Blood pressure response to treatment of obese vs non-obese adults with sleep apnea
Funding information
Support: NIH HL094307 and the Icelandic Research Fund (174067-051). Erna Arnardóttir reports personal fees from Nox Medical and personal fees from ResMed, outside the submitted work. Julio A. Chirinos reports non-financial support from ResMed; grants and personal fees from Bristol-Myers Squibb; personal fees from Merck; personal fees from Akros; grants, personal fees, and non-financial support from Fukuda Denshi; grants and personal fees from Microsoft; personal fees from Bayer; personal fees from Pfizer; non-financial support from AtCor Medical; non-financial support from Uscom; personal fees from OPKO; personal fees from Sanifit; and personal fees from Ironwood Pharmaceuticals, outside the submitted work. Thorarinn Gislason owns 0.76% of stocks in Nox Medical, outside the submitted work. Allan I. Pack reports that he is the John Miclot Professor of Medicine. Funds for this endowment were provided by the Phillips Respironics Foundation, outside the submitted work. Raymond R. Townsend reports personal fees from Medtronic, personal fees from Rox, and personal fees from UpToDate, outside the submitted work. The following authors have nothing to disclose: Bryndís Benediktsdóttir, Sigrun Gudmundsdóttir, Xiaofeng Guo, Brendan T. Keenan, Samuel Kuna, David Maislin, Greg Maislin, Frances M. Pack, Richard Schwab, Andrea Sifferman, and Beth Staley.
Abstract
Many patients with obstructive sleep apnea (OSA), but not all, have a reduction in blood pressure (BP) with positive airway pressure (PAP) treatment. Our objective was to determine whether the BP response following PAP treatment is related to obesity. A total of 188 adults with OSA underwent 24-hour BP monitoring and 24-hour urinary norepinephrine collection at baseline. Obesity was assessed by waist circumference, body mass index, and abdominal visceral fat volume. Participants adherent to PAP treatment were reassessed after 4 months. Primary outcomes were 24-hour mean arterial pressure (MAP) and 24-hour urinary norepinephrine level. Obstructive sleep apnea participants had a significant reduction in 24-hour MAP following PAP treatment (−1.22 [95% CI: −2.38, −0.06] mm Hg; P = .039). No significant correlations were present with any of the 3 obesity measures for BP or urinary norepinephrine measures at baseline in all OSA participants or for changes in BP measures in participants adherent to PAP treatment. Changes in BP measures following treatment were not correlated with baseline or change in urinary norepinephrine. Similar results were obtained when BP or urinary norepinephrine measures were compared between participants dichotomized using the sex-specific median of each obesity measure. Greater reductions in urinary norepinephrine were correlated with higher waist circumference (rho = −0.21, P = .037), with a greater decrease from baseline in obese compared to non-obese participants (−6.26 [−8.82, −3.69] vs −2.14 [−4.63, 0.35] ng/mg creatinine; P = .027). The results indicate that the BP response to PAP treatment in adults with OSA is not related to obesity or urinary norepinephrine levels.
1 INTRODUCTION
Numerous randomized controlled trials demonstrate that some individuals with OSA are more likely than others to have a blood pressure (BP) response following treatment with positive airway pressure (PAP).1-4 Existing evidence indicates that improvement in BP with PAP treatment is more likely to occur in individuals who are younger, hypertensive, have more severe OSA, and adhere to PAP treatment.1-5 Although obesity is a risk factor for OSA and an independent risk factor for hypertension,6, 7 the influence of obesity on the BP response to PAP treatment in adults with OSA is unknown. The current study was designed to determine whether the BP response to PAP treatment is related to obesity.
We also evaluated the relationship of sympathetic activity, as assessed by 24-hour urinary norepinephrine level, to the BP response following 4 months of PAP treatment. Both obesity and OSA are associated with increased sympathetic activity.8, 9 We hypothesized that a greater reduction in urinary norepinephrine level following PAP treatment would occur in more obese adults with OSA and that the improvements in BP following PAP treatment would be associated with decreased urinary norepinephrine excretion.
2 METHODS
2.1 Study population
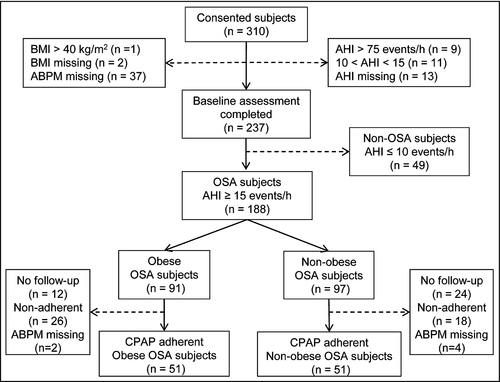
Consecutive adults referred for evaluation of suspected OSA were invited to participate. Inclusion criteria included age 40-65 years and apnea-hypopnea index (AHI) ≥15 to <75 events/h. Individuals were excluded for the following reasons: diagnosis of another sleep disorder in addition to OSA; previous surgical or PAP treatment for OSA; oxygen treatment; and BMI > 40 kg/m2. All inclusion and exclusion criteria are detailed in the online supplement. Written informed consent was obtained from all participants, and the protocol was approved by the Institutional Review Board at the University of Pennsylvania and the University of Iceland. The project was registered at https://clinicaltrials.gov (NCT01578031). Processing of all assessment procedures, including polysomnograms (PSG), ambulatory blood pressure monitoring (ABPM), abdominal magnetic resonance imaging (MRI), and urine assays, was performed at the University of Pennsylvania.
