KITLG is a novel target of miR-34c that is associated with the inhibition of growth and invasion in colorectal cancer cells
Abstract
MiR-34c is considered a potent tumour suppressor because of its negative regulation of multiple target mRNAs that are critically associated with tumorigenesis and metastasis. In the present study, we demonstrated a novel target of miR-34c, KITLG, which has been implicated in colorectal cancer (CRC). First, we found a significant negative relationship between miR-34c and KITLG mRNA expression levels in CRC cell lines, including HT-29, HCT-116, SW480 and SW620 CRC cell lines. In silico analysis predicted putative binding sites for miR-34c in the 3′ untranslated region (3′UTR) of KITLG mRNA. A dual-luciferase reporter assay further confirmed that KITLG is a direct target of miR-34c. Then, the cell lines were infected with lentiviruses expressing miR-34c or a miR-34c specific inhibitor. Restoration of miR-34c dramatically reduced the expression of KITLG mRNA and protein, while silencing of endogenous miR-34c increased the expression of KITLG protein. The miR-34c-mediated down-regulation of KITLG was associated with the suppression on proliferation, cellular transformation, migration and invasion of CRC cells, as well as the promotion on apoptosis. Knockdown of KITLG by its specific siRNA confirmed a critical role of KITLG down-regulation for the tumour-suppressive effects of miR-34c in CRC cells. In conclusion, our results demonstrated that miR-34c might interfere with KITLG-related CRC and could be a novel molecular target for CRC patients.
Introduction
The 20–24 nucleotide microRNAs post-transcriptionally regulate the expression of their target genes via either translational repression or mRNA degradation by binding to the complementary seed sites within the 3′ untranslated region (3′UTR) of the target mRNA. It is widely believed that microRNAs modulate diverse biological processes, and aberrant expression of microRNAs has been implicated in multiple pathological processes, including tumorigenesis and metastasis, as these genes typically serve as either oncogenes or tumour suppressor genes, depending on their targets 1-5. Recent works have shown that Overexpression of miR-34c induces apoptosis and inhibits cell proliferation and invasion in a variety of tumours 6-10. Therefore, miR-34c is considered a potent tumour suppressor. The tumour-suppressive role of miR-34c can be attributed to the down-regulation of its target genes that are critically associated with cell apoptosis, proliferation and invasion. For example, miR-34c negatively regulates the oncogenes E2F3 and BCL-2 and subsequently suppresses proliferation and stimulates apoptosis in prostate cancer cells 8. MiR-34c was also found to silence BMF and c-MYC, which contribute to the cell's resistance to paclitaxel-induced apoptosis 10. Ectopic expression of miR-34c inhibits the epithelial-mesenchymal transition (EMT) and suppresses migration of breast tumour-initiating cells through the silencing of NOTCH4 9. Additionally, a few publications have shown that miR-34c is down-regulated in colorectal cancer (CRC) specimens and plays a tumour-suppressive role in CRC by targeting SNAIL1, a transcriptional repressor linked to the EMT programme and tissue-invasive activity, and some components of the canonical Wnt signalling cascades, which control cancer stem cell generation and cancer progression 11-14.
Colorectal cancer is the 3rd most common gastrointestinal cancer and one of the leading causes of death worldwide. With emerging evidence supporting the tumour-suppressive role of miR-34c, miR-34c down-regulation could be one of the causes and novel therapeutic strategies for CRC. However, because the neoplastic growth and invasion of CRC are a result of a network of multiple signalling molecules, efforts are being made to figure out how miR-34c is involved in CRC suppression. KITLG, also known as stem cell factor, steel factor and mast cell growth factor, has multiple biological functions during the development of mice, rats and humans by triggering its receptor tyrosine kinase, c-KIT 15-17. Interest in KITLG has been bolstered by work showing that aberrant expression of KITLG has been implicated in the development of several cancers. For instance, elevated KITLG expression contributes to the carcinogenesis of uveal melanoma 18, neurofibromatosis type 1 19, gliomagenesis 20, breast cancer 21 and non-small-cell lung cancer 22, presumably because of the c-KIT/KITLG autocrine/paracrine stimulation-loop mechanism 23, 24. Moreover, Bellone et al. 25-27 reported that CRC tissues over-express KITLG, which is required for cancer cell growth, migration and invasion. However, the regulation of KITLG expression in CRC still remains largely unexplored.
