Between the Poles: Rethinking Global Patterns in Sea Anemone Biodiversity
ABSTRACT
Aim
To assess global sampling coverage of sea anemones and global species richness across four different spatial resolutions, and analyse these along latitudinal gradients to investigate true bimodality and the extent to which these patterns have been influenced by uneven sampling efforts.
Location
The study encompasses a global scale.
Time Period
Occurrence data included in this study were collected from 1900 to present.
Taxon
Sea anemones (Actiniaria).
Methods
Using 247,542 global occurrence records, we estimated species richness and sampling coverage across four resolutions of grid cells: 800 km, 600 km, 400 km and 200 km. We employed a standardised rarefaction-extrapolation approach to mitigate biases introduced by uneven sampling efforts and to ensure comparability across spatial scales, then compared these species richness estimates across latitudes.
Results
Across all resolutions, we find a discernible peak in species richness in temperate latitudes, however, the latitudinal peak in diversity shifts dependent on the resolution; our coarsest resolution reveals the most pronounced bimodality, with peaks especially pronounced around 40° N and 40° S, while our finest resolution reveals species richness peaks at 40°–60° N and a subtler increase around 40° S. We find highest observed species richness consistently in temperate regions across resolutions, particularly in southern California, United States and northern Europe. Across all resolutions, we find a discernible peak in species richness in temperate latitudes, however, the latitudinal peak in diversity shifts dependent on the resolution; our coarsest resolution reveals the most pronounced bimodality, with peaks especially pronounced around 40° N and 40° S, while our finest resolution reveals species richness peaks at 40°–60° N and a subtler increase around 40° S.
Main Conclusions
Sea anemones display an asymmetrically bimodal pattern of global diversity and display the highest species richness at temperate latitudes around 40° N and 40° S. Our study underscores the need for targeted exploration in undersampled environments and understudied marine invertebrates to continue refining biogeographic theories, such as the frequency of bimodal distribution patterns.
1 Introduction
Sea anemones (Actiniaria, Hexacorallia, Anthozoa, Cnidaria) are a widely ecologically successful and diverse clade, with approximately 1200 species that occur across all latitudinal gradients, from polar regions to mid-latitudinal temperate zones to equatorial latitudes in tropical zones (see Rodríguez et al. 2025). Members of this order also occur at all ocean depths. Many sea anemones function as essential members of tidepool communities (e.g., Bedgood et al. 2023) and have adaptations inferred to be useful in these variable, often extreme environments (e.g., Hart and Crowe 1977; Shick et al. 1979; Shick and Dykens 1984; Zamer 1986; Amado et al. 2011; Bingham et al. 2011; Cubillos et al. 2023; Clarke et al. 2024). Conversely, a large amount of sea anemone diversity exists in the deep ocean and many species are adapted to survive in extreme conditions such as high pressure, low temperatures, lack of sunlight and even hydrothermal vent systems (Rodríguez et al. 2008; Zelnio et al. 2009; Goffredi et al. 2021; Zhou et al. 2023). Many sea anemones show high phenotypic plasticity and diversity (e.g., Chomsky et al. 2009; Hoeksema and Crowther 2011; González-Muñoz et al. 2015; Glon et al. 2020; Porro et al. 2020; Clarke et al. 2024), which may contribute to broad geographic and bathymetric geographic ranges.
The broad global pattern of increasing species diversity from the poles towards the equator, the well-established latitudinal diversity gradient (LDG), is defined by unimodality with a tropical peak (Jablonski et al. 2006). However, increasingly, vertebrate and invertebrate taxa have demonstrated contrasting patterns; vertebrates tend to hold true to the LDG and have increased diversity near the equator, whereas invertebrate species richness tends to peak in temperate regions (Edgar et al. 2017). The picture becomes more complicated in marine systems, with many marine lineages including sea anemones (see Fautin et al. 2013), largely showing a trend of bimodality with a distinct dip near the equator. These bimodal distributions, defined as a type of interruption in the distribution of taxa in both hemispheres and characterised by their absence in the tropics (Chaudhary et al. 2016; Rodríguez et al. 2009), have been well documented across diverse marine lineages. For example, bimodality has been observed in spiny dogfish (Veríssimo et al. 2010), copepods (Havermans et al. 2013), planktonic foraminifera, bivalves, razor clams, ophiuroidea and brachiopoda, among others. Further, the acceleration of climate change-induced ecological changes is already reshaping species distributions and community compositions (Moullec et al. 2022).