2.2 Obesity measurements
Obesity measurements were obtained at baseline and the 4-month follow-up. Baseline waist circumference was chosen as the primary measure to define obesity in order to better capture visceral obesity level, which is often used as a clinical determinant of OSA and associated with increased risk for cardiometabolic diseases.10, 11 BMI and abdominal visceral fat volume (captured with MRI) at baseline were used as secondary measures to assess obesity status (see online supplement).12
2.3 Diagnostic polysomnogram
Overnight PSGs were performed to diagnose OSA using current practice guidelines.13 Apneas were defined as an absence of airflow on the oronasal thermistor and nasal pressure signals for at least 10 seconds. Hypopneas were defined as a greater than 30% reduction from baseline in airflow for at least 10 seconds associated with at least a 3% oxygen desaturation and/or arousal. Apnea-hypopnea index was calculated as the mean number of apneas and hypopneas per hour of sleep.
2.4 Ambulatory blood pressure monitoring
At baseline and the 4-month follow-up, participants underwent 24-hour ABPM (Spacelabs Model 90217-1A, Spacelabs) using current guidelines (see online supplement).14 Wake and sleep periods were determined from simultaneous wrist-worn actigraph recordings (Actiwatch 2, Philips Respironics) and sleep diaries obtained during ABPM data collection. Our primary ABPM outcome measure was 24-hour mean arterial pressure (MAP). We chose MAP as our primary outcome because ABPM measures MAP directly and meta-analyses report a statistically significant decrease in mean systemic arterial pressure following CPAP treatment.1, 2 Daytime and nocturnal ABPM measurements were considered secondary. Participants with a 24-hour systolic BP > 130 mm Hg at baseline were classified as hypertensive in secondary analyses. Office BP measurements were obtained at baseline and the 4-month follow-up (see online supplement for results).
2.5 Urinary norepinephrine level
At baseline and the 4-month follow-up, participants performed a 24-hour urine collection for measurement of 24-hour urinary norepinephrine using liquid chromatography.15 Creatinine levels were measured using a commercial colorimetric kit (Cayman Chemical), and norepinephrine concentration was expressed as ng/mg creatinine to control for collection issues.
2.6 Positive airway pressure treatment
Following baseline assessment, participants were started on auto-adjusting or fixed PAP treatment (ResMed S9, ResMed, Inc). The PAP device's SD card was downloaded to obtain daily hours of mask on time, an objective measure of treatment use. The outcome assessments were repeated following 4 months of treatment in participants who had an average daily PAP use ≥4 hours/d over at least 90 days. Participants adherent to PAP at the 4-month end point were scheduled for a PSG with PAP at the pressure setting they used at home to document treatment efficacy. See online supplement for additional methods.
2.7 Statistical methods
Quality assurance checks were performed by reviewing raw data and performing graphical screening. Descriptive statistics are presented using frequencies and percentages for categorical variables and means, standard deviations, and/or 95% confidence intervals (CI) for continuous variables. Variables were compared between groups using two-sided t tests (continuous) and chi-square or Fisher's exact tests (categorical) at baseline. To assess the potential impact of non-normality in 24-hour urinary norepinephrine and to facilitate comparisons to prior literature, values were assessed untransformed and using a natural log transformation; results were similar with and without transformation.
Primary analyses of the relationship between baseline BP measures or 24-hour urinary norepinephrine levels and obesity metrics were performed using Pearson's correlations. Secondary analyses compared obese and non-obese participants using linear regression models, with BP or norepinephrine measures as outcome variables. Obesity status was defined relative to the sex-specific median values for waist circumference and abdominal visceral fat and a clinically meaningful cut-point of 30 kg/m2 for BMI. Analyses were controlled for relevant covariates, including age, sex, clinical site, number of hypertension medications (0, 1, or ≥2), and baseline AHI. In all of the above analyses, waist circumference was considered the primary measure of obesity with abdominal visceral fat and BMI being secondary (see online supplement).
To examine the effect of PAP on BP measures or 24-hour urinary norepinephrine, we calculated participant-specific change scores as follow-up minus baseline values among OSA participants adherent to treatment. Analyses were performed in a similar manner to baseline associations controlling for similar covariates, as well as the baseline value of the outcome of interest to improve statistical efficiency and reduce bias (eg, regression to the mean).16 Assessment of the overall effect of PAP on BP or norepinephrine levels was performed using linear mixed models evaluating the effect of time (0 = baseline, 1 = follow-up), restricted to individuals with observed values at both baseline and follow-up.
Given a single primary ABPM outcome measure (24-hour MAP), statistical significance was based on a P < .05. For the secondary ABPM measures, statistical significance was determined using the Hochberg step-up method to correct for multiple comparisons (see online supplement).17, 18 An uncorrected P < .05 was considered nominal evidence of an association in secondary analyses. For analyses of sympathetic activity, a P < .05 was considered significant given a single outcome measure (24-hour urinary norepinephrine). Additional information can be found in the online supplement. All analyses were performed using SAS, version 9.4 (SAS Institute), Stata/SE 14 (StataCorp), or R3.4 Software (www.r-project.org).
