Was the Dry Diagonal of South America a Barrier for Dispersing Pteridaceae (Polypodiopsida) Species Between the Brazilian Atlantic Forest and Amazon Forest?
Funding: This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
ABSTRACT
Aim
We sought to assess how phylogenetic patterns within the fern family Pteridaceae are related to the history of the Amazon Forest and the Brazilian Atlantic Forest, as well as the Dry Diagonal of South America. The age of taxa present in these regions was estimated, as well as those found in previously identified areas of endemism for the family. We verified whether the Dry Diagonal constitutes a barrier to the dispersal of taxa between the Brazilian Atlantic Forest and the Amazon Forest, and investigated whether the latter domain is a source area of species.
Location
Neotropics, including the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal.
Taxon
Pteridaceae (Polypodiopsida).
Methods
We compiled rbcL and atpA sequences for all Pteridaceae species available in GenBank and obtained a dated phylogeny using fossil information and secondary calibrations. Using the resulting chronogram, we performed ancestral area reconstructions using BioGeoBEARS.
Results
Species from the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal tend to originate during the Eocene/Oligocene transition, in the Miocene or the Pleistocene. Vicariance events explain the origin of many of these species; however, dispersal events also played a significant role in the biogeographic history of the group. The areas of endemism of Pteridaceae in Brazil have distinct biogeographic histories. The areas of southeastern Brazil and southeastern Bahia likely originated more recently, driven by Pleistocene climatic changes and habitat specialisations. In contrast, southern Brazil and the Guiana Shield have older taxa, which originated in regions with climatic and (or) geological stability through habitat specialisations.
Main Conclusions
The origin of most species present in the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal most likely resulted from climatic changes during the Miocene and Pleistocene. The Dry Diagonal does not constitute a barrier to the dispersal of Pteridaceae species, as taxa shared between the Brazilian Atlantic Forest and Amazon Forest are common. The Amazon Forest is not the primary source area of species within the global context of the family.
1 Introduction
The Neotropics are notable for their immense biological diversity, harbouring the highest number of plant and animal species in the world (Antonelli and Sanmartín 2011; Antonelli et al. 2018; Colli-Silva and Pirani 2019; Thode et al. 2019). This extensive area encompasses a wide diversity of vegetation types and habitats, including seasonally dry forests, arid zones, high-altitude grasslands, mountain systems and tropical forests (Antonelli et al. 2018). Among the tropical forests, the Amazon Forest and Brazilian Atlantic Forest stand out for their high richness of species and endemisms. They are currently isolated but are believed to have been continuous in the past (Costa 2003; Sobral-Souza et al. 2015; Matos-Maraví et al. 2021).
From the separation of South America from Africa (ca. 65 Mya) until the Eocene, the Neotropics were warm, humid and predominantly covered by extensive and continuous tropical forests (Zachos et al. 2001; Burnham and Johnson 2004; Musher et al. 2019; Peres et al. 2020). Periods of global cooling and increased aridity towards the end of the Eocene and into the Oligocene led to the fragmentation of these vast tropical forests and, at the same time, promoted the expansion of open and dry vegetation in the central portion of South America (Prado and Gibbs 1993; Simon et al. 2009; Pinheiro and Monteiro 2010; Sobral-Souza et al. 2015; Thode et al. 2019; Bacci et al. 2022).
This open vegetation, which composes the ‘Dry Diagonal’ (also referred to as ‘the open formations diagonal’ or ‘the main South American disjunction’), currently encompasses the distinct phytogeographical domains of the Caatinga, Cerrado and Chaco (Prado and Gibbs 1993; Pennington et al. 2000; Santos et al. 2007; Werneck 2011; Côrtes et al. 2015; Sobral-Souza et al. 2015). Fossil pollen records support the young age of these open vegetation types, particularly the Caatinga, which would have been established in the early to middle Holocene (Thomé et al. 2016; Costa et al. 2018; Lima et al. 2018). Thus, open formations are more recent than tropical forests in the Neotropics (Burnham and Johnson 2004; Pinheiro and Monteiro 2010), and taxa occurring in open areas of the Cerrado likely differentiated from humid forest lineages and began diversifying in the last 10–4 Mya (Simon et al. 2009; Pinheiro and Monteiro 2010).
The Dry Diagonal acted as a barrier geographically segregating the tropical forest into two ‘blocks’, leading to the formation of the Amazon to the west and the Atlantic Forest to the east (Bigarella et al. 1975; Pennington et al. 2000; Hoorn et al. 2010; Costa 2003; Simon et al. 2009; Batalha-Filho et al. 2013; Peres et al. 2020; Bacci et al. 2022). Furthermore, this barrier restricted (and continues to restrict) the dispersal of species between these forest blocks, which would explain the floristic differences between them (Prado and Gibbs 1993; Costa 2003; Silva et al. 2004; Fouquet et al. 2012; Collevatti et al. 2020).
Studies have verified the correspondence between the period of expansion of open vegetation, the vicariance of humid forests and the estimated age for taxa occurring disjunctively in the Atlantic Forest and the Amazon (Batalha-Filho et al. 2013; Côrtes et al. 2015). For example, Thode et al. (2019) found that the divergence between Amazonian and Atlantic Forest clades of the genus Amphilophium Kunth occurred around 28.4–32.2 Mya, corresponding to the cooling phase of the Eocene–early Oligocene, associated with the formation of the Dry Diagonal. Despite this, authors consider that high levels of biotic exchange should occur among the vegetation types in the Neotropical region (Costa 2003; Werneck 2011; Sobral-Souza et al. 2015; Antonelli et al. 2018; Musher et al. 2019; Peres et al. 2020). In this context, Antonelli et al. (2018) considered the Amazon as the most important source area of biodiversity in the Neotropics for all taxa analysed, including ferns. Thus, the Amazon was understood to not only generate great diversity in situ but also provide lineages for all areas of the Neotropics throughout the Cenozoic (Antonelli et al. 2018; Musher et al. 2019). For ferns, the second-largest source of species was the Atlantic Forest (Antonelli et al. 2018), although the authors included a low sampling of this group (four clades of ferns—53 spp.—1.16% of the group). Gentry (1982) also suggested that the Atlantic Forest was a source area of species (Fiaschi and Pirani 2009).
The use of different taxa in biogeographic studies can unveil specific details of the intricate history of these phytogeographic domains (Sobral-Souza et al. 2015). Therefore, we considered Pteridaceae, a fern family still underexplored in this sense, as our study model. Pteridaceae, in a broadly accepted classification for ferns (PPG I 2016), comprises 53 genera and 1211 species, which represent about 10% of extant fern diversity (Schuettpelz et al. 2007). It is a monophyletic group traditionally consisting of five subfamilies: Cheilanthoideae, Cryptogrammoideae, Parkerioideae, Pteridoideae and Vittarioideae (Prado et al. 2007; Schuettpelz et al. 2007; PPG I 2016). The family exhibits a wide range of morphological characteristics. However, it can be defined by sporangia distributed along veins or at the leaf margin (protected by pseudo-indusium), or even covering the entire abaxial surface of the frond; and by the number of chromosomes, x = 29 or 30, or multiples of these (Sehnem 1972; Tryon and Tryon 1982; Schuettpelz et al. 2007).