Therefore, in the present study, we investigated the interaction between miR-34c and KITLG in CRC cell lines, and demonstrated that KITLG is a direct target of miR-34c and mediates the role that miR-34c plays in proliferation, migration and invasion in CRC cells. Our results might be helpful in improving the current understanding of CRC development, as well as improving the diagnosis and treatment of CRC.
Materials and methods
Cell culture
The human CRC cell lines HT-29, HCT-116, SW480 and SW620, as well as the African green monkey kidney fibroblast COS-7 cell line, were purchased from ATCC. The HT-29 and HCT-116 cell lines were cultured in DMEM (Gibco, Carlsbad, CA, USA); the SW480 and SW620 cells were grown in RPMI-1640 (Gibco); the COS-7 cells were grown in Opti-MEM (Gibco). All mediums were supplemented with 10% foetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin (P/S, Gibco). The cells were grown at 37°C in the presence of 5% CO2.
Immunofluorescence
The cells were fixed with 4% paraformaldehyde for 30 min. Non-specific binding was blocked by incubation with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) for 1 hr. The cells were incubated with the mouse anti-KITLG (1:200; Santa Cruz, Dallas, TX, USA) or rabbit anti-c-KIT (1:200; Cell Signaling Technology, Beverly, MA, USA) primary antibody overnight at 4°C, followed by incubation with the corresponding secondary antibodies for 1 hr at 25°C. The cells were mounted by using DAPI mounting medium (Zhongshan Jinqiao Biotechnology, Beijing, China) and were observed with a fluorescence microscope (Nikon, 80i, Tokyo, Japan). The negative control cells were incubated with an isotype control antibody or without a primary antibody.
Lentiviral vector construction and infection
The full length pre-miR-34c was chemically synthesized by GeneChem and was introduced into the GV217 lentiviral vector (GeneChem, Shanghai, China, Fig. S1) in the unique EcoRI site to construct a lentivirus encoding miR-34c (lenti-miR-34c); this construct was confirmed by using nucleotide sequencing. The specific inhibitor of miR-34c was constructed by cloning the complementary nucleotides of miR-34c into the GV280 lentiviral vector (GeneChem, Fig. S1) between the AgeI and EcoRI sites. The cells were seeded in a 6-well plate at a density of 5 × 104 cells/well and were infected with lenti-miR-34c or its inhibitor when the cells reached 30% confluence. Three days after infection, the efficiency of infection was evaluated by observing the EGFP expression by using a fluorescence microscope (Nikon, 80i).
RNA extraction and Real-time PCR
Total RNA was extracted from the cultured cells by using the TRIzol reagent (Invitrogen), and microRNA was extracted by using the miRNApure Mini Kit (CWBiotech, Beijing, China), according to the manufacturers’ instructions.
For mRNA, the reverse transcription reactions were performed with the Super cDNA First-Strand Synthesis Kit (CWBiotech). Real-time PCR was performed in an ABI 7500 real-time PCR system (Applied Biosystems, Carlsbad, CA, USA) by using the Ultra SYBR Mixture with ROX (CWBiotech). The following primers were used: KITLG (Forward: CAGAGTCAGTGTCACAAAACCATT, Reverse: TTGGCCTTCCTATTACTGCTACTG); c-KIT (Forward: GATTATCCCAAGTCTGAGAATGAA; Reverse: CGTCAGAATTGGACACTAGGA); GAPDH (Forward: AGAAGGCTGGGGCTCATTTG, Reverse: AGGGGCCATCCACAGTCTTC). The reactions were incubated at 95°C for 10 min., followed by 40 cycles of 95°C for 15 sec. and 60°C for 1 min.