3 RESULTS
3.1 Characteristics of obese and non-obese participants with OSA
Of the 237 adults who completed the baseline assessment, 188 participants (mean age 53.5 ± 6.9 [SD] years, mean BMI 31.7 ± 4.2 kg/m2, 83.5% male, 73.9% Caucasian) had an AHI ≥ 15 events/h (Figure 1, Table 1). The mean AHI was 36.1 ± 15.8 events/h, and oxygen desaturation index (ODI) was 23.7 ± 14.9 events/h. Table 1 also compares baseline characteristics of the obese (n = 91) and non-obese (n = 97) individuals with OSA based on a sex-specific median split of waist circumference measures. The mean waist circumference was 118.4 ± 6.8 cm in the obese participants and 100.0 ± 7.6 cm in the non-obese (P < .0001). As expected, there were also differences in BMI (34.7 ± 3.1 vs 28.8 ± 3.0 kg/m2; P < .0001) and abdominal visceral fat volume (5157 ± 1848 vs 3328 ± 1485 cm3; P < .0001) between the obese and non-obese groups. There was no statistically significant difference between obesity groups in AHI (P = .076), but the obese group had a higher ODI (P = .025) and a greater percentage time of SaO2 < 90% (P = .019). Similar baseline results were found when limiting the above comparisons to participants who were adherent to PAP treatment (n = 108), although the difference in the oxygen saturation measures between the obese vs non-obese groups was no longer significant (P = .627, see Table S1). Baseline characteristics of participants with OSA adherent to PAP and those non-adherent or lost to follow-up are shown in Table S2.
| Variable | OSA | Non-obese OSA | Obese OSA | P † | |||
|---|---|---|---|---|---|---|---|
| N | Estimate | N | Estimate | N | Estimate | ||
| Age, y | 188 | 53.5 ± 6.9 | 97 | 53.1 ± 6.9 | 91 | 54.0 ± 6.9 | .409 |
| Caucasian, % | 188 | 73.9% | 97 | 71.1% | 91 | 76.9% | .366 |
| Male, % | 188 | 83.5% | 97 | 83.5% | 91 | 83.5% | .998 |
| BMI, kg/m2 | 188 | 31.7 ± 4.2 | 97 | 28.8 ± 3.0 | 91 | 34.7 ± 3.1 | <.0001 |
| Weight, kg | 188 | 97.6 ± 15.5 | 97 | 87.9 ± 11.1 | 91 | 108.0 ± 12.5 | <.0001 |
| Waist circumference, cm | 188 | 108.9 ± 11.7 | 97 | 100.0 ± 7.6 | 91 | 118.4 ± 6.8 | <.0001 |
| Abdominal visceral fat, cm3 | 147 | 4174 ± 1893 | 79 | 3328 ± 1485 | 68 | 5157 ± 1848 | <.0001 |
| Baseline SBP > 130 mm Hg, % | 188 | 50.5% | 97 | 53.6% | 91 | 47.3% | .384 |
| Anti-hypertensive med use, % | 188 | 44.1% | 97 | 39.2% | 91 | 49.5% | .156 |
| Number anti-hypertensive meds | 188 | 97 | 91 | .346 | |||
| 0 Meds, % | 55.9% | 60.8% | 50.5% | ||||
| 1 Med, % | 15.4% | 14.4% | 16.5% | ||||
| ≥2 Meds, % | 28.7% | 24.7% | 33.0% | ||||
| ESS score | 183 | 9.9 ± 4.8 | 94 | 9.3 ± 4.9 | 89 | 10.5 ± 4.6 | .073 |
| AHI, events/h | 188 | 36.1 ± 15.8 | 37 | 34.1 ± 14.5 | 91 | 38.3 ± 17.0 | .076 |
| ODI, events/h | 155 | 23.7 ± 14.9 | 79 | 21.1 ± 14.0 | 76 | 26.5 ± 15.4 | .025 |
| % time SaO2 < 90% | 166 | 6.6 ± 10.4 | 87 | 4.8 ± 6.4 | 79 | 8.6 ± 13.2 | .019 |
- Abbreviations: AHI, apnea-hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; ODI, oxygen desaturation index; OSA, obstructive sleep apnea participants with 15 ≤ AHI <75 events/h; SaO2, arterial oxygen saturation.
- † P-value from t test or chi-square test comparing non-OSA and OSA participants.