This family has a broad geographic distribution, occurring on all continents, although it is especially concentrated in humid tropical and arid regions (Sánchez-Baracaldo 2004; Prado et al. 2007). Pteridaceae species occupy various habitats, including open areas, forest interiors, rocky, xeric and aquatic environments, and mangroves, being terrestrial, rupicolous, epiphytic and aquatic (Prado et al. 2007, 2020; Schuettpelz et al. 2007). According to Flora of Brazil 2020, the Amazon Forest has14 genera and 70 species (30 spp. endemic), the Atlantic Forest 20 genera and 146 species (72 spp. endemic), in the Cerrado 11 genera and 50 species (24 spp. endemic) and the Caatinga 5 genera and 6 species (1 endemic) (Prado et al. 2020). The Atlantic Forest comprises three areas of endemism for the Pteridaceae: southern Brazil, southeastern Brazil and southeastern Bahia. These areas suffer human pressure for deforestation to expand agriculture, and additionally, they are close to the biggest cities in Brazil. All species from these areas are considered to some degree threatened. The Amazon Forest presents only one area of endemism, the Guiana Shield, a habitat very peculiar and isolated, and fortunately better preserved as National Parks (Della and Prado 2024).
In this study, we present a calibrated phylogenetic hypothesis for the family Pteridaceae, based on the molecular markers rbcL and atpA, and assess how phylogenetic patterns are related to the history of Neotropical phytogeographic domains, focusing on the relationship among species from the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal. We seek to answer the following questions: (1) What is the age of the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal members of the Pteridaceae? (2) What is the age of the taxa that are associated with the areas of endemism obtained by Della and Prado (2024)? (3) During which period did the greatest diversification of taxa occur? (4) Was the Dry Diagonal a barrier to dispersal between tropical forest domains for Pteridaceae? (5) Was the Amazon region a source area for Pteridaceae species, as generically suggested for ferns by Antonelli et al. (2018)?
2 Materials and Methods
2.1 Taxonomic Sampling
We included a sampling of 154 species of Pteridaceae occurring in Brazil (approximately 74%), in addition to 648 Pteridaceae species found in other regions and/or continents. All rbcL and atpA sequences were obtained through searches on GenBank (see Supporting Information S1). As an outgroup, we considered 17 species belonging to the families Aspleniaceae, Blechnaceae, Didymochlaenaceae, Dryopteridaceae, Lindsaeaceae, Nephrolepidaceae, Polypodiaceae, Saccolomataceae, Thelypteridaceae and Woodsiaceae, whose sequences were also obtained from GenBank (see Supporting Information S1).
2.2 Sequence Alignment
For each marker, sequences were aligned using MAFFT v. 7.505 (Katoh and Toh 2010) and subsequently reviewed manually. Regions with a large amount of missing data, primarily those located at the ends of each alignment, were excluded. Additionally, for each marker, a preliminary phylogenetic tree was generated (see parameters in the next section) using maximum likelihood (ML) in IQTree software (Nguyen et al. 2015; Trifinopoulos et al. 2016) to identify anomalous sequences, which were subsequently removed.
2.3 Phylogenetic Analyses
From the concatenated dataset, we generated an ML phylogeny in IQTree software. ModelFinder (Kalyaanamoorthy et al. 2017), implemented in IQTree, was used to select the most suitable substitution model for the data. For all phylogenetic analyses performed, the GTR+F+I+G4 model presented the best fit and was automatically selected by IQTree according to the Bayesian Information Criterion and Corrected Akaike Information Criterion. The other parameters used in the analysis were: bootstrap analysis ultrafast with 1000 alignments, maximum iterations = 1000, minimum correlation coefficient = 0.99 and single branch test with 1000 replicates; for IQTree search parameters, perturbation strength = 0.5 and stopping rule = 100.
2.4 Divergence Time Estimation
The calibration of the ML tree obtained in IQTree was performed using penalised likelihood in treePL (Smith and O'Meara 2012). Three fossil constraints extracted from the Fern Tree of Life (Nitta et al. 2022) were used in this analysis: Heinrichsia cheilanthoides Regalado, A.R. Schmidt, M. Krings & H. Schneid., with an estimated age of 100.5–113 Mya (Regalado et al. 2019), positioned at the crown Pteridaceae; Acrostichum intertrappeum Bonde & Kumaran, with 66–72.1 Mya (Bonde and Kumaran 2002), in the stem Acrostichum+Ceratopteris; and Acrostichum palaeoaureum Beauchamp, Lemoigne & Petrescu, with 23.03–27.82 Mya (García-Massinni et al. 2006), in the Acrostichum stem. The position of the fossils in the phylogeny is in agreement with Nitta et al. (2022). In addition, we incorporated a secondary calibration point at the root node of Pteridaceae. Age estimates for the family obtained from previous studies were used to fix the age of this node. We considered 110.8 Mya obtained by Schuettpelz and Pryer (2009), 184.5 Mya from Testo and Sundue (2016) and 227.9 Mya from Nitta et al. (2022). We chose to test these three estimates as they represent the minimum and maximum ages previously recorded for Pteridaceae. Finally, we applied a smoothing parameter of 0.000001 based on the smallest chi-squared value obtained from treePL.
2.5 Biogeographical Analyses
We explored the biogeographical history of Pteridaceae and estimated the variation of ancestral distribution across the three chronograms obtained in treePL. Due to computational limitations and the maximum number of taxa allowed in each analysis, subfamilies were pruned from each calibrated tree. Thus, a total of 15 analyses were performed for the 5 subfamilies from each of the three chronograms.
The current geographical distribution for each species (see Supporting Information S1) considered in the analyses was obtained from herbaria reviews (for species occurring in Brazil), online databases (Tropicos [https://www.tropicos.org/home], GBIF [https://www.gbif.org] and speciesLink [https://specieslink.net/search/]), and the literature. We considered seven areas: A: North and Central America, Mexico and the Antilles; B: South America (excluding G, H and I); C: Africa and Madagascar; D: Eurasia and Australasia; E: Amazon; F: Atlantic Forest; G: Dry Diagonal (Huiet et al. 2018; Pereira et al. 2021). Only a single discrete area was specified for Eurasia and Australasia, and another one for North and Central America, Mexico and the Antilles due to computational limitations.
We employed the BioGeoBEARS package (Biogeography with Bayesian Evolutionary Analysis in R Scripts; Matzke 2013) in RASP 4.0 (Yu et al. 2020). This package implements three commonly employed approaches: dispersal-extinction-cladogenesis (DEC; Ree and Smith 2008), dispersal-vicariance analysis (DIVA; Ronquist 1997) and bayesian inference for discrete areas (BayArea; Landis et al. 2013). Since BioGeoBEARS uses probability-based versions of DIVA and BAYAREA, they are referred to as DIVALIKE and BAYAREALIKE, respectively.
Analyses were conducted using these three methods, also considering the additional free parameter j (thus, DEC+j, DIVALIKE+j, BAYAREALIKE+j). The parameter j represents the additional speciation process from the founder effect, which occurs when a daughter lineage disperses to a new area (where the ancestor did not occur) (Matzke 2014). This is particularly relevant for ferns, which generally exhibit a high dispersal capacity due to their small and light spores (Barrington 1993; Wolf et al. 2001). From the six models employed, the most suitable for each subfamily tree was selected based on the Akaike Information Criterion (AIC). Using the selected model, distribution probabilities for ancestral nodes were inferred. The maximum number of coded areas was set to seven, as there are species in the analyses that occur in all seven areas.