The current picture of global patterns of diversity in sea anemones is built on previous studies that are regionally focused (e.g., Manuel 1988; Häussermann and Försterra 2005; Fautin et al. 2005, 2015; Rodríguez et al. 2007; Kostina 2011; González-Muñoz et al. 2016; Laird and Griffiths 2016; Targino and Gomes 2020; Gusmão and Rodríguez 2021; Anushma et al. 2022; Ramírez-Orellana et al. 2024) lineage specific (e.g., Grajales and Rodriguez 2014; Hancock et al. 2017; Titus et al. 2019; Bennett-Smith et al. 2021; Izumi and Fujii 2021; Glon et al. 2023; Barragán et al. 2024) or otherwise limited in terms of comprehensiveness and number of data points. Because these studies are not synthetic or comparative across regions, drawing holistic conclusions about biodiversity trends from them is challenging. The only previous global assessment of sea anemone distribution found that sea anemone diversity peaks in richness at 30°–40° N and S, with lower numbers at the tropical latitudes and fewest species in polar areas; however, these results lacked the benefit of many recently described sea anemones and included relatively few occurrence points (Fautin et al. 2013).
In this study, we utilise publicly available species occurrence records and standardise these data with a combined rarefaction-extrapolation method (Chao et al. 2014) to evaluate global sampling coverage for sea anemones and assess species richness at four different spatial resolutions. We then analyse these results along latitudinal gradients to determine whether a true bimodal distribution exists and the extent to which these patterns have been influenced by uneven sampling efforts. Finally, we revisit the latitudinal diversity gradient and other scale-dependent geographic patterns of sea anemone diversity to deepen our understanding of their global biodiversity.
2 Methods
2.1 Occurrence Data
Occurrence data were obtained from the Global Biodiversity Information Facility (GBIF) using the R package ‘rgbif’ and from the Ocean Biodiversity Information System (OBIS). The dataset underwent a series of cleaning steps to ensure data quality and relevance using the R package ‘CoordinateCleaner’ (Zizka et al. 2019). We first retained only occurrences identified to the species level to prioritise accuracy in identification, then standardised species names following the World Register of Marine Species (WoRMS, 2025).
Filtering included removing all occurrences recorded before the year 1900 to ensure accurate identifications. To ensure a level of spatial accuracy, occurrences were filtered to include only those with coordinate precision less than 0.01° and occurrences where precision information was not available were excluded. Furthermore, occurrences were only included if the coordinate uncertainty was less than 10,000 m. Occurrences with coordinate uncertainties of 301, 3036, 999 and 9999 m were removed, as these specific values often indicate systematic errors or placeholders not based on actual measurements. Additionally, occurrences with zero values for either latitude or longitude were discarded. To further refine the dataset, country centroids within a 2 km buffer and capital centroids within a 2 km buffer around each occurrence were removed. All terrestrial coordinates were removed to focus on marine occurrences. Considering the intertidal nature of many sea anemone lineages, we used the Natural Earth land shapefile to adjust the coastline inwards by a 10 km buffer to retain all coastal occurrence points. After these cleaning procedures, our final dataset comprised 247,542 occurrence data points.
2.2 Defining Species Incidences
To avoid the potential problem of underrepresentation of common species and overrepresentation of rare species, a possible result of the heterogenous nature of our raw occurrence sampling data, we converted the species occurrence records into species incidence data for downstream processing. We first transformed our occurrence data into species incidence data at the scale of approximately 2 km-by-2 km cells using the Behrmann projection. For generating our incidence-frequency data, we treated each grid cell as an ‘assemblage’ and each sub-grid cell as a ‘sampling unit.’ We opt for use of the Behrmann projection following Kusumoto et al. 2023, favouring the equal-area projection to minimise area distortions in temperate and polar regions, allowing for accurate comparisons across geographies. Sea anemones are cosmopolitan and exist in every marine environment and so have occurrence data for every latitude. We then define four coarser grids at 200 km-by-200 km, 400 km-by-400 km, 600 km-by-600 km and 800 km-by-800 km resolution. To obtain reliable estimates of species diversity, we filtered grid cells with few occurrence records from analysis, removing cells if the observed number of species (Sobs) was < 2, the number of sub-gridded cells with at least one incidence (T) was < 3, or the total number of species incidences (U) was equal to the number of unique species (Q1—species that are each detected in only one sub-grid cell).