3.2 Baseline BP and norepinephrine measures
No significant unadjusted (see Table S3) or adjusted (Table 2) correlations were present between any of the 3 obesity measures and BP or urinary norepinephrine values at baseline among all OSA participants. Higher nocturnal HR and nocturnal:daytime HR ratio were associated with greater waist circumference in adjusted comparisons (Table 2, P-values = .002). However, in the OSA participants who were adherent to PAP, greater obesity was associated with lower 24-hour MAP (see Table S4). Baseline 24-hour MAP had negative relationships with waist circumference (rho = −0.32, P = .001), BMI (rho = −0.24, P = .017), and abdominal visceral fat (rho = −0.22, P = .045). Greater waist circumference was also associated with lower values of several secondary BP measures and higher HR values (see Table S4).
| Outcome | Waist circumference | Body mass index | Abdominal visceral fat | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Rho† | P | N | Rho† | P | N | Rho† | P | |
| ABPM measure | |||||||||
| MAP 24 h, mm Hg | 188 | −0.09 | .225 | 188 | −0.08 | .254 | 147 | −0.11 | .181 |
| MAP daytime, mm Hg | 188 | −0.08 | .265 | 188 | −0.09 | .244 | 147 | −0.11 | .202 |
| MAP nocturnal, mm Hg | 188 | −0.09 | .232 | 188 | −0.06 | .427 | 147 | −0.10 | .222 |
| MAP N:D ratio | 188 | −0.03 | .661 | 188 | 0.02 | .789 | 147 | −0.03 | .751 |
| SBP 24 h, mm Hg | 188 | −0.05 | .520 | 188 | −0.04 | .632 | 147 | −0.09 | .297 |
| SBP daytime, mm Hg | 188 | −0.03 | .646 | 188 | −0.03 | .641 | 147 | −0.07 | .378 |
| SBP nocturnal, mm Hg | 188 | −0.07 | .336 | 188 | −0.03 | .708 | 147 | −0.11 | .197 |
| SBP N:D ratio | 188 | −0.07 | .382 | 188 | 0.00 | .963 | 147 | −0.07 | .437 |
| DBP 24 h, mm Hg | 188 | −0.13 | .075 | 188 | −0.14 | .064 | 147 | −0.14 | .093 |
| DBP daytime, mm Hg | 188 | −0.13 | .081 | 188 | −0.14 | .051 | 147 | −0.15 | .083 |
| DBP nocturnal, mm Hg | 188 | −0.11 | .138 | 188 | −0.09 | .231 | 147 | −0.10 | .237 |
| DBP N:D ratio | 188 | −0.01 | .918 | 188 | 0.05 | .511 | 147 | 0.02 | .789 |
| HR 24 h, min−1 | 188 | 0.14 | .056 | 188 | 0.04 | .607 | 147 | 0.05 | .524 |
| HR daytime, min−1 | 188 | 0.11 | .152 | 188 | 0.01 | .922 | 147 | 0.04 | .661 |
| HR nocturnal, min−1 | 188 | 0.23 | .002 | 188 | 0.12 | .093 | 147 | 0.10 | .257 |
| HR N:D ratio | 188 | 0.23 | .002 | 188 | 0.20 | .006 | 147 | 0.09 | .315 |
| Urinary norepinephrine | |||||||||
| Norepinephrine, ng/mg creatinine | 140 | 0.07 | .416 | 140 | 0.00 | .955 | 112 | −0.04 | .691 |
| Norepinephrine, log ng/mg creatinine | 140 | 0.06 | .510 | 140 | −0.02 | .853 | 112 | −0.06 | .519 |
Note
- P-values in bold significant in primary comparisons (P < .05 for 24-h MAP or norepinephrine) or after Hochberg correction (for secondary ABPM measures).
- Abbreviations: ABPM, ambulatory blood pressure monitor; HR, heart rate; MAP, mean arterial pressure; N:D ratio, nocturnal BP measure divided by daytime measure; SBP, systolic blood pressure.
- † Pearson's linear correlation adjusted for site, age, sex, race, AHI, and number of anti-hypertensive medications (0, 1, or ≥2).
No differences in BP measurements were noted between all obese and non-obese participants at baseline, but nocturnal heart rate (P = .002) and nocturnal:daytime HR ratio (P = .003) were significantly greater in obese vs non-obese participants (see Table S5). Similar results were present when comparing obese vs non-obese participants adherent to PAP, with the exception that the difference in nocturnal HR became nominally significant (P = .005, Table 3).