3 Results
3.1 Phylogenetic Relationships
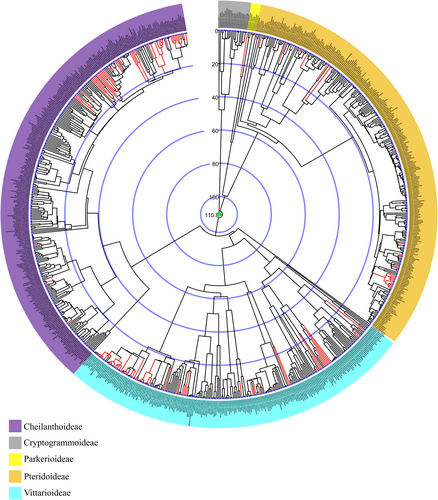
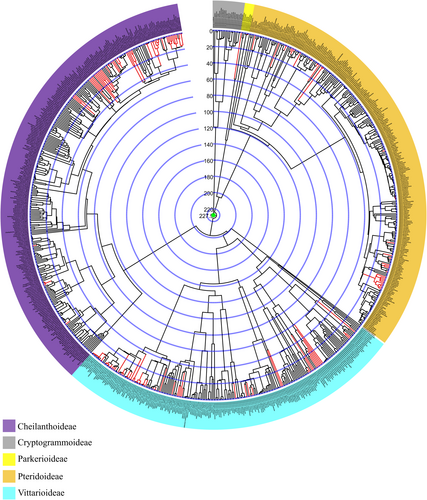
The atpA alignment included 583 species and 1515 bp, while the rbcL alignment presented 813 taxa and 1309 bp. ML analyses for these gene regions resulted in congruent topologies (see Supporting Information S1), with good bootstrap support. These markers were subsequently concatenated, resulting in a matrix with 819 taxa and 2824 bp, comprising 1918 distinct patterns, 1293 parsimony-informative sites, 191 singleton sites and 1340 constant sites. The phylogeny based on the concatenated data, constructed using ML, is available in the Supporting Information S1.
Pteridaceae is monophyletic, as are the subfamilies Parkerioideae, Cryptogrammoideae, Pteridoideae and Vittarioideae. Subfamily Cheilanthoideae was not resolved as monophyletic, due to the phylogenetic positioning of Calciphilopteris ludens (Wall. ex Hook.) Yesilyurt & H. Schneid., which in our analysis appeared as the sister group to Vittarioideae, instead of being the sister group to the remainder of Cheilanthoideae. The relationships among the subfamilies and between most genera are well-resolved and well-supported. The majority of the genera are monophyletic, with a few exceptions (mainly Cheilanthoideae).
3.2 Divergence Time Estimates
Three chronograms (Figures 1-3) were obtained using fossil information and a secondary calibration point. The differences between these trees are due to the fixed age of the Pteridaceae root. In analysis 1, we fixed this node at 110.8 Mya; in the second analysis at 184.5 Mya; and in the third at 227.9 Mya. The secondary calibration of this node generates variations in the ages of the family, as well as the subfamilies and genera; see Table 1.

| Taxa (area of endemism) | Ages (Mya) | ||
|---|---|---|---|
| Analysis 1 | Analysis 2 | Analysis 3 | |
| Pteridaceae | 110.8 | 184.5 | 227.9 |
| Cheilanthoideae | 98 | 162 | 203 |
| Adiantopsis Fée | 21 | 33 | 45 |
| Adiantopsis alata Prantl (SB) | 1 | 1.5 | 2 |
| Adiantopsis dichotoma (Sw.) T. Moore (SE) | 6.5 | 11 | 13 |
| Argyrochosma (J.Sm.) Windham | 22 | 36 | 48 |
| Doryopteris J.Sm. | 20 | 32 | 43 |
| Doryopteris rivalis Sehnem (SE) | 3.5 | 5 | 7 |
| Hemionitis L. | 19 | 28 | 40 |
| Lytoneuron (Klotzsch) Yesilyurt | 15 | 24 | 34 |
| Lytoneuron itatiaiense (Fée) Yesilyurt (SA) | 3.5 | 5.64 | 6.97 |
| Lytoneuron paradoxum (Fée) Yesilyurt (SA) | 0.6 | 1.15 | 1.35 |
| Mineirella Ponce & Scataglini | 20 | 32 | 43 |
| Ormopteris J.Sm. ex J.Sm. | 15 | 24 | 34 |
| Ormopteris gleichenioides (Gardner) J.Sm (SA) | 0.6 | 1 | 1.34 |
| Trachypteris André ex Chris | 21 | 33 | 45 |
| Cryptogrammoideae | 110 | 184 | 227 |
| Llavea Lag. | 48 | 96 | 119 |
| Cryptograma R.Br. | 38.5 | 75 | 88 |
| Coniogramme Fée | 38.5 | 75 | 88 |
| Parkerioideae | 99 | 164 | 205 |
| Acrostichum L. | 67 | 115 | 134 |
| Ceratopteris Brongn. | 67 | 115 | 134 |
| Pteridoideae | 99 | 164 | 205 |
| Anogramma lorentzii (Hieron.) Diels (SE) | 19 | 24 | 32 |
| Pityrogramma Link | 41 | 68 | 88 |
| Jamesonia Hook. & Grev. | 35 | 58 | 75 |
| Jamesonia brasiliensis Christ (SA) | 0.7 | 0.7 | 1.17 |
| Jamesonia cheilanthoides (Sw.) Christenh. (SA) | 0.3 | 0.5 | 1 |
| Jamesonia osteniana (Dutra) J.G. Gastony (SE) | 10 | 17 | 19 |
| Pterozonium Fée (GS) | 27 | 43 | 56 |
| Tryonia Schuettp., J. Prado & A.T. Cochran | 25 | 40 | 52 |
| Pteris L. | 54.5 | 89 | 116 |
| Pteris congesta J. Prado (SA) | 2.5 | 4.5 | 6 |
| Vittarioideae | 98 | 162 | 203 |
| Adiantum L. | 68 | 149 | 187 |
| Adiantum digitatum Hook. (SE) | 24 | 43 | 47 |
| Ananthacorus Underw. & Maxon | 25 | 44 | 52 |
| Hecistopteris J.Sm. | 29 | 48 | 62 |
| Polytaenium Desv. | 44 | 72 | 91 |
| Radiovittaria (Benedict) E.H. Crane | 29 | 48 | 62 |
| Vittaria Sm. | 40 | 65 | 83 |
- Note: In Analysis 1, the root node was fixed at 110.8 Mya (following Schuettpelz and Pryer 2009); in Analysis 2, it was fixed at 184.5 Mya (Testo and Sundue 2016); and in Analysis 3, it was fixed at 227.9 Mya (Nitta et al. 2022).
- Abbreviations: GS: Guiana Shield; SA: Southeastern Brazil; SB: Southeastern Bahia; SE: Southern Brazil.
Overall, the period of greatest diversification for the Pteridaceae (with the highest number of cladogenetic events) encompasses the last 10 Mya. Regarding the species from the areas of endemism defined by Della and Prado (2024), it is observed that those delimiting southeastern Brazil and southeastern Bahia are quite recent compared to the taxa from southern Brazil and the Guiana Shield (see Table 1). The species from southeastern Brazil emerged in the last 8 Mya, and the only species sampled from southeastern Bahia, Adiantopsis alata Prantl, is at most 2 Mya. In contrast, the taxa from southern Brazil range between 47 and 10 Mya (except for Doryopteris rivalis Sehnem, which in the most recent estimate has 3.5 Mya and Adiantopsis dichotoma (Sw.) T. Moore with 6.5–13 Mya). For the Guiana Shield, it can be noted that Pterozonium Fée dates to approximately 55–27 Mya.