For microRNA, reverse transcription was performed with the Taqman microRNA RT Kit (Invitrogen) and the Taqman microRNA Assay with specific stem-loop primers (Assay ID 428 for miR-34c and 1973 for RNU6B; Invitrogen). Real-time PCR was performed with the Taqman Universal Master Mix II (Invitrogen) and the Taqman microRNA Assay (Invitrogen). The reactions were incubated at 95°C for 10 min., followed by 40 cycles of 95°C for 15 sec. and 60°C for 1 min.
All reverse transcription reactions included no-template controls, and all PCR reactions were run in triplicate. Relative gene expression was determined by using the comparative CT (2−ΔΔCt) method.
Western blot analysis
The harvested cells were suspended in cell lysis buffer. After 12% SDS-PAGE, the proteins were transferred onto a PVDF membrane (Millipore, Temecula, CA, USA) and blocked with Tris-buffered saline containing 0.05% Tween-20 (TBST) and 5% non-fat dry milk for 1 hr. The membrane was incubated with the rabbit anti-KITLG (1:500; Abcam, Cambridge, UK) or rabbit anti-c-KIT (1:1000; Cell Signaling Technology) primary antibodies at 4°C overnight. Then, the membrane was incubated with HRP-conjugated secondary goat anti-rabbit IgG (1:2000; Santa Cruz) for 1 hr. The proteins were detected by using ECL chemiluminescence. A mouse anti-GAPDH (1:1000; Applygen, Beijing, China) antibody was used as an internal control.
Soft agar colony formation assay for anchorage-independent growth
The bottom of a 6-well plate was coated with 0.6% low melting agarose (Promega, Madison, WI, USA), which was then covered with 1.4 ml of 0.35% agarose containing 1000 cells either infected with miR-34c or its inhibitor. The 6-well plate was incubated for 14 days at 37°C with 5% CO2. Colonies were photographed by using an inverted phase contrast microscope (Leica DMI3000 B, Wetzlar, Germany). Ten visual fields were sampled with a systemic random sampling method, and the colonies were counted through stereological technique.
Real-time monitoring of cellular proliferation, migration and invasion
We employed the ‘xCELLigence’ system (ACEA, Biosciences, San Diego, CA, USA), which consists of E-plates and the Real Time Cell Analyzer Dual Purpose (RTCA-DP) instrument, to monitor cell proliferation by measuring the cell index (CI), which is proportional to the number of cells 28. The cells were seeded in E-plates at a density of 8000 cells/well. The E-plates were then transferred to the RTCA-DP instrument for automated real-time monitoring at standard incubator conditions; CI measurements were collected every 15 min. for the following 96 hrs.
Cellular migration and invasion were also monitored by using the ‘xCELLigence’ system on CIM (Cell Invasion-and-Migration)-plates, instead of E-plates. The CIM-plates are made by integrating microelectronic sensors onto the underside of the microporous membrane of the upper chamber. Cells capable of migrating from the upper chamber through the microporous membrane into the bottom chamber will contact and adhere to the sensors, leading to an increase in the impedance signals detected by the sensors. The impedance increase is correlated with numbers of cells that have migrated to the underside of the membrane. Therefore, cell migration activity can be monitored with the impedance readouts 28. Our migration assays were performed by seeding cells in the upper chambers of the CIM-plates in serum-free medium at a density of 20,000 cells per well. The bottom chambers of the CIM-plates were filled with serum-containing medium to promote migration across the membranes towards the serum gradient. After seeding, the CIM-plates were transferred into the RTCA-DP instrument for real time read-outs during 48 hrs. Impedance or the CI was registered only from the cells that were capable of migrating through the membranes. Four independent experiments were performed. For the invasion assays, the protocol was identical to that for the migration assay, except that the upper chambers were loaded with 30 μl of a 10% Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) solution to create a 3D biomatrix film in each well prior to cell seeding.
Apoptosis analysis
Cell apoptosis was detected with the Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit with Alexa® Fluor 488 Annexin V and PI for Flow Cytometry (Invitrogen) according to the manufacturers’ instruction.