| Outcome | Non-obese | Obese | P ‡ | ||
|---|---|---|---|---|---|
| N | Estimate (95% CI)† | N | Estimate (95% CI)† | ||
| ABPM measure | |||||
| MAP 24 h, mm Hg | 55 | 97.2 (95.1, 99.4) | 53 | 94.6 (92.5, 96.8) | .097 |
| MAP daytime, mm Hg | 55 | 100.1 (97.8, 102.3) | 53 | 97.7 (95.5, 100.0) | .153 |
| MAP nocturnal, mm Hg | 55 | 89.1 (86.6, 91.6) | 53 | 85.8 (83.3, 88.3) | .070 |
| MAP N:D ratio | 55 | 0.89 (0.87, 0.91) | 53 | 0.88 (0.86, 0.90) | .355 |
| SBP 24 h, mm Hg | 55 | 131.1 (128.3, 134.0) | 53 | 128.3 (125.5, 131.2) | .182 |
| SBP daytime, mm Hg | 55 | 134.3 (131.3, 137.2) | 53 | 131.7 (128.7, 134.7) | .242 |
| SBP nocturnal, mm Hg | 55 | 122.1 (119.0, 125.2) | 53 | 118.7 (115.6, 121.9) | .142 |
| SBP N:D ratio | 55 | 0.91 (0.89, 0.93) | 53 | 0.90 (0.89, 0.92) | .516 |
| DBP 24 h, mm Hg | 55 | 80.5 (78.5, 82.5) | 53 | 77.8 (75.7, 79.8) | .064 |
| DBP daytime, mm Hg | 55 | 83.3 (81.2, 85.4) | 53 | 80.7 (78.6, 82.8) | .094 |
| DBP nocturnal, mm Hg | 55 | 72.5 (70.2, 74.7) | 53 | 69.4 (67.1, 71.7) | .068 |
| DBP N:D ratio | 55 | 0.87 (0.85, 0.89) | 53 | 0.86 (0.84, 0.88) | .498 |
| HR 24 h, min−1 | 55 | 73.3 (70.6, 76.0) | 53 | 76.1 (73.4, 78.9) | .153 |
| HR daytime, min−1 | 55 | 75.9 (73.1, 78.7) | 53 | 77.8 (74.9, 80.6) | .365 |
| HR nocturnal, min−1 | 55 | 65.8 (63.1, 68.5) | 53 | 71.6 (68.8, 74.3) | .005 |
| HR N:D ratio | 55 | 0.87 (0.85, 0.89) | 53 | 0.92 (0.90, 0.94) | .0004 |
| Urinary norepinephrine | |||||
| Norepinephrine, ng/mg creatinine | 55 | 28.4 (25.3, 31.5) | 52 | 29.0 (25.8, 32.2) | .799 |
| Norepinephrine, log ng/mg creatinine | 55 | 3.32 (3.22, 3.41) | 52 | 3.31 (3.22, 3.41) | .963 |
Note
- P-values in bold significant in primary comparisons (P < .05 for 24-h MAP or norepinephrine) or after Hochberg correction (for secondary ABPM measures).
- Abbreviations: ABPM, ambulatory blood pressure monitor; HR, heart rate; MAP, mean arterial pressure; N:D ratio, nocturnal BP measure divided by daytime measure; SBP, systolic blood pressure.
- † Beta-estimate from linear regression model represents the estimated value at baseline; Models adjusted for site, age, sex, race, AHI, and number of anti-hypertensive medications (0, 1, or ≥2).
- ‡ P-value for significance of values between non-obese and obese participants with OSA.
No correlation was present between any of the 3 obesity measures and baseline urinary norepinephrine level (Table 2). The 24-hour urinary norepinephrine levels at baseline were similar between all obese and non-obese OSA participants (P = .409; Table S5) and between obese and non-obese OSA participants adherent to PAP (P = .799; Table 3).
3.3 PAP adherence and efficacy
Participants who were assessed at the 4-month follow-up used their PAP treatment for 5.8 ± 1.0 hours/d and on 93.7 ± 7.8% of days. The AHI was 5.5 ± 5.2 events/h, and there was no difference between individuals based on type of PAP unit (P = .517). The ODI was 2.3 ± 2.4 events/h on the PSG performed on PAP treatment at the 4-month follow-up. There were no differences in hours (P = .768) or percentage of days (P = .945) of treatment use between the obese and non-obese OSA groups. There were also no significant differences between groups in AHI (P = .260) or ODI (P = .056) on the PSG with PAP; the borderline difference in ODI among the obese (2.7 ± 2.3 events/h) and non-obese (1.8 ± 2.4 events/h) was not clinically meaningful. There were no significant changes in waist circumference (P = .391) or abdominal visceral fat (P = .156) among those who adhered to treatment. There was a statistically significant, but small, increase in weight following PAP treatment (mean [95% CI] change = 1.47 [0.35, 2.58] kg; P = .011) among those compliant to treatment; this change in weight was similar in obese and non-obese participants (P = .519).
3.4 BP changes in participants adherent to PAP treatment
Following PAP treatment, OSA participants adherent to PAP had a significant reduction in 24-hour MAP of −1.22 mm Hg (95% CI: −2.38, −0.06; P = .039; see Table S6). No significant unadjusted (see Table S7) or adjusted (Table 4) correlations were present between the baseline obesity measures and changes in any of the BP measures.