3.3 Ancestral Areas Estimation
From the chronograms of each subfamily (considering the three calibrations), we identified the models that best fit the data according to the AIC (Table 2). For Cheilanthoideae and Vittarioideae, in all three analyses, the best model was BAYAREALIKE+J; for Cryptogrammoideae, it was DEC; for Parkerioideae, it was BAYAREALIKE and DEC; and for Pteridoideae, it was DEC and BAYAREALIKE+J. The reconstructions of Cryptogrammoideae are not discussed here, since they do not include species that occur in South America.
| Taxa/analysis | Model | LnL | N | d | e | J | AICc |
|---|---|---|---|---|---|---|---|
| Cheilanthoideae | |||||||
| 1 | BAYAREALIKE+J | −467.8 | 3 | 0.0056 | 0.11 | 0.0023 | 941.7 |
| 2 | BAYAREALIKE+J | −469 | 3 | 0.0034 | 0.067 | 0.0025 | 944.1 |
| 3 | BAYAREALIKE+J | −468.2 | 3 | 0.0027 | 0.051 | 0.0023 | 942.4 |
| Cryptogrammoideae | |||||||
| 1 | DEC | −20.66 | 2 | 0.025 | 0.28 | 0 | 46.03 |
| 2 | DEC | −20.93 | 2 | 0.019 | 0.22 | 0 | 46.57 |
| 3 | DEC | −20.8 | 2 | 0.014 | 0.16 | 0 | 46.31 |
| Parkerioideae | |||||||
| 1 | BAYAREALIKE | −21.2 | 2 | 1.01 | 1.97 | 0 | 50.41 |
| 2 | BAYAREALIKE | −21.21 | 2 | 0.48 | 0.93 | 0 | 50.42 |
| 3 | DEC | −21.2 | 2 | 2.16 | 4.22 | 0 | 50.41 |
| Pteridoideae | |||||||
| 1 | DEC | −518.3 | 2 | 0.019 | 0.10 | 0 | 1041 |
| 2 | BAYAREALIKE+J | −518.1 | 3 | 0.010 | 0.11 | 0.0038 | 1042 |
| 3 | DEC | −518 | 2 | 0.0091 | 0.052 | 0 | 1040 |
| Vittarioideae | |||||||
| 1 | BAYAREALIKE+J | −595.4 | 3 | 0.012 | 0.11 | 0.0067 | 1197 |
| 2 | BAYAREALIKE+J | −595.3 | 3 | 0.0071 | 0.064 | 0.0069 | 1197 |
| 3 | BAYAREALIKE+J | −595.9 | 3 | 0.0055 | 0.049 | 0.0073 | 1198 |
- Note: In Analysis 1, the root node was fixed at 110.8 Mya (Schuettpelz and Pryer 2009), in Analysis 2, it was fixed at 184.5 Mya (Testo and Sundue 2016) and in Analysis 3, it was fixed at 227.9 Mya (Nitta et al. 2022).
- Abbreviations: +J: additional free parameter j; AICc: Akaike Information Criterion corrected; BAYAREALIKE: Bayesian inference for discrete areas; d: rate of dispersal; DEC: dispersal-extinction-cladogenesis; e: rate of extinction; j: the relative probability of founder-event speciation; LnL: log marginal likelihood; N: number of parameters.
Figures 4 and 5 present the biogeographic history of only a few clades of Pteridaceae, focusing primarily on the largest genera from each subfamily and the groups most relevant to the discussion of the hypotheses tested here. These figures were created using the oldest estimated age for the Pteridaceae root node, ensuring that the most recent estimates are also covered. Complete reconstructions for each subfamily are provided in the Supporting Information S1.
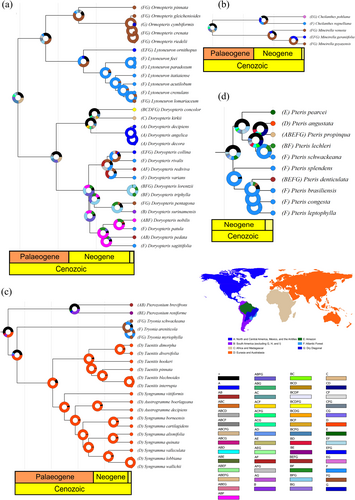
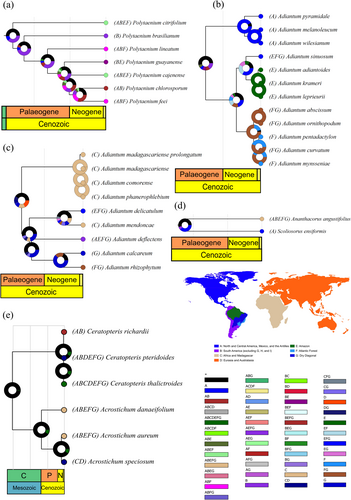
The biogeographical histories obtained through the reconstructions show us that species from the Dry Diagonal, Brazilian Atlantic Forest and Amazon Forest tend to originate in three periods: before 20 Mya, around 10 Mya and in the last 2 Mya. Vicariance events explain the origin of many of these species. However, dispersal events play an important role in the evolutionary history of all subfamilies.
The Dry Diagonal does not constitute a barrier to species dispersal, as it is common to find shared taxa with the Brazilian Atlantic Forest and (or) Amazon Forest. Species occurring in the Cerrado, Caatinga and Chaco are mostly recent, dating from the Late Miocene to the Pleistocene. Additionally, some taxa with disjunct distributions in the Amazon Forest and the Brazilian Atlantic Forest, primarily within the subfamily Vittarioideae, are ancient, originating between 28 and 62 Mya.
The Amazon region does not appear to be the primary source area for species of the Pteridaceae subfamilies. Table 3 shows the estimates of dispersal frequency and speciation in each area. This table does not show data on Cryptogrammoideae, as they do not occur in South America, and on Parkerioideae, as they have few species, which are widely distributed. The primary source areas are North and Central America, and Africa for Cheilanthoideae; South America (excluding the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal) and Eurasia and Australasia for Pteridoideae and Vittarioideae. The sink areas are South American vegetation (excluding the Amazon Forest and Brazilian Atlantic Forest) and the Dry Diagonal for Cheilanthoideae; North, Central and South America (excluding the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal) for Pteridoideae; and North and Central America, and the Brazilian Atlantic Forest for Vittarioideae.
| Subfamilies/area | Dispersal | Speciation within areas | |
|---|---|---|---|
| From | To | ||
| Cheilanthoideae | |||
| A | 45.33–45.83 | 15.00–16.00 | 19,223 |
| B | 16.33–17.33 | 73.00 | 151 |
| C | 45.50–48.00 | 9.00 | 28 |
| D | 20.00 | 10.00 | 36 |
| E | 9.00 | 11.00–12.00 | 4 |
| F | 23.50–25.50 | 25.00–27.00 | 29 |
| G | 10.33–11.83 | 29.00–30.00 | 12 |
| Pteridoideae | |||
| A | 1.50–2.50 | 69.00–76.00 | 72–102 |
| B | 79.50–92.50 | 26.00–30.00 | 60–88 |
| C | 13.50–18.50 | 25.00–27.00 | 26–27 |
| D | 77.50–91.50 | 6.00 | 157–159 |
| E | 1.00 | 16.00–23.00 | 1–7 |
| F | 5.00–6.00 | 25.00–29.00 | 13–15 |
| G | 0.00 | 15.00–17.00 | 1–2 |
| Vittarioideae | |||
| A | 8–9.00 | 67.00 | 179–180 |
| B | 105.50–106.50 | 27.00 | 61 |
| C | 6.50–10.00 | 18.00 | 11 |
| D | 53.00–55.50 | 3–4.00 | 79–80 |
| E | 32.00 | 32.00 | 27–28 |
| F | 11.50 | 41.00 | 22–23 |
| G | 6.00 | 38.00 | 17–18 |
- Note: Some values are presented as intervals due to the consideration of the three reconstructions performed for each subfamily. Data for Parkerioideae and Cryptogrammoideae are not presented as they are less diverse groups, and the latter does not occur in South America.
- Abbreviations: A: North America, Central America, Mexico and Antilles; B: South America (excluding G, H and I); C: Africa and Madagascar; D: Eurasia and Australasia; E: Amazon; F: Atlantic Forest; G: Dry Diagonal.
