood flow was evaluated by PSI at day 3, 7, 14, 21, 28 and 35 after surgery. The results showed that sitagliptin treatment time dependently improved blood perfusion in db/db mice underwent HLI (Fig. 1A), which was accompanied by promoted angiogenesis mirrored by increased capillary density (Fig. 1B).
Sitagliptin increases proangiogenic factor expression levels and enhances EPCs mobilization in type 2 diabetic mice
To evaluate the effect of DPP-4 inhibition on the expression levels of proangiogenic factors in plasma and ischaemic tissue, peripheral blood and gastrocnemius muscles in ischaemic limbs were collected at day 7 after surgery. The ELISA results showed that mice that received sitagliptin had elevated levels of GLP-1 and SDF-1 in plasma (Fig. 2A and B). Proangiogenic factors in gastrocnemius muscle were detected by Western blotting, and the results showed that VEGF and SDF-1 increased significantly in sitagliptin-treated mice (Fig. 2C).
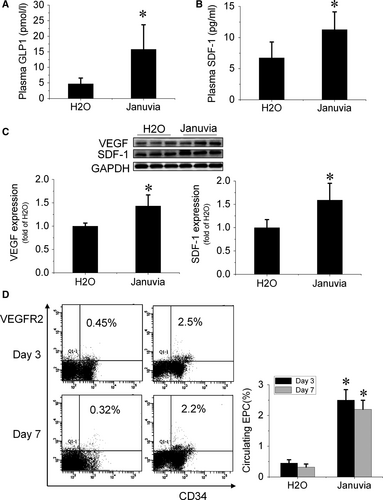
At days 3 and 7 after surgery, peripheral blood was collected to evaluate the number of EPCs in circulation. A flow cytometry assay showed that the number of circulating EPCs (CD34+/VEGFR2+) significantly increased in sitagliptin-treated mice (Fig. 2D), indicating that sitagliptin promoted EPCs mobilization from bone marrow to peripheral blood.
Characterization of bone marrow-derived EPCs
MNCs isolated from bone marrow were cultured in EGM-2 medium on vitronectin-coated culture dishes. By 14–21 days of culture, endothelium-like cells with the cobblestone-like morphology of late EPCs were nearly confluent. Late EPCs were further characterized by Dil-ac-LDL uptake and FITC-UEA-1 lectin binding. As shown in Figure 3A, most late EPCs were positive for Dil-ac-LDL and FITC-UEA-1 lectin staining. To further confirm the identity of these cells, immunofluorescence staining was performed to analyse the expression of specific cell markers (Fig. 3B, upper panel). The results showed that the majority of cells are both VEGFR2 and Sca-1 positive (Fig. 3B, lower panel).
Sitagliptin improves the survival and angiogenic function of EPCs treated with HG
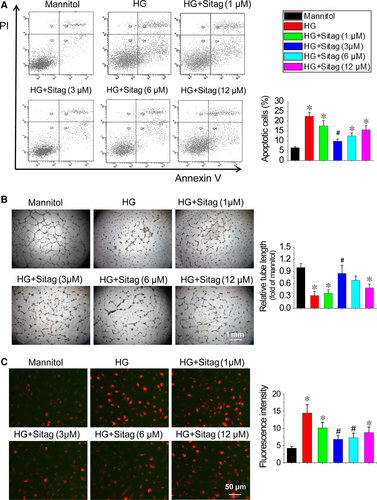
To validate the potency of sitagliptin, DPP-4 activity was measured in the medium of EPCs using a DPP-4 Activity Assay Kit. As expected, HG increased the DPP-4 activity of EPCs, which was inhibited by sitagliptin treatment in a dose-dependent manner (Figure S1). To determine whether DPP-4 inhibition could restore the survival and angiogenic function of EPCs under diabetic conditions, EPCs were exposed to HG to mimic the dysmetabolic stress of hyperglycaemia. The results demonstrated that HG significantly increased EPC apoptosis, as measured by Annexin V/PI staining (Fig. 4A). Sitagliptin treatment dose dependently prevented HG-induced apoptosis of EPCs within the lower dose range, although this protection was blunted at higher doses (Fig. 4A). Tube formation assays were used as one measure of angiogenic function in vitro. Similarly, HG significantly impaired the tube formation abilities of EPCs, and sitagliptin treatment significantly prevented the detrimental effects of HG on EPCs function at the same dose levels that prevented apoptosis (Fig. 4B).