| Outcome | Waist circumference | Body mass index | Abdominal visceral fat | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Rho† | P | N | Rho† | P | N | Rho† | P | |
| ABPM measure | |||||||||
| MAP 24 h, mm Hg | 102 | 0.03 | .782 | 102 | −0.05 | .633 | 84 | −0.07 | .538 |
| MAP daytime, mm Hg | 102 | 0.05 | .647 | 102 | −0.03 | .770 | 84 | −0.02 | .839 |
| MAP nocturnal, mm Hg | 101 | −0.05 | .603 | 101 | −0.11 | .286 | 83 | −0.16 | .157 |
| MAP N:D ratio | 101 | −0.09 | .387 | 101 | −0.09 | .404 | 83 | −0.16 | .174 |
| SBP 24 h, mm Hg | 102 | 0.06 | .553 | 102 | −0.01 | .904 | 84 | −0.02 | .870 |
| SBP daytime, mm Hg | 102 | 0.07 | .522 | 102 | 0.00 | .994 | 84 | 0.02 | .834 |
| SBP nocturnal, mm Hg | 101 | 0.00 | .990 | 101 | −0.07 | .488 | 83 | −0.13 | .260 |
| SBP N:D ratio | 101 | −0.05 | .600 | 101 | −0.07 | .473 | 83 | −0.16 | .162 |
| DBP 24 h, mm Hg | 102 | 0.02 | .866 | 102 | −0.02 | .864 | 84 | −0.10 | .374 |
| DBP daytime, mm Hg | 102 | 0.05 | .614 | 102 | 0.02 | .867 | 84 | −0.03 | .781 |
| DBP nocturnal, mm Hg | 101 | −0.10 | .324 | 101 | −0.13 | .211 | 83 | −0.23 | .042 |
| DBP N:D ratio | 101 | −0.14 | .186 | 101 | −0.14 | .177 | 83 | −0.23 | .049 |
| HR 24 h, min−1 | 102 | −0.18 | .079 | 102 | −0.13 | .219 | 84 | −0.08 | .507 |
| HR daytime, min−1 | 102 | −0.15 | .142 | 102 | −0.08 | .422 | 84 | −0.05 | .672 |
| HR nocturnal, min−1 | 101 | −0.22 | .029 | 101 | −0.23 | .027 | 83 | −0.13 | .260 |
| HR N:D ratio | 101 | −0.14 | .166 | 101 | −0.21 | .038 | 83 | −0.09 | .430 |
| Urinary norepinephrine | |||||||||
| Norepinephrine, ng/mg creatinine | 107 | −0.21 | .037 | 107 | −0.09 | .396 | 88 | −0.20 | .073 |
| Norepinephrine, log ng/mg creatinine | 107 | −0.22 | .025 | 107 | −0.10 | .304 | 88 | −0.19 | .089 |
Note
- P-values in bold significant in primary comparisons (P < .05 for 24-h MAP or norepinephrine) or after Hochberg correction (for secondary ABPM measures).
- Abbreviations: ABPM, ambulatory blood pressure monitor; HR, heart rate; MAP, mean arterial pressure; N:D ratio, nocturnal BP measure divided by daytime measure; SBP, systolic blood pressure.
- † Pearson's linear correlation adjusted for site, age, sex, race, AHI, and number of anti-hypertensive medications (0, 1, or ≥2).
Adjusted changes from baseline in 24-hour MAP between obese (n = 67) and non-obese (n = 35) participants did not differ (P = .699; Table 5). Unadjusted results were similar to the adjusted results (see Table S8). An analysis restricted to participants with hypertension at baseline showed no significant correlation between any of the 3 obesity measures and changes in the BP or urinary norepinephrine values (see Table S9). As expected, we observed larger changes in BP measures following PAP treatment among those with hypertension at baseline, although differences between obese and non-obese were not statistically significant in this subset (see Table S10). Across all BP measures, absolute effect sizes were on average 2.1-fold larger among obese participants with hypertension at baseline (Table S10) when compared to obese participants in the full sample (Table 5).
| Outcome | Non-obese OSA | Obese OSA | P § | ||||
|---|---|---|---|---|---|---|---|
| N | Estimate (95% CI)† | P ‡ | N | Estimate (95% CI)† | P ‡ | ||
| ABPM measure | |||||||
| MAP 24 h, mm Hg | 51 | −0.99 (−2.61, 0.64) | .231 | 51 | −1.45 (−3.08, 0.18) | .080 | .699 |
| MAP daytime, mm Hg | 51 | −1.03 (−2.75, 0.70) | .240 | 51 | −1.52 (−3.25, 0.20) | .083 | .694 |
| MAP nocturnal, mm Hg | 51 | −0.32 (−2.24, 1.61) | .746 | 50 | −1.63 (−3.58, 0.32) | .100 | .356 |
| MAP N:D ratio | 51 | 0.006 (−0.01, 0.02) | .471 | 50 | −0.004 (−0.02, 0.01) | .634 | .409 |
| SBP 24 h, mm Hg | 51 | −0.47 (−2.64, 1.69) | .665 | 51 | −1.11 (−3.28, 1.06) | .312 | .689 |
| SBP daytime, mm Hg | 51 | −0.27 (−2.59, 2.04) | .815 | 51 | −1.17 (−3.48, 1.15) | .319 | .597 |
| SBP nocturnal, mm Hg | 51 | −0.51 (−2.95, 1.92) | .677 | 50 | −1.33 (−3.79, 1.13) | .286 | .649 |
| SBP N:D ratio | 51 | −0.002 (−0.02, 0.01) | .771 | 50 | −0.003 (−0.02, 0.01) | .736 | .973 |
| DBP 24 h, mm Hg | 51 | −1.18 (−2.63, 0.27) | .109 | 51 | −1.65 (−3.10, −0.20) | .026 | .658 |
| DBP daytime, mm Hg | 51 | −1.42 (−2.95, 0.12) | .071 | 51 | −1.75 (−3.29, −0.22) | .026 | .764 |
| DBP nocturnal, mm Hg | 51 | 0.06 (−1.73, 1.85) | .944 | 50 | −1.75 (−3.56, 0.05) | .057 | .171 |
| DBP N:D ratio | 51 | 0.015 (−0.00, 0.03) | .110 | 50 | −0.003 (−0.02, 0.02) | .722 | .178 |
| HR 24 h, min−1 | 51 | −0.08 (−1.99, 1.83) | .937 | 51 | −2.26 (−4.17, −0.35) | .021 | .120 |
| HR daytime, min−1 | 51 | 0.08 (−2.00, 2.15) | .941 | 51 | −2.12 (−4.20, −0.05) | .045 | .148 |
| HR nocturnal, min−1 | 51 | −0.45 (−2.58, 1.68) | .678 | 50 | −2.56 (−4.72, −0.41) | .020 | .184 |
| HR N:D ratio | 51 | −0.019 (−0.04, 0.00) | .075 | 50 | 0.003 (−0.02, 0.02) | .754 | .165 |
| Urinary norepinephrine | |||||||
| Norepinephrine, ng/mg creatinine | 55 | −2.14 (−4.63, 0.35) | .092 | 52 | −6.26 (−8.82, −3.69) | <.0001 | .027 |
| Norepinephrine, log ng/mg creatinine | 55 | −0.092 (−0.18, 0.00) | .045 | 52 | −0.247 (−0.34, −0.15) | <.0001 | .022 |
Note
- P-values in bold significant in primary comparisons (P < .05 for 24-h MAP or norepinephrine) or after Hochberg correction (for secondary ABPM measures).