4 Discussion
4.1 Phylogenetic Relationships
The phylogeny we obtained is generally consistent with previously published studies of the Pteridaceae (Prado et al. 2007; Schuettpelz et al. 2007), its subfamilies (Zhang et al. 2005, 2017), and genera therein (Sánchez-Baracaldo 2004; Link-Pérez et al. 2011; Lu et al. 2011; Sigel et al. 2011; Chao et al. 2014; Grusz and Windham 2014; Cochran et al. 2014; Yesilyurt et al. 2015; Zhang et al. 2015; Schuettpelz et al. 2016; Regalado et al. 2017; Huiet et al. 2018). The position of Calciphilopteris ludens was perhaps most discordant relative to what are now understood to be its affinities. Although unusual, this Asian species has typically been considered to be part of the Cheilanthoideae. While most molecular phylogenetic analyses have revealed it to be sister to the remainder of that subfamily, support is often poor (e.g., Schuettpelz et al. 2007) and, in some cases, the species is instead resolved as sister to Vittarioideae (e.g., Pryer et al. 2016); as we find it here. Ultimately, this inconsistency reflects the distinct nature of Calciphilopteris and most likely stems from a deep and rapid divergence separating it from the (other) Cheilanthoideae and Vittarioideae. It is likely that additional sequencing (of both chloroplast and nuclear loci) will be necessary to find strong support for its phylogenetic placement.
4.2 Divergence Time Estimates
The selection and positioning of fossil constraints is a critical step in dating phylogenies, which can lead to markedly different age estimates (Sauquet et al. 2012). This can be seen in the different age estimates already published for Pteridaceae in the works of Schuettpelz and Pryer (2009), Testo and Sundue (2016) and Nitta et al. (2022). Additionally, the statistical methods used to obtain the chronogram must be considered, as discussed by Bauret et al. (2017). In the aforementioned studies, two used penalised likelihood (the first and third studies) and one employed an uncorrelated lognormal relaxed clock. Another relevant factor is the use of secondary calibrations, which can negatively influence results and lead to the propagation of errors if the original primary calibration includes fossils that are phylogenetically misplaced or assigned incorrect ages (Parham et al. 2012; Schenk 2016). Furthermore, using secondary calibration points tends to produce younger age estimates (Sauquet et al. 2012).
In a study with Pteris, Chao et al. (2014) estimated that the genus diverged from other Pteridoideae lineages around 47 million years ago. The methodology used was an uncorrelated lognormal relaxed model, with two secondary calibration points obtained from Schuettpelz and Pryer (2009). Therefore, our estimate, which used a fixed age for the root node of Pteridaceae at 110.8 Mya, obtained the closest age, 54.5 Mya. However, under the other analyses, we obtained ages of 89 and 116 Mya. This demonstrates that the secondary calibration point directly impacts the estimated age of the genus.
Two other examples, using Adiantum as a study model, can also illustrate this. Regalado et al. (2017) suggested that the crown group of Adiantum originated in the Palaeocene (~70 Mya), using three local clocks and secondary calibrations from Rothfels and Schuettpelz (2014). Our three analyses showed that the crown Adiantum ages were 68, 111 and 143 Mya. Lu et al. (2011) estimated the age of the A. pedatum complex as 4.27 Mya (2.2–6.5) using a lognormal relaxed clock model, fossils and secondary calibrations (according to Schuettpelz and Pryer (2009)). The result of our analysis 1, with secondary calibration from Schuettpelz and Pryer (2009), obtained similar results for the complex, 6 Mya, while analyses 2 and 3 obtained 10.5 and 13.5 Mya, respectively. Therefore, we prefer to consider the three chronograms to perform the reconstructions of the ancestral areas, even if this implies large intervals for maximum and minimum ages.
4.3 Origin of Pteridaceae Species Occurring in the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal
4.3.1 Cheilanthoideae
Myriopteris Fée and Argyrochosma (J.Sm.) Windham are genera that frequently occur in North and Central America, Mexico and the Antilles. Only M. myriophylla (Desv.) Sm. and A. nivea (Poir.) Windham are found in the locations highlighted in this section. The first species arose 3.0–7.1 Mya, and its presence in the Brazilian Atlantic Forest results from dispersal events (see Supporting Information S1). The second arose in the last 2.7 Mya in the Brazilian Atlantic Forest and the Dry Diagonal, also due to dispersal events.
Hemionitis L. has a wide distribution across the Americas, and the ancestor of this lineage probably had a similar occurrence. The species present in the Amazon Forest, Brazilian Atlantic Forest and/or Dry Diagonal are H. palmata L., with 8.9–18.8 Mya; H. rufa (L.) Sw., with 11.3–22.4 Mya; and H. tomentosa (Lam.) Raddi, with 11.3–22.4 Mya (see Supporting Information S1). These taxa may have arisen from vicariance events resulting from climatic changes in the Miocene (Zachos et al. 2001).
Adiantopsis Fée is also a genus with a broad distribution across the Americas. According to Link-Pérez et al. (2011), it would have originated in South America. A. flexuosa (Kunze) Link-Pérez & Hickey, the sister species to the rest of the genus, occurs only in the Dry Diagonal and originated 18.0–38.3 Mya (see Supporting Information S1). The origin of this taxon coincides with the increase in aridity existing in the late Eocene and part of the Oligocene, which led to the formation of the Dry Diagonal and the fragmentation of tropical forests (Prado and Gibbs 1993; Simon et al. 2009; Thode et al. 2019).
The clade composed of Adiantopsis regularis (Mett.) T. Moore (14.3–30.1 Mya), A. dichotoma (Sw.) T. Moore (6.2–13.7 Mya) and A. tweediana (Hook.) Link-Pérez & Hickey (6.2–13.7 Mya) includes species that occur only in the Brazilian Atlantic Forest, in this and other South American vegetation and in the Amazon Forest, respectively. Subsequently, 14.9–31.5 Mya, A. senae (Baker) Schuettp. & Al. Davila appeared, present in the Amazon Forest and the Dry Diagonal. The origin of A. senae can also be associated with the increase in aridity in the late Eocene and part of the Oligocene (Prado and Gibbs 1993; Simon et al. 2009; Thode et al. 2019).
Next came Adiantopsis timida Link-Pérez Hickey, endemic to the Amazon Forest (7.9–16.8 Mya), A. monticola (Gardner) T. Moore, endemic to the Dry Diagonal (7.6–16.0 Mya) and A. chlorophylla (Sw.) Fée and A. perfasciculata Sehnem, both present in the Brazilian Atlantic Forest and the Dry Diagonal (4.1–7.5 Mya). Finally, in the last 2 Mya, A. alata, endemic to the Brazilian Atlantic Forest, and A. radiata (L.) Fée, which, on the other hand, is widely distributed across the Americas, diverged. These last two species may have arisen due to climatic changes during the Pleistocene (Zachos et al. 2001).
The genus Mineirella Ponce & Scataglini is frequently found in the Brazilian Atlantic Forest and the Dry Diagonal (Figure 4b). The first species to appear was M. venusta (Brade) Ponce & Scataglini, occurring in both regions 7.8–14.8 Mya. M. geraniifolia (Weath.) Ponce & Scataglini and M. goyazensis (Taub.) Ponce & Scataglini diverged 3–5 Mya, with the former also occurring in the Amazon Forest.
Ormopteris J.Sm. ex J.Sm. is an endemic genus to Brazil, currently found in the Brazilian Atlantic Forest and the Dry Diagonal (Figure 4a). The ancestor of this group was probably present in both areas, 9.4–20.4 Mya. Most of the species are recent, arising from vicariance events in the last 1.3 Mya. Ormopteris cymbiformis (J. Prado) T. Barbará, which originated in the last 900,000 years, is the only species that is exclusively distributed in the Dry Diagonal.