Dual-luciferase reporter assay
The 3′UTR sequences of KITLG containing the predicted seed regions of miR-34c were chemically synthesized by GeneChem. The fragment was introduced into the GV306 luciferase reporter vector at the unique XbaI site, which is downstream of the Firefly luciferase stop codon and is followed by the Renilla luciferase sites (GeneChem, Fig. S1). The seed regions of miR-34c in the KITLG 3′UTR were mutated to construct GV306-KITLG-MUT. The GV262-miR-34c construct was created by introducing the miR-34c sequence into the GV262 vector at the XhoI/EcoRI sites (GeneChem, Fig. S1). A construct containing an unrelated microRNA was used as a negative control (GV262-miR-control).
The GV262-miR-34c construct was transiently transfected with GV306-KITLG or GV306-KITLG-MUT into COS-7 cells by using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Twenty-four hours after transfection, the Firefly and Renilla luciferase activities were measured by using the Dual Luciferase Assay System (Promega) in a Modulus™ II Microplate Multimode Reader (Promega), according to the manufacturers’ protocols. The ratio of Firefly/Renilla activity was calculated.
KITLG-specific small interfering RNA (siRNA) treatment
For specific gene knockdown on KITLG mRNA, siRNA targeting KITLG and negative control siRNA were purchased from Ribobio Co., Ltd (Guangzhou, China). siRNAs were transfected into HCT-116 cells by using riboFECT™ CP Transfection Kit (Ribobio) according to the manufacturer's instructions. Total RNA and total protein were prepared 48 hrs post-transfection and used for real-time PCR and western blot analysis, respectively. Furthermore, cellular proliferation, invasion and apoptosis after siRNA treatment were performed.
Statistics
The results were presented as the mean ± SEM and analysed by using a Student's t-test or one-way anova with the SPSS 13.0 software (Chicago, IL, USA). Bivariate correlation was analysed by using the Spearman method with SPSS 13.0. A P-value of 0.05 or less was considered statistically significant.
Results
Expression of KITLG and c-KIT in CRC cell lines
The autocrine/paracrine stimulation loop of c-KIT/KITLG signalling is one mechanism of malignant cells 25. Therefore, we first screened four CRC cell lines for the presence of the mRNA and protein of KITLG and its receptor c-KIT The mRNA and protein expressions of KITLG and c-KIT were determined in these CRC cell lines (Fig. 1A and B).
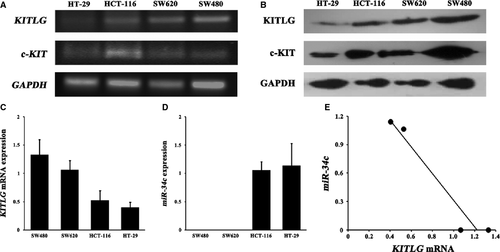
miR-34c is inversely correlated with KITLG mRNA expression
All CRC cells showed apparent but variable levels of KITLG mRNA expression (Fig. 1C). Although miR-34c was not detectable in the SW480 and SW620 cells, it was expressed in the HCT-116 and HT-29 cells (Fig. 1D). Notably, statistical analysis revealed a significant inverse correlation between the endogenous KITLG mRNA and miR-34c expression levels (rs = −0.913, P < 0.05; Fig. 1E).
KITLG is a direct target of miR-34c
We performed in silico analysis with the TargetScanHuman, MiRanda and miRBase programmes to search for all putative targets of miR-34c, and a cross-checked list revealed hundreds of predicted targets. For the purposes of uncovering the relevant targets that would modulate the phenotypic effects in CRC, we then performed a Functional Annotation Clustering of the combined predicted targets by using the DAVID program (http://david.abcc.ncifcrf.gov/). The KEGG pathways that were enriched with potential miR-34c targets (enrichment score = 2.15) were cancer related (P = 0.015), and KITLG was one of the most likely targets within these pathways in cancer. We further predicted a potential miR-34c seed region at nt 2644-2651 of the KITLG 3′UTR (NM_000899). To determine whether KITLG is a bona fide target of miR-34c, we constructed a luciferase reporter plasmid containing the seed region of the KITLG 3′UTR that was complementary to miR-34c (Fig. 2A). Additionally, we generated a mutant reporter in which the whole 8 nucleotides of the seed region in the KITLG 3′UTR were mutated (Fig. 2A). Using a dual-luciferase reporter assay, transfection of the KITLG 3′UTR in combination with miR-34c significantly reduced the luciferase activity, whereas miR-34c had no influence on the luciferase activity of the mutated KITLG 3′UTR construct (P < 0.01, Fig. 2B), suggesting that the inserted fragment from the KITLG 3′UTR (nt 2644-2651) is a direct target of miR-34c.