- Abbreviations: ABPM, ambulatory blood pressure monitor; CI, confidence interval; HR, heart rate; MAP, mean arterial pressure; N:D ratio, nocturnal BP measure divided by daytime measure; OSA, obstructive sleep apnea; PAP, positive airway pressure; SBP, systolic blood pressure.
- † Beta-estimate from linear regression model represents the estimated change from baseline.
- ‡ P-value for significance of within-group change; Models adjusted for site, age, sex, race, AHI, number of anti-HTN medications (0, 1, or ≥2), and baseline ABPM or catecholamine value.
- § P-value for significance of between non-obese and obese group comparison of change scores.
3.5 Urinary norepinephrine changes in participants adherent to PAP treatment
In all participants adherent to PAP treatment, greater baseline waist circumference was associated with a greater reduction in 24-hour urinary norepinephrine level (rho = −0.21, P = .037, Table 4). The correlations with BMI and abdominal visceral fat were not significant.
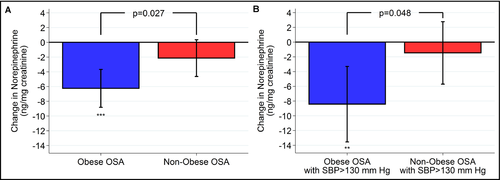
In obese vs non-obese participants with OSA adherent to PAP, the decrease in mean 24-hour urinary norepinephrine level following treatment was significantly greater in the obese participants (P = .027, Figure 2, Table 5). The adjusted decrease was 6.26 ng/mg creatinine (95% CI: −8.82, −3.69; P < .0001) in the obese participants compared to no significant change in the non-obese apneics (P = .092). Participants with hypertension at baseline showed no significant correlation between any of the 3 obesity measures and changes in urinary norepinephrine values (see Table S9). Obese participants adherent to PAP who were hypertensive at baseline had a greater reduction in urinary norepinephrine than their non-obese counterparts (P = .048, Figure 2, Table S10). The change in urinary norepinephrine among obese participants with hypertension at baseline (−8.43 ng/mg creatinine; Table S10) was 35% larger than the effect among all obese participants (−6.26 ng/mg creatinine; Table 5).
3.6 Changes in BP and 24-hour urinary norepinephrine measures
In all PAP-adherent participants with OSA, 24-hour urinary norepinephrine levels at baseline were not associated with changes of any BP measures following PAP treatment (see Table S11). In addition, no significant relationship was present between change in urinary norepinephrine level and change in BP measures (see Table S11). When the analysis was restricted to obese participants adherent to PAP (see Table S12), higher baseline norepinephrine levels were significantly associated with greater increases in the nocturnal:daytime MAP ratio (rho = 0.44, P = .003). There were no statistically significant correlations between changes in norepinephrine and changes in BP measures among obese OSA participants adherent to PAP.
3.7 Changes in BP or 24-hour urinary norepinephrine and amount of PAP adherence
To assess whether there was evidence of additional benefit of greater adherence to PAP, we examined correlations of hours/night and percentage of nights using PAP with either BP measures or urinary norepinephrine (Table S13). No significant correlations were found between PAP adherence and changes in either urinary norepinephrine levels or BP measures in participants with OSA adherent to PAP.
4 DISCUSSION
Our primary results indicate that obesity is not a factor that influences the BP response to treatment of adults with OSA and that the BP response to PAP treatment is not related to either baseline 24-hour urinary norepinephrine or change in 24-hour urinary norepinephrine following treatment. Although we found that the participants with OSA had a significant reduction in 24-hour MAP following PAP treatment, there were no significant correlations between the 3 obesity measures (waist circumference, BMI, and abdominal visceral fat) and change in BP measures. In addition, we found no differences in BP responses comparing obese and non-obese participants. However, the comparison of the obese vs non-obese groups was underpowered and more well-powered studies are needed to confirm this finding. We found a greater reduction in 24-hour urinary norepinephrine following PAP treatment in obese compared to non-obese participants adherent to PAP treatment (based on the sex-specific waist circumference median). Obese participants had almost a 22% reduction in urinary norepinephrine level following PAP treatment compared to less than an 8% reduction in non-obese participants. Including all adherent participants, there were no correlations between the reductions in BP following PAP treatment and either baseline urinary norepinephrine or decrease in urinary norepinephrine levels following treatment.