The age estimates of Mineirella and Ormopteris species are consistent with those obtained for angiosperm groups present in the Cerrado (most of which emerged in the last 4 Mya) and the Campos Rupestres (diversification occurred in the last 5 Mya) (Simon et al. 2009; Rando et al. 2016). Furthermore, they are also in agreement with results from Fabaceae, which show that Cerrado lineages are recently derived from forest ancestors (Simon et al. 2009).
Lytoneuron (Klotzsch) Yesilyurt, the sister group of Ormopteris, occurs frequently in the Brazilian Atlantic Forest and the Dry Diagonal (Figure 4a). The ancestor of this lineage was probably present in both locations 8.7–18.6 Mya. Lytoneuron ornithopus (Mett. ex Hook. & Baker) Yesilyurt, the first species to diverge from the group, has the widest distribution, also occurring in the Amazon Forest. The other species, mostly from the Brazilian Atlantic Forest, arose from vicariance events in the last 10.6 Mya. In the Late Miocene, there was global cooling, which led to the fragmentation of tropical forests, as well as during the Pleistocene, with repeated glacial and interglacial cycles, which could be associated with the emergence of these taxa (Zachos et al. 2001).
Doryopteris J.Sm. is a predominantly American genus. Some clades occur only in the three regions highlighted in this section, among them the clade formed by D. collina (Raddi) J.Sm., D. rivalis Sehnem, D. rediviva Fée and D. varians (Raddi) J.Sm., which dates back to 4.3–10.0 Mya (Figure 4a). Another group, composed of D. lorentzii (Hieron.) Diels and D. triphylla (Lam.) Christ, emerged in the last 700,000 years, presumably due to Quaternary climatic changes (Zachos et al. 2001). Lastly, the clade formed by D. nobilis (T. Moore) J.Sm. ex C.Chr., D. patula (Fée) Fée and D. sagittifolia (Raddi) J.Sm. has ages between 5.4 and 11.5 Mya, with origins likely associated with climate changes in the Miocene (Zachos et al. 2001).
4.3.2 Parkerioideae
Ceratopteris Brongn. and Acrostichum L. are relatively ancient genera, estimated to have originated 66–134 Mya, and they currently have a wide geographical distribution (Figure 5e). The ancestor of these lineages was probably also widely distributed across all continents. The species of Ceratopteris found in the Brazilian Atlantic Forest, Amazon Forest and Dry Diagonal emerged recently, in the last 1.1 Mya. In contrast, species of Acrostichum emerged between 26.9 and 58.0 Mya. The broad distributions of species in these genera were achieved through long-distance dispersal.
4.3.3 Pteridoideae
The lineage that gave rise to the genus Jamesonia Hook. & Grev. likely originated from dispersals from Eurasia and Australasia to South America 25–75 Mya. The first species of the genus to appear was J. osteniana (Dutra) J.G. Gastony, with 10.2–19.7 Mya, which occurs exclusively in the Brazilian Atlantic Forest. The other species of the group, mostly Andean, arose 7.8–16.0 Mya during the development of the Andes mountain range (Sánchez-Baracaldo 2004). Jamesonia brasiliensis Christ arose from dispersal from the Andes and/or Central America in the last one Mya (see Supporting Information S1). Additional dispersals to the Brazilian Atlantic Forest likely occurred around two Mya, leading to the emergence of J. flexuosa (Kunth) Christenh. and J. cheilanthoides (Sw.) Christenh. This disjunction between the Andes and the southeastern mountains of Brazil is shared by numerous plant and animal genera (Sánchez-Baracaldo 2004; Della and Prado 2020).
The lineage that gave rise to Tryonia Schuettp. et al., a genus endemic to the Brazilian Atlantic Forest and Dry Diagonal, likely also originated from dispersals from Eurasia and Australasia, 25.2–51.4 Mya (Figure 4c). However, the species of this genus are quite recent, having emerged in the last 1.6 Mya, probably as a result of Pleistocene climatic changes (Zachos et al. 2001).
Pteris, the largest genus of this subfamily, has a wide geographical distribution, occurring in all defined areas. Reconstructions suggest that some dispersals from Eurasia and Australasia to the Americas occurred, giving rise to clades that include species from the Brazilian Atlantic Forest, Amazon Forest and Dry Diagonal. This is supported by Chao et al. (2014), who highlighted long-distance dispersal as the main process that shaped the global distribution of Pteris. A first dispersal likely occurred 14–30 Mya, leading to the formation of groups with a wide distribution across the Americas (see Supporting Information S1). Among the species that occur in the regions considered in this section, there are P. deflexa Link 5.2–11.8 Mya; P. decurrens C. Presl 1.9–4.2 Mya; P. podophylla Sw. 5.0–10.6 Mya; P. haenkeana C. Presl 3.4–6.4 Mya; and P. altissima Poir. 1.1–2.8 Mya. The origin of these taxa may be associated with the climatic changes of the Miocene and Pleistocene (Zachos et al. 2001).
Another dispersal event probably occurred 13.4–28.4 Mya, leading to the formation of two groups. The first group is composed of Pteris pearcei Baker, P. angustata (Fée) C.V. Morton, P. propinqua J. Agardh, P. lechleri Mett. and P. schwackeana Christ, whose ancestor was widely distributed across the Americas, with the species ages 4.0–9.4 Mya, and currently distributed in the Amazon Forest and Brazilian Atlantic Forest. The second group includes P. splendens Kaulf., P. denticulata Sw., P. brasiliensis Raddi, P. congesta J. Prado and P. leptophylla Sw., with an ancestor likely occurring in the Brazilian Atlantic Forest around 5.6–11.9 Mya (Figure 4d). The emergence of taxa in these two groups may also be attributed to climatic changes during the Miocene and Pliocene. The Pliocene was characterised by a gradual decrease in temperature and humidity, resulting in the contraction of humid forests and the expansion of dry forests (Zachos et al. 2001; Machado et al. 2018).
4.3.4 Vittarioideae
Radiovittaria (Benedict) E.H. Crane and Hecistopteris J.Sm. are sister genera, endemic to the Americas, whose origins probably resulted from dispersals from Eurasia and Australasia, 45–95 Mya. Hecistopteris pumila (Spreng.) J.Sm. dates to 29–62 Mya and is widely distributed throughout the Americas. Radiovittaria, on the other hand, is a more diverse group. Among the species occurring in the Amazon Forest and (or) Brazilian Atlantic Forest, there are R. gardneriana (Fée) E.H. Crane and R. stipitata (Kunze) E.H. Crane, which diverged from each other 3.5–8.8 Mya (see Supporting Information S1).
Ananthacorus Underw. & Maxon, Vittaria Sm. and Polytaenium Desv., genera widely distributed in the Americas, also likely originated through dispersals from Eurasia and Australasia, 59–124 Mya. Ananthacorus angustifolius (Sw.) Underw. & Maxon, the only species of the genus, dates to 24.8–51.0 Mya and occurs throughout the Americas (Figure 5d). In Vittaria, there are two species occurring in the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal, V. lineata (L.) Sm. 9.3–18.1 Mya, and V. graminifolia Kaulf. 1.6–3.5 Mya. Of the seven sampled Polytaenium species, six occur in the Amazon Forest and Brazilian Atlantic Forest (Figure 5a): P. citrifolium (L.) Schuettp. (28.4–59.6 Mya), P. brasilianum (Desv.) Benedict (25.1–52.5 Mya), P. lineatum (Sw.) J.Sm. (18.1–37.4 Mya), P. guayanense (Hieron.) Alston (12.6–26.6 Mya), P. cajenense (Desv.) Benedict (11.1–23.5 Mya) and P. feei (W. Schaffn. ex Fée) Maxon (7.9–16.8 Mya). These species are mostly older than the Dry Diagonal; therefore, they likely established in the Amazon Forest and Brazilian Atlantic Forest when these domains were still connected, forming an extensive forest (Zachos et al. 2001; Burnham and Johnson 2004; Musher et al. 2019; Peres et al. 2020).