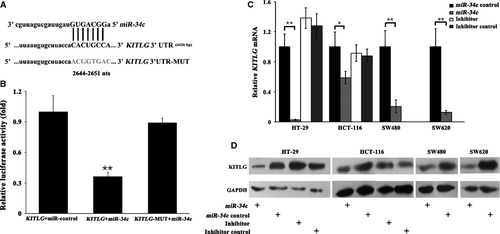
To assess the consequences of the up- or down-regulation of miR-34c, CRC cells were infected with miR-34c or its inhibitor, and the mRNA and protein levels of KITLG were analysed. Three days post-infection, the infection efficiency of miR-34c was determined by immunofluorescence staining and real-time PCR analysis and suggested that the lentiviral-mediated miR-34c constructs were efficiently transduced into the CRC cells; consequently, the expression level of miR-34c was significantly increased compared to the controls (P < 0.01), whereas the level of the unrelated miR-214 remained unchanged (P > 0.05; Fig. S2). As expected, both the mRNA and protein levels of KITLG in the cells infected with miR-34c were significantly decreased (P < 0.05 or 0.01, Fig. 2C and D). In contrast, the silencing of endogenous miR-34c by its specific inhibitor resulted in the increased expression of KITLG protein (P < 0.05, Fig. 2D), but not KITLG mRNA, in the HCT-116 and HT-29 cells (P > 0.05, Fig. 2C). Because miR-34c was undetectable in the SW480 and SW620 cells, we did not perform the miR-34c inhibitor infection in these two cell lines. These results indicated that miR-34c negatively regulates the expression of KITLG in CRC cells.
miR-34c-mediated down-regulation of KITLG is associated with cellular proliferation and apoptosis
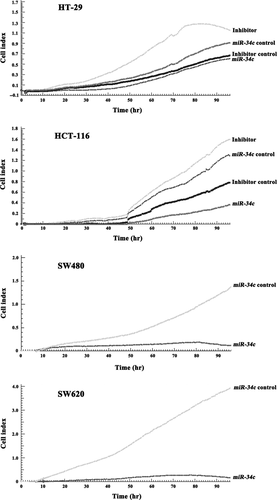
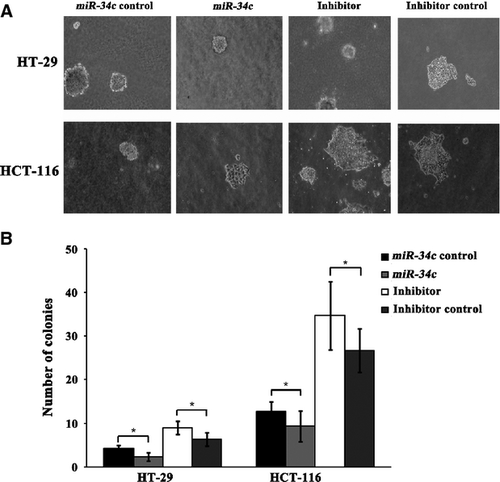
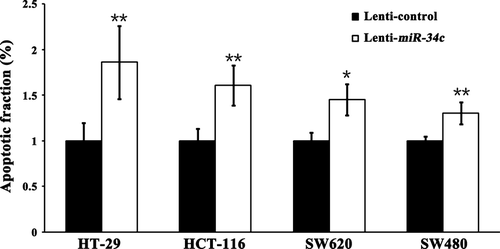
Next, we investigated the consequences of ectopic miR-34c expression on cellular proliferation and apoptosis. Overexpression of miR-34c resulted in a decrease in cellular proliferation by approximately 32.3% in HT-29 cells (P < 0.01), 71.1% in HCT-116 cells (P < 0.01), 91.5% in SW480 cells (P < 0.01) and 93.2% in SW620 cells (P < 0.01); on the other hand, silencing of miR-34c increased cell proliferation by 66.7% in HT-29 cells (P < 0.01) and 50.3% in HCT-116 cells (P < 0.01; Fig. 3). Meanwhile, to determine whether the miR-34c-mediated down-regulation of KITLG could affect the capacity of CRC cells to grow in a semisolid medium, HT-29 and HCT-116 cells were seeded in soft agar and allowed to grow for 14 days, and the number of colonies from the HT-29 and HCT-116 cells infected with miR-34c was significantly less than those of the controls, while the number of colonies from cells infected with the miR-34c