Sitagliptin attenuates ROS levels in EPCs treated with HG
Oxidative stress is a major factor responsible for the dysfunction of diabetic EPCs 19, 20. We used DHE staining to show superoxide production in EPCs. The results demonstrated that HG significantly increased superoxide production levels, whereas sitagliptin treatment attenuated HG-induced superoxide production in EPCs at the same optimal dose level that prevented apoptosis and protected angiogenic function (Fig. 4C). Therefore, the optimal dose of sitagliptin at 3 μmol/l in EPCs protection was used in the following mechanistic studies.
Sitagliptin improves the survival and function of EPCs treated with HG via augmenting autophagy
Under diabetic conditions, impaired autophagy contributes to endothelial dysfunction, and restoration of autophagy can improve endothelial cell survival and function 21. In this study, we found that sitagliptin improved the survival and function of EPCs treated with HG. Therefore, we investigated whether the protective effects of sitagliptin on EPCs were mediated by autophagy activation in the following studies. EPCs were treated with HG (25 mmol/l) in the presence or absence of sitagliptin (3 μmol/l), and cells treated with 5.5 mmol/l glucose plus 20.5 mmol/l mannitol were used as a control. Immunofluorescence staining analysis demonstrated that elevated glucose levels reduced the number and distribution of LC3 puncta staining, suggesting a reduction in autophagosome formation, which was significantly prevented by sitagliptin treatment (Fig. 5A). In addition, we also detected the expression of p62 and the ratio of LC3II to LC3I by Western blotting. The results showed that the ratio of LC3II to LC3I decreased in EPCs with HG treatment, which was reversed by sitagliptin, whereas p62 expression exhibited the opposite pattern (Fig. 5B). These results indicate that sitagliptin activates autophagy in EPCs under HG treatment conditions.

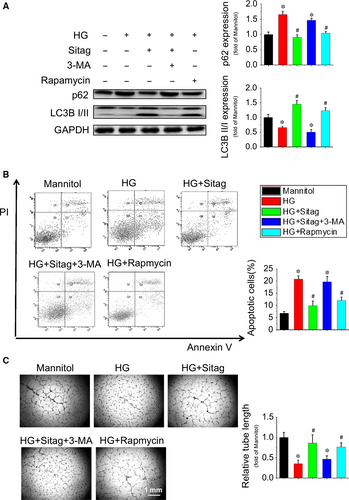
To confirm whether the protective effects of sitagliptin on EPCs were dependent on augmented autophagy, the autophagy inhibitor 3-MA and the activator rapamycin were tested against the protection of sitagliptin. The result showed that inhibition of autophagy by 3-MA completely abolished the sitagliptin-stimulated increase in LC3II and decrease in p62 expression in EPCs (Fig. 6A), resulting in complete abolishment of sitagliptin-induced cell survival (Fig. 6B) and tube formation (Fig. 6C) in EPCs under HG treatment conditions, whereas activation of autophagy by rapamycin almost completely prevented HG-induced pathological changes in EPCs, which are comparable to that of sitagliptin treatment (Fig. 6A–C). These results suggest that sitagliptin preserves EPC function via restoring autophagy in EPCs.