Adiantum, the largest genus of this subfamily, currently has a very broad distribution worldwide. The lineages present in the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal also likely arose from dispersals originating in Eurasia and Australasia. One of these dispersals must have occurred between 60 and 132 Mya, giving rise to two species, A. subcordatum Sw. (present in the Brazilian Atlantic Forest and Dry Diagonal) and A. digitatum Hook. (Brazilian Atlantic Forest and other South American regions), which diverged from each other 24.6–48.4 Mya (see Supporting Information S1). This divergence may be associated with the temperature reduction in the late Eocene and early Oligocene, which led to the fragmentation of tropical forests (Prado and Gibbs 1993; Simon et al. 2009).
A second dispersal event probably occurred between 4.7 and 31 Mya, leading to the formation of clades with a broad distribution across the Americas. The species found in the regions described in this section are A. lorentzii Hieron. (with 5.0–10.8 Mya), A. raddianum C. Presl (1.3–2.5 Mya), A. concinnum Willd. (8.2–17.2 Mya) and A. pseudotinctum Hieron. (7.7–15.6 Mya). The origin of these taxa may be associated with climatic changes during the Miocene and Pleistocene (Zachos et al. 2001).
A third dispersal event from Eurasia and Australasia to Africa likely occurred 20–43 Mya, leading to the emergence of species on the African continent. Subsequent dispersals from Africa to South America gave rise to species, such as A. delicatulum Mart. (Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal) with an age of 5.2–11.0 Mya, A. deflectens Mart. (Amazon Forest, Brazilian Atlantic Forest, Dry Diagonal and other South American vegetations) with 14.2–30.5 Mya, A. calcareum Gardner (Dry Diagonal) with 6.1–13.0 Mya and A. rhizophytum Schrad. (Atlantic Forest and Dry Diagonal) with 6.1–13.0 Mya (Figure 5c). These diversifications may be associated with Miocene climatic changes (Zachos et al. 2001).
A fourth dispersal event from Eurasia and Australasia to the Americas occurred 63–134 Mya, giving rise to several clades widely distributed in the Americas. Two groups are endemic to the Amazon Forest; the first includes A. adiantoides (J.Sm.) C.Chr., A. krameri B. Zimmer and A. leprieurii Hook., all of which are less than 1.6 Mya (Figure 5b); the second includes A. fuliginosum Fée and A. paraense Hieron., both less than 1.2 Mya. Young radiations in the Amazon Forest have been observed in other plant groups and are interpreted as recent colonisation events and high rates of diversification after the disappearance of mega wetlands in this region (Hoorn et al. 2010; Thode et al. 2019). Another explanation could be the origin of these species due to repeated glacial cycles in the Pleistocene and edaphic specialisations (Ribas et al. 2012; Matos-Maraví et al. 2021).
The other four groups are formed by species that occur mainly in the Brazilian Atlantic Forest and the Dry Diagonal. The first group includes A. abscissum Schrad., A. ornithopodum C. Presl ex Kuhn, A. pentadactylon Langsd. & Fisch., A. curvatum Kaulf. and A. mynsseniae J. Prado, with estimated ages 1.7 and 4.3 Mya (see Supporting Information S1); the second group includes A. discolor J. Prado (4–9 Mya), A. platyphyllum Sw. (last 1.6 Mya) and A. intermedium Sw. (last 600,000 years); the third group includes A. villosum L., which appeared in the last 800,000 years and A. tetraphyllum Willd. with an age between 2.5 and 5.3 Mya; and the fourth group includes A. gracile Fée, A. serratodentatum Willd., A. windischii J. Prado, A. humile Kunze, A. terminatum Kunze ex Miq., A. obliquum Willd., A. argutum Splitg., A. latifolium Lam. and A. petiolatum Desv., all with ages between 4.3 and 9.0 Mya. Finally, some taxa occur in the Amazon Forest and the Brazilian Atlantic Forest, such as A. cajennense Willd. ex Klotzsch (2–6.4 Mya), A. dolosum Kunze (last 900,000 years) and A. argutum Splitg. (last 1.7 Mya). In general, the origin of these species could be explained by climatic changes during the Miocene and Pleistocene (Zachos et al. 2001).
4.4 Was the Dry Diagonal a Barrier to Species Dispersal?
Based on our ancestral reconstructions within subfamilies, it can be concluded that the Dry Diagonal of South America did not constitute a barrier to the dispersal of Pteridaceae species. On the contrary, many taxa that occur in the Brazilian Atlantic Forest or Amazon Forest are also found in the Dry Diagonal. Examples include Adiantum subcordatum Sw., Lytoneuron ornithopus and Adiantum mathewsianum Hook., which are present in the Brazilian Atlantic Forest and Dry Diagonal; and Adiantopsis senae, Cheilanthes pohliana Mett. and Adiantum cinnamomeum Lellinger & J. Prado, which are found in the Amazon Forest and Dry Diagonal. Some of these species are relatively old, originating in the last 48 Mya. However, the vast majority appeared within the past 10 Mya or even within the last 1 Mya. These younger ages agree with previous studies that found the Cerrado flora was assembled from numerous plant lineages from different phytogeographic domains, mainly in the last 4–5 Mya (Simon et al. 2009; Rando et al. 2016; Antonelli et al. 2018). Furthermore, earlier work estimates that the biotic exchange from the Brazilian Atlantic Forest to the Dry Diagonal may have been a geologically recent event, occurring at least 5 Mya (Batalha-Filho et al. 2013; Thomé et al. 2016; Antonelli et al. 2018; Matos-Maraví et al. 2021).
Endemic taxa of the Dry Diagonal predominantly emerged in the last 10 Mya, with exceptions such as Adiantum patens, which is very recent, estimated to have originated in the last 400,000 years, and Adiantopsis flexuosa, which is quite ancient, with an age ranging from 18 to 38 Mya. This also aligns with previous studies that dated the formation of the Cerrado to around 10 Mya (Simon et al. 2009; Rando et al. 2016).
For some relatively ancient groups, the disjunct distributions between the Atlantic Forest and Amazon could be explained by the formation of the Dry Diagonal. It is estimated that periods of global cooling and increased aridity in the late Eocene and part of the early Oligocene led to the formation of the Dry Diagonal (Prado and Gibbs 1993; Simon et al. 2009; Pinheiro and Monteiro 2010; Sobral-Souza et al. 2015; Thode et al. 2019; Bacci et al. 2022). Therefore, species such as Hecistopteris pumila, with an origin estimated between 29 and 62 Mya, and Polytaenium citrifolium, with an age of 28–59 Mya (see Figure 5), probably arose before the separation of the Amazon and Atlantic Forest. The establishment of the Caatinga, Cerrado and Chaco therefore led to the formation of these disjunct populations.
Species that occur in the Brazilian Atlantic Forest, as well as in the Dry Diagonal and Amazon Forest, are mostly very young, having appeared in the last 2 Mya (such as Adiantum latifolium L. and Adiantum terminatum). However, some taxa arose around 10 Mya (e.g., Vittaria lineata and Adiantum delicatulum), and a few that are older than 20 Mya (e.g., Ananthacorus angustifolius and Adiantum deflectens). Some of these species are epiphytic, but the majority are terrestrial and found in moist areas. In the Dry Diagonal, they occur mainly in riparian forests (Xavier 2007; Xavier et al. 2012; Prado et al. 2020).