inhibitor was significantly increased compared to the controls (Fig. 4). Moreover, infection with miR-34c induced more pronounced apoptosis, as determined by Annexin V/PI double-staining by using flow cytometry. The apoptotic fraction was elevated by 86.0% in HT-29 cells (P < 0.01), 60.5% in HCT-116 cells (P < 0.01), 45.2% in SW620 cells (P < 0.05) and 29.9% in SW480 cells (P < 0.05; Fig. 5). These results support an inhibitory effect of miR-34c on CRC tumorigenesis, with variable extents in the different CRC cell lines.
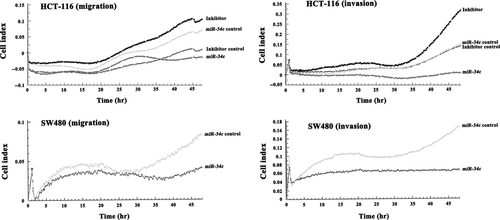
Disruption of KITLG by miR-34c abrogates cellular migration and invasion
It has been reported that exogenous KITLG enhanced the cellular migration and invasion of the Colo320 CRC cell line 29. Here, we tested whether infection with miR-34c might interfere with the migratory and invasive capacities of HCT-116 and SW480 cells. As determined by RTCA, the ectopic expression of miR-34c led to a significant reduction in the migratory capacity of HCT-116 (52.0%, P < 0.01) and SW480 cells (70.0%, P < 0.01; Fig. 6). The invasive capacity of the cells to migrate across a Matrigel biofilm was decreased by 77.8% (P < 0.01) in SW480 cells and 95.7% in HCT-116 cells (P < 0.01) after miR-34c infection. Conversely, the silencing of miR-34c enhanced the migratory (50.0%, P < 0.05) and invasive (80.8%, P < 0.01) abilities of HCT-116 cells (Fig. 6).
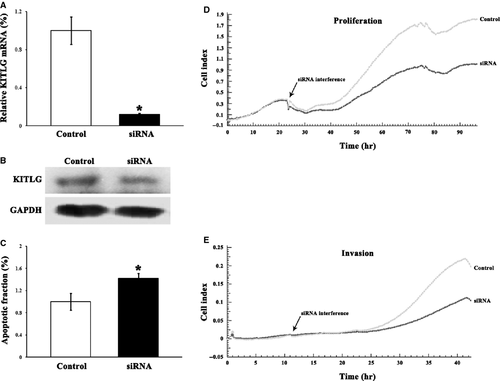
Knockdown of KITLG by specific siRNA contributes to tumour suppression
To observe that the tumour-suppressive effects of miR-34c are caused by regulation of KITLG, KITLG was effectively knocked down by 88.1% in HCT-116 cells by its specific siRNA (Fig. 7). The down-regulation of KITLG suppressed cellular proliferation by 31.8%, invasion by 46.1%, and increased apoptosis by 42.2%, respectively (Fig. 7).
Discussion
A growing number of studies have shown that the overexpression of KITLG in CRC cells is correlated with tumorigenesis and metastasis 25, 27, 29, which prompted us to examine the regulation of KITLG expression in CRC cells. MicroRNAs are one of the important post-transcriptional regulators of their target genes. In the current study, we provided evidence that KITLG is a new target of miR-34c, and miR-34c activation negatively regulates KITLG-mediated cellular proliferation, transformation, apoptosis, migration and invasion of CRC cells.