Sitagliptin restores autophagy in EPCs treated with HG via activating the AMPK/ULK1 signalling pathway
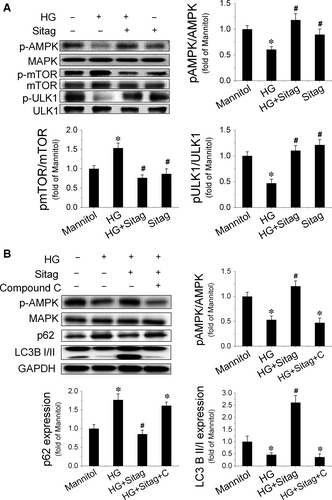
AMPK/ULK1 signalling is critical for autophagy regulation under diabetic conditions 22, 23. A previous study has shown that sitagliptin can activate AMPK 24. Therefore, we first detected AMPK activation in EPCs. The results showed that HG inhibited AMPK activity, as estimated by the decreased phosphorylation of AMPK, whereas sitagliptin treatment prevented HG-induced decreases in the phosphorylation of AMPK (Fig. 7A). Meanwhile, we also found that sitagliptin prevented HG-induced phosphorylation of mTOR, which was accompanied by an increase in the phosphorylation of ULK1 at Ser555 (Fig. 7A). To further confirm the critical role of AMPK activation in sitagliptin-induced augmentation of autophagy in EPCs, the AMPK inhibitor compound C was tested against sitagliptin-induced activation of autophagy. The result showed that compound C completely abolished the sitagliptin-stimulated increase in LC3II and decrease in p62 expression in EPCs (Fig. 7B). These results suggest that AMPK is a critical mediator of the protective effects of sitagliptin on EPC function via augmenting autophagy.
Discussion
The present study provides three new lines of evidence demonstrating the benefits of sitagliptin in ischaemic angiogenesis in type 2 diabetic mice in vivo and the angiogenic function of EPCs in vitro. The first novel finding is that sitagliptin treatment enhances blood perfusion and angiogenesis in HLI in db/db type 2 diabetic mice by increasing the circulating number of EPCs; the second innovative finding is that sitagliptin protects EPCs against diabetic stress conditions, which is mediated by restoring the autophagy of EPCs; and the third novel finding is that sitagliptin restores EPC autophagy, which is mediated by the AMPK/mTOR/ULK1 signalling pathway.
Previous studies have shown that DPP-4 inhibitors can improve blood flow recovery and angiogenesis in critical limb ischaemia models, which are accompanied by an increase in circulating EPCs 8, 9. In the present study, for the first time, we revealed that sitagliptin treatment improves blood perfusion and enhances angiogenesis in HLI in db/db type 2 diabetic mice (Fig. 1), which is consistent with previous findings in normal mice 9, 25. We also found that sitagliptin treatment increased the number of circulating EPCs (Fig. 2D) and elevated the expression of the chemokine SDF-1 in plasma (Fig. 2B). SDF-1 is a substrate of DPP-4, which proteolytically cleaves SDF-1 and attenuates the interaction of SDF-1 with its receptor CXCR4 26, 27. SDF-1 is a critical factor in EPC mobilization from bone marrow to peripheral blood and homing to ischaemic sites via interacting with its receptor CXCR4 28. Therefore, sitagliptin-induced enhancement of EPC mobilization and promotion of ischaemic blood perfusion and angiogenesis are most likely mediated by protecting SDF-1 from DPP-4 cleavage. In addition, our study demonstrated that sitagliptin also increased the proangiogenic factor SDF-1 and VEGF expression in ischaemic tissue (Fig. 2C), indicating that sitagliptin-augmented ischaemic angiogenesis may also attribute to a proangiogenic factor-mediated paracrine mechanism.
Accumulating studies have demonstrated that the beneficial effects of DPP-4 inhibition on vascular damage are mainly mediated by mobilization of EPCs 7, 8, 25; however, the direct effects of DPP-4 inhibition on EPCs biological function have been largely neglected, especially under diabetic conditions. In a previous study, researchers have presented preliminary data showing the direct effects of the DPP-4 inhibitors sitagliptin and vildagliptin on human EPCs under basal conditions 29. The authors found that DPP-4 inhibition prevented the spontaneous apoptosis, enhanced cell proliferation and the expression levels of VEGF, VEGFR-2 and eNOS in EPCs, which were accompanied by SDF-1/CXCR4 signalling activation, and blockade of the SDF-1/CXCR4 signalling pathway by AMD3100 resulted in increased apoptosis and the inhibition of cell proliferation and the expression levels of VEGF, VEGFR-2 and eNOS in EPCs 29. In the present study, we further evaluated the direct effects of sitagliptin on EPC survival and angiogenic function under diabetic conditions for the first time (Fig. 4). We found that direct incubation of EPCs with sitagliptin significantly attenuated HG-induced apoptosis and angiogenic dysfunction (Fig. 4A and B), which is consistent with previous findings that direct incubation of mesenchymal stem cells with sitagliptin remarkably attenuates hypoxia-induced apoptosis30. More recently, Pujadas et al. 31 found that teneligliptin, another DPP-4 inhibitor, reduced pro-apoptotic gene expression levels and ameliorated oxidative stress in human umbilical vein endothelial cells under HG conditions. In the present study, we also found that sitagliptin attenuated the ROS accumulation in EPCs induced by HG (Fig. 4C). These results indicate that sitagliptin-mediated improvement of the survival and angiogenic function of EPCs may attribute to sitagliptin-induced attenuation of ROS production and accumulation under HG conditions.