4.5 Was the Amazon a Source Area for Pteridaceae Species?
The Amazon was not recovered as the primary source for Pteridaceae species when considering the biogeographic history of their subfamilies in a global context. North, Central and South America (excluding the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal), as well as Eurasia and Australasia, are the main source areas for these subfamilies. The sink areas are the vegetation of South America (excluding the Brazilian Atlantic Forest and Amazon Forest), as well as North and Central America (see Table 3 and Supporting Information S1).
However, in the context of South America, the Brazilian Atlantic Forest (for Cheilanthoideae) and the Amazon Forest (for Vittarioideae) are areas where many species originate and from which many lineages disperse. On the other hand, the Dry Diagonal acts as a sink area for Cheilanthoideae, Pteridoideae and Vittarioideae. Antonelli et al. (2018) identified the Amazon as the most important primary source of Neotropical for several groups of organisms, including ferns in general. The second main source area for ferns was the Brazilian Atlantic Forest (Antonelli et al. 2018). Gentry (1982) had already suggested that the Brazilian Atlantic Forest was a source area of species for various Gondwanan vegetation. For ferns, the Amazon Forest is considered an area poor in species, mainly due to the homogeneous relief (Tryon and Conant 1975; Tryon 1986). In contrast, the Brazilian Atlantic Forest hosts a large number of species, many of which are endemic (Prado et al. 2020; Della and Prado 2024). Antonelli et al. (2018) also found that dispersals from forested/wet environments to open/dry habitats are relatively common. This would explain the recent origin of many endemic taxa in the Dry Diagonal and the high number of species that co-occur in this region as well as in the Brazilian Atlantic Forest and (or) the Amazon Forest.
4.6 Areas of Endemism of Pteridaceae in Brazil
The areas of endemism of Pteridaceae in Brazil have relatively distinct biogeographical histories. Regarding the Guiana Shield, although none of the taxa listed by Della and Prado (2024) were sampled in our phylogeny, there are two species belonging to the genus Pterozonium (Pterozonium brevifrons (A.C.Sm.) Lellinger and Pterozonium reniforme (Mart.) Fée) that are endemic to the Pantepuis. These two species diverged between 12 and 25.5 Mya; however, the origin of the genus is dated to 27–55 Mya. Thus, although the Pantepuis are formed by very ancient rocks from the Precambrian, the genus Pterozonium is seemingly more recent (see Table 1). The age estimates obtained here for Pterozonium are older than those found for Stegolepis (Rapateaceae), Brocchinia and Lindmannia (both Bromeliaceae) (Givnish et al. 2000, 2004, 2007; Rull and Vegas-Vilarrúbia 2020). Stegolepis would have occupied the Pantepuis around 12 Mya through dispersal and began local diversification around 6 Mya. Brocchinia species would have emerged between 12 and 17 Mya, while Lindmannia diverged in the last 2.5 Mya (early Pleistocene). Based on our results from ancestral area reconstructions, dispersals from the Andes to the Pantepuis (between 27 and 55 Mya) likely occurred, followed by habitat specialisation.
Southern Brazil encompasses part of the Serra Geral (Della and Prado 2024), which dates back approximately 133 Mya (Cretaceous) (Reis 2013). The endemic taxa in this area are also relatively old, with estimated ages ranging from 47 to 10 Mya (except Doryopteris rivalis, which in the most recent estimate has 3.5 Mya, and Adiantopsis dichotoma with 6.5–13 Mya—see Table 1). Based on reconstructions, it is estimated that all sampled species from this area originated from vicariance events. Since paleoclimatic models predicted the existence of forest refugia with relative climatic stability along the Serra Geral (Carnaval et al. 2014), these lineages may have diversified and specialised due to the microclimatic conditions present in the mountain's altitude gradients. More recent taxa, such as Doryopteris rivalis, may have originated from glacial cycles, which led to the extinction of subtropical forests and the expansion of vegetation from arid and semi-arid areas (Arana et al. 2013).
The only sampled species from southeastern Bahia, Adiantopsis alata, is about 1–2 Mya and likely originated from vicariance. This region has been interpreted as a forest refuge (‘Bahia refuge’) because it showed climatic stability during the last glacial maximum (Mori et al. 1983; Carnaval and Moritz 2008; Carnaval et al. 2009). Thus, this area likely retained lineages that diversified (in situ speciation) later due to habitat specialisation (Staggemeier et al. 2015; Machado et al. 2018).
The taxa from southeastern Brazil are also very recent, with most having originated in the last 2 Mya (except for Lytoneuron itatiaiense (Fée) Yesilyurt and Pteris congesta J. Prado, which, according to older estimates, may be 8 and 6 Mya, respectively—see Table 1). This area includes Brazil's coastal mountains, the Serra do Mar and Serra da Mantiqueira, which emerged about 65 Mya during the transition from the Cretaceous to the Paleogene (Almeida and Carneiro 1998; Vieira and Gramani 2015). Most species in this area arose through vicariance events, which may have resulted from specialisation to the heterogeneous microclimatic and soil conditions along the altitude gradients of these mountains (Kluge and Kessler 2006; Rahbek et al. 2019; Vasconcelos et al. 2020). An alternative explanation would be the diversification of these lineages due to Pleistocene glacial cycles, which may have led to repeated vicariance events (Haffer 1969). Jamesonia brasiliensis and J. cheilanthoides likely originated through long-distance dispersal (in the last 1 Mya) from the Andes and (or) Central America.
5 Conclusions and Perspectives
This is the first biogeographic analysis of Pteridaceae species from the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal. This study provided three calibrated phylogenies of Pteridaceae, which allow us to address questions about the evolutionary history of the group, as well as hypotheses explaining the origin of these taxa and areas of endemism. Although we included a considerable number of species, only two markers, rbcL and atpA, were used in the phylogeny reconstruction. Future studies should therefore incorporate additional genetic markers, not only from the chloroplast but also from the nuclear genome. In this way, better phylogenetic resolution would be obtained for some species.
Despite the uncertainty in some species relationships, this study demonstrates that Pteridaceae taxa from the Amazon Forest, Brazilian Atlantic Forest and Dry Diagonal mainly originated during the Eocene/Oligocene transition, in the Miocene or the Pleistocene. Climatic changes in the Miocene and Pleistocene may have acted as drivers of diversification for these lineages. The Amazon does not appear to have served as the main ‘species pump’ for all Pteridaceae lineages in a global context, as it is seen for other groups of organisms. Even within the context of South America, the Brazilian Atlantic Forest seems to have been a more important source area for Pteridaceae. On the other hand, the Dry Diagonal is considered a receiver area for lineages. The areas of endemism of Pteridaceae in Brazil have relatively distinct biogeographic histories. Southeastern Brazil and Bahia have very recent species, which originated mainly from vicariance events due to Pleistocene climatic changes and habitat specialisation. In contrast, the Guiana Shield and southern Brazil comprise older taxa that originated in areas with climatic and (or) geological stability through habitat specialisation.
Author Contributions
A.P.D. and J.P. conceived the original research idea. A.P.D. performed searches for DNA sequences in GenBank, carried out the alignments, phylogenetic and biogeographical analyses, and wrote the first version of the article. J.P., E.S. and K.P. assisted in all phylogenetic and biogeographical analyses and revised the paper.
Acknowledgements
The first author thanks the Coordination for the Improvement of Higher Education Personnel (CAPES—Finance Code 001, Brazil) for the scholarship provided and Rafael Ribeiro for assistance with the execution of the biogeographic analyses. We also acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq (Proc. EXC n. 010239/2009-0) for providing permits to conduct fieldwork in Brazil. The Article Processing Charge for the publication of this research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (ROR identifier: 00x0ma614).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Information from the molecular data is available in the Supporting Information S1, as well as the distribution data employed in BioGeoBEARS.