KITLG is absent in normal colonic epithelium, but it has been detected in most cancer specimens 27. In contrast, miR-34c is hypo-expressed in CRC tumour tissues because of CpG methylation of its promoter region 7, 14, 30. In the present study, we showed that KITLG was detected in HT-29, HCT-116, SW480 and SW620 cells, and the level of KITLG mRNA was reversely correlated with the level of miR-34c. In silico analysis predicted that the KITLG 3′UTR contains miR-34c binding sites, and a dual-luciferase reporter assay further confirmed that miR-34c represses luciferase activity by targeting the binding sites in the KITLG 3′UTR, indicating a direct interaction between miR-34c and KITLG mRNA. MicroRNAs control their target mRNAs through degradation and/or translational repression. Kaller et al. showed that one member of the miR-34 family, miR-34a, mediates its major target mRNAs through both degradation and translational repression 31. In line with Kaller et al. 31, Siemens et al. 32 suggested that the up-regulation of miR-34a is sufficient to reduce the expression of its target, c-KIT, at the mRNA and protein levels 32. Here, we demonstrated that the ectopic expression of another member of the miR-34 family, miR-34c, caused a reduction in KITLG mRNA and protein in CRC cells, suggesting that miR-34c could affect both KITLG mRNA degradation and translational repression. The miR-34c-mediated down-regulation of KITLG protein could be counteracted by the introduction of a miR-34c specific inhibitor. Collectively, our results suggested that miR-34c critically regulates the expression of KITLG in CRC cells.
Several lines of evidence have established that KITLG and its tyrosine kinase receptor, c-KIT, are involved in tumorigenesis. One general mechanism of c-KIT activation in malignant cells has been described as an autocrine/paracrine stimulation loop triggered by its ligand KITLG 25. Tumorigenesis results from a disruption of the balance between proliferation and apoptosis. Krasagakis et al. found that the proliferation index is in generally high and the apoptosis rate is low in Merkel cell carcinoma, which co-expresses c-KIT and KITLG 33. The c-KIT/KITLG axis plays an important role in the self-renewal and proliferation of cancer stem cells in non-small-cell lung cancer 22. In this study, we showed that KITLG expression was significantly suppressed after infection with miR-34c, and the miR-34c-induced down-regulation of KITLG inhibited proliferation but promoted apoptosis in CRC cells that concomitantly express c-KIT and KITLG. To evaluate the potential role of miR-34c in affecting anchorage-independent growth, which is an important malignant property of tumour cells, the four CRC cell lines were infected with miR-34c or its inhibitor; these experiments showed that miR-34c could inhibit the growth of tumour cell colonies. Moreover, our results demonstrated that the migratory and invasive abilities of the HCT-116 and SW480 cells were significantly suppressed following infection with miR-34c. We performed an additional in silico analysis via the cBio Cancer Genomics Portal (http://cbioportal.org). There are 59 invasive colorectal adenocarcinoma cases available in the database; among them, 1 case (TCGA-AA-3680) shows homozygous deletion of miR-34c and 3 cases (TCGA-AA-3561, TCGA-AG-3586 and TCGA-AG-3892) exhibit KIT mRNA up-regulation but none cases show decreased miR-34a or KITLG alteration. These in silico data along with the results from Siemens et al. 32 and our present study suggest that miR-34 and KIT/KITLG signalling might be involved in the invasive CRC, which still needs further experiment to confirm. Furthermore, to show a critical role of KITLG down-regulation for the tumour-suppressive effects of miR-34c in CRC cells, siRNA-mediated knockdown of KITLG was applied. siRNA against KITLG in HCT-116 cells resulted in significant inhibition in cellular proliferation and invasion, but promotion in apoptosis. Thus, we proposed that miR-34c might function as a tumour suppressor in CRC by targeting KITLG to disrupt the c-KIT/KITLG autocrine/paracrine stimulation loop. It was noted that the outcome of KITLG knockdown was not completely parallel with what miR-34c overexpression brought about, indicating that there are likely other targets of miR-34c implicated in the CRC suppression.