Autophagy plays an important role in cellular homeostasis through the degradation and recycling of organelles, such as mitochondria or the endoplasmic reticulum, that are closely related to the pathogenesis of diabetes 32, 33. Autophagy can be induced by conditions such as starvation or inflammation but can also occur constitutively under non-starvation conditions in basal autophagy, a process critical for the maintenance of cellular homoeostasis in the vasculature 33. The level of autophagy in EPCs from diabetic patients is decreased, and upregulating autophagy can improve the survival and function of EPCs under diabetic conditions34. Recently, the DPP-4 inhibitor vildagliptin has been reported to reduce acute mortality after myocardial infarction with the restoration of autophagy in type 2 diabetes 11. In the present study, we found that HG treatment decreased the autophagy level in EPCs and that sitagliptin could restore the autophagy levels (Fig. 5A and B). Furthermore, the protective effects of sitagliptin on EPCs are orchestrated by the induction of autophagy as the autophagy inhibitor, 3-MA, abrogated sitagliptin-induced protection against apoptosis and angiogenic dysfunction under HG conditions (Fig. 6).
However, the role of ROS in the induction of autophagy still remains controversial. For example, recent studies have found that ROS inhibits autophagy 35, 36, whereas other studies have shown that natural compounds can protect cells against ROS damage via elevating autophagy 37, 38. In our study, we found that sitagliptin induced the autophagy of EPCs under HG conditions, which was accompanied by reduced ROS levels, indicating a possible link between sitagliptin-induced autophagy and the reduced ROS levels of EPCs. The underlying mechanism needs to be investigated in future studies.
AMPK is a serine/threonine kinase that stimulates catabolic processes and inhibits anabolic processes to restore ATP levels when cellular energy is low 39. It is well-known that activation of AMPK promotes starvation-induced autophagy through interactions with mTORC1 and/or ULK-1 40, 41. However, whether AMPK regulates basal autophagy in the excess nutrient environment is controversial. He et al. 42 found that activation of AMPK overcomes the autophagy impairment in myoblasts induced by HG; in addition, the authors also found that activation of AMPK restores autophagy in rodent macrophages exposed to palmitate, following stimulation with lipopolysaccharide 43. However, in human aortic endothelial cells, AMPK activation cannot restore autophagy impaired by HG and palmitate 44. In the present study, we showed that sitagliptin preserved the phosphorylation of AMPK and ULK1 and inhibited the phosphorylation of mTOR under HG treatment conditions, eventually resulting in the restoration of autophagy in EPCs, which could be blocked by the AMPK inhibitor compound C (Fig. 7). These findings indicate that sitagliptin-induced restoration of autophagy in EPCs under HG conditions is mediated by the activation of the AMPK signalling pathway. However, how sitagliptin activates AMPK-mediated autophagy signalling in EPCs under diabetic conditions warrants further investigation.
Acknowledgements
This study was supported by Natural Science Foundation of Zhejiang Province (LY16H070006, LY14H070007, LY16H070005), Key Science and Technology Development Plan from Wenzhou City (Y20100001, Y20140654), Research Development Fund of Wenzhou Medical University (QTJ15014, QTJ13007), a Junior Faculty Award from American Diabetes Association (1-13-JF-53), the National Natural Science Foundation of China grants (91639111, 81573435, 81273509, 81200239, 30971209, 81470061, 81671378), a Key New Drug Development Grants (2012ZX09103-301-016), a Changjiang Innovation Team Program (2010R50042-17), and the Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences (YKFJ002), Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (CQKLBST-2015-007), and ‘Scientific Research Fund of Sichuan Provincial Education Department (17ZA0101)’.
Conflict of interest
The authors confirm that there are no conflicts of interest.