Sympathetic over-activity is believed to play a major role in the elevation of BP in OSA.9, 19 Previous studies report that both obesity and OSA are associated with an increase in sympathetic activity,20-26 and patients with severe OSA have higher urinary norepinephrine levels than patients with mild-moderate OSA, independent of obesity.25, 27 In agreement with the results of Pinto et al25 we found no difference in urinary norepinephrine excretion at baseline between obese and non-obese participants with comparable OSA severity. However, an increase in sympathetic activity in individuals with OSA is supported by our finding of a 14% reduction in mean 24-hour urinary norepinephrine at follow-up among all PAP-adherent OSA participants. Previous studies report decreases in urinary norepinephrine excretion of similar magnitude.21, 23-25, 28-31 Extending those findings, our results, controlling for baseline AHI, indicate that OSA participants who are obese have the greatest reduction in urinary norepinephrine excretion following PAP treatment.
Marrone et al22 reported that reduction in nocturnal diastolic BP and HR following 2 months of PAP treatment in a small sample of 17 participants with OSA was correlated with a decrease in nocturnal urinary catecholamine. However, most previous studies report that change in sympathetic activity, measured by plasma or urinary catecholamine levels, is not correlated with the BP response to PAP treatment (see online supplement for more details).21, 28, 29, 32, 33 Our study confirms and extends these findings by showing that the lack of correlation exists when examining either obese or non-obese adults with OSA. We hypothesized that a correlation might be present in obese adults with OSA since obesity is associated with increased sympathetic activity. Although measurement of urinary catecholamine levels did not capture the effect of sympathetic over-activity on BP in adults with OSA, it is possible that measuring muscle sympathetic nerve activity might reveal a significant relationship. Other factors such as the known increased oxidative stress and renin-angiotensin activation in adults with OSA may be responsible for the BP response.
The strengths of our study are the novel evaluation of BP response to PAP treatment in obese vs non-obese adults with OSA, the multiple assessments of obesity (ie, waist circumference, body mass index, and abdominal fat volume), and exclusion of individuals with PAP use <4 hours. However, our study has a number of limitations. The study was projected to need a sample size of 64 participants in both the obese and non-obese groups to achieve 80% power. We did not achieve this goal in either group. Therefore, the absence of significant differences in BP response between obese and non-obese participants may have been due to a lack of statistical power. The study did not include individuals that were non-adherent to PAP (eg, a ‘control’ group). However, there is a strong body of literature supporting a BP response to PAP and the goal was to evaluate the effects of obesity on this response. Similarly, the study included some participants who were not hypertensive. Blood pressure improvements with PAP are more likely to occur in hypertensive patients, particularly those with resistant hypertension.3, 34 Given the stronger effects seen in our analyses restricted to participants with SBP > 130 mm Hg at baseline, greater changes in BP may have been observed if recruitment had been limited to individuals with OSA who were hypertensive. Another limitation of the study is the low number of women included. The effect of obesity might be different according to sex.
In summary, the participants with OSA had a significant reduction in 24-hour MAP following PAP treatment, but there was no association between obesity measures and changes in BP in all adherent participants. Relatedly, there were no differences in BP response following PAP treatment between obese and non-obese participants. However, the sample size in the dichotomized analysis comparing obese and non-obese participants was underpowered and more well-powered studies are needed to confirm this finding. More obese adults with OSA had greater reductions in 24-hour urinary norepinephrine levels following PAP treatment compared to non-obese adults with OSA, based on waist circumference. However, the BP reductions were not correlated with reductions in urinary norepinephrine levels. Previous studies have shown younger adults who have hypertension, greater OSA severity, and adequate use of PAP treatment are more likely to have a BP reduction following PAP treatment. Our results suggest increased obesity levels do not help to better characterize the patients with OSA who will have a BP response to PAP treatment.
ACKNOWLEDGMENTS
The authors express their grateful appreciation to the work on this project performed by William Wieland, RPSGT, Yi Sun, RPSGT, and Robert Hachadoorian.
AUTHOR CONTRIBUTIONS
The authors have made the following contributions to this research project: Samuel T. Kuna, Raymond R Townsend, Brendan T. Keenan, David Maislin, Thorarinn Gislason, Richard J. Schwab, Greg Maislin, Julio A. Chirinos, and Allan I. Pack designed the study. Samuel T. Kuna, Raymond R Townsend, Thorarinn Gislason, Bryndis Benediktsdottir, Sigrun Gudmundsdottir, Erna Sif Arnardottir, Andrea Sifferman, Bethany Staley, Frances M. Pack, Xiaofeng Guo, and Richard J. Schwab collected and processed the data. Brendan T. Keenan, David Maislin, Greg Maislin, and Samuel T. Kuna performed the statistical analysis. Samuel T. Kuna, Raymond R Townsend, Brendan T. Keenan, David Maislin, Thorarinn Gislason, Bryndis Benediktsdottir, Erna Sif Arnardottir, Richard J. Schwab, Greg Maislin, Julio A. Chirinos, and Allan I. Pack prepared the manuscript.