The miR-34 family is composed of miR-34a, miR-34b and miR-34c and mediates tumour cell activities through cell cycle arrest, apoptosis or senescence, and metastasis 34-36, depending on its targets, such as the oncogenes BCL-2, c-MYC and c-MET 8, 10, 35. Recently, Siemens et al. demonstrated that the miR-34 family, particularly miR-34a, represses c-KIT in CRC cell lines, which interferes with several c-KIT-mediated effects in CRC cells 32. They further found that, after stimulation with KITLG, Colo320 CRC cells displayed increased migration and invasion, whereas ectopic expression of miR-34a inhibited the KITLG-induced migration and invasion 32. They concluded that the tumour-suppressive role of miR-34a may be potentially relevant to the repressed c-KIT expression in CRC cells 32. We found that both miR-34a and miR-34c could repress the expressions of c-KIT and KITLG by the use of in silico analysis and dual-luciferase reporter assays; and miR-34a more significantly down-regulated c-KIT expression than miR-34c did, while miR-34c played a more robust role in down-regulating KITLG expression than miR-34a did (data not shown). It seems that miR-34a mainly down-regulated the c-KIT expression, while miR-34c mainly down-regulated the KITLG expression; and the mechanism is needed to be studied in the future. Since Siemens et al. 32 have reported the regulation of c-KIT by miR-34 family, we here only reported the regulation of KITLG by miR-34c which showed a more significant suppression of KITLG expression to avoid the re-publication of similar data. Suppressed KITLG expression by miR-34c in CRC cell lines consequently inhibited cellular transformation, proliferation, migration and invasion, as well as promoted apoptosis. Clinical observations revealed that the aberrant expression of the c-KIT/KITLG system is involved in a subgroup of CRC. Although only a small proportion of CRC patients expressed c-KIT in their tumours 37, 38, the concomitant expression of c-KIT and KITLG is significantly associated with worse clinical outcome 27, most likely caused by the high proliferation rate and the low apoptosis rate of these tumour cells. Given that proliferation and apoptosis, as well as invasion, are critically correlated with the c-KIT/KITLG system, we propose that miR-34a/c might suppress these activities in CRC cells by inhibiting the expression of ' and KITLG.
Activation of c-KIT by KITLG results in the activation of several downstream cascades, such as the MAPK, STAT and PI3K/Akt pathways. The KITLG-enhanced proliferation and invasion of KIT-positive CRC cells has been shown to be achieved mainly through the PI3K/Akt pathway 29. Interestingly, miR-34c also directly down-regulates the expression of c-MET, which causes similar alterations in its downstream target, p-Akt 39. Collectively, the PI3K/Akt pathway might be influenced by the miR-34c-mediated down-regulation of KITLG expression in CRC, and this possibility should be investigated. Moreover, the miR-34 family is involved in Wnt signalling cascades by suppressing the transcriptional activity of the β-catenin-T cell factor/lymphoid enhancer factor (LEF) complexes by targeting a set of Wnt pathway-regulated genes, including WNT1, WNT3, LRP6, β-catenin, LEF1 and Axin2. We also noticed that there was cross-talk between the PI3K/Akt and Wnt pathways via GSK-3β, which can possibly expand and enhance the inhibitory effects of miR-34 in cancers 11, 12.
In conclusion, our present study demonstrated the tumour-\suppressive abilities of miR-34c in CRC cells by targeting KITLG in vitro. Restoration of miR-34c expression resulted in the inhibition of cell growth, transformation, migration and invasion, accompanied by decreased KITLG expression. The miR-34c-KITLG pathway may be helpful in enhancing our understanding of the molecular mechanisms of CRC tumorigenesis and metastasis. Taken together, our findings suggested that miR-34c likely has a diagnostic advantage and may be a novel molecular target for CRC patients.
Acknowledgements
This study was supported by the National Science Foundation of China (81300285, 31371220, 31271290, 81101769), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20111107110012, 20121107120020), the Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201310025020), the Science Foundation of Capital Medical University (2014JS11) and the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of Beijing Municipality (PHR201007113).
Conflicts of interest
The authors declare that they have no conflicts of interest.