2.3 Diversity Estimation
We assessed the sample coverage and species diversities of our coarse grid cells (200 km-by-200 km, 400 km-by-400 km, 600 km-by-600 km and 800 km-by-800 km) by using the 2 km-by-2 km sub-grid cell as the fundamental unit for incidence frequency. We calculated incidence-based species diversities, q = 0, 1 and 2, corresponding to species richness, Shannon diversity and Simpson diversity, respectively, in each grid cell. The parameter ‘q’ determines the sensitivity to species incidence frequencies. For the purposes of this study, we focus on species richness. Species richness (q = 0) counts the number of different species present in an assemblage, only accounting for number of species and not including abundance or evenness.
Empirical diversity estimates depend on a combination of sampling effort and sample completeness, so we then estimated diversity using a combination of rarefaction and extrapolation based on our standardised sample completeness. We used the sample coverage in each coarse grid cell as a measure of sample completeness. Based on incidence data, sample coverage is the proportion of detected species occurrences relative to the total (detected and undetected) occurrences in that grid cell.
2.4 Sampling Coverage
To ensure fair comparisons of diversity across grid cells, we then standardised our species richness (q = 0) by sampling coverage (SC). This ensures comparability and reduces the effects introduced by uneven sampling effort and the tendency to overrepresent diversity at certain localities. The inherently uneven sampling efforts for sea anemones can lead to inflated diversity estimates in well-sampled areas, like the Pacific coast of the United States, and underestimation of diversity in poorly sampled areas, like Western Africa. We standardised by SC by first calculating seven different percentiles; 0.01, 0.05, 0.10, 0.20, 0.30, 0.40, 0.50, then recalculating diversity estimates using the R package ‘iNEXT’ version 2.0.20 (Hseih et al. 2020) at each of these values. Using an extremely low standard, such as an SC of 0.01, leads to far too few species included at each grid cell, while using an SC too high, such as 1, can lead to a severe negative bias of true diversity. To mitigate these effects, we compared our observed species richness (obs.D0), and our SC standardised richness at 0.05 (SC2.D0), 0.20 (SC4.D0) and 0.50 (SC7.D0). These three bracket a realistic range of sampling conditions while displaying enough variance to be informative and have been previously used in macroecological studies,
2.5 Biogeographical Patterns
We mapped sample coverage (SC) and diversity estimates at different spatial resolutions and examined geographical patterns. We then investigated patterns of diversity estimates across a latitudinal gradient with LOWESS (locally weighted scatterplot smoothing) curves, using both observed and extrapolated species richness estimates at each of our four resolutions (800 km, 600 km, 400 km, 200 km) for obs.D0, SC2.D0, SC4.D0 and SC7.D0.
3 Results
3.1 Global Sampling Coverage
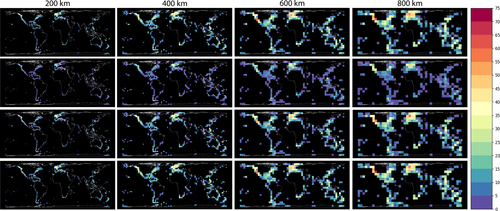
Sampling completeness (measured here by sampling coverage or SC) was consistent across our four spatial resolutions, averaging 0.85 (Figure 1). We recovered the highest SC at our finest resolution (SC = 0.861 at 200 km) and our lowest SC (0.830) at 400 km, with the intermediate value of 0.846 at 800 km. Observed SC was especially high along particular coastal areas and overlapped with some areas of higher observed species richness, such as Northern Europe and the Pacific coast of the United States. Our average SC value indicates a reasonably good representation of the diversity captured in our grids where samples occurred; however, there remain rare or elusive species not represented by this sampling.
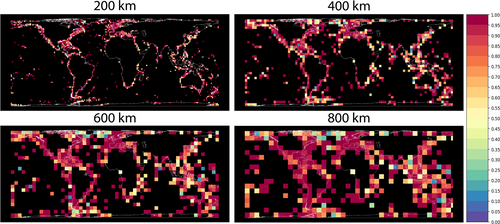
3.2 Global Species Richness
Across resolutions, temperate areas in the Northern Hemisphere consistently emerge as centres of sea anemone species richness. At our coarsest resolutions 800 km and 600 km (highest grid Sobs of 64 and 61, respectively), highest species richness was observed along the southern coast of California extending northward along the eastern Pacific coast, as well as in cells spanning the United Kingdom, Ireland, Denmark and Sweden. Finer resolutions 400 km and 200 km (highest grid Sobs of 64 and 50, respectively) provided more granular detail: the southern California coast, in particular the Channel Islands, remained a notable hotspot, along with the northern Pacific coast and Salish sea. The United Kingdom and Ireland, especially the Celtic Sea, Irish Sea and English Channel, also exhibited high species richness. At our finest level of granularity (200 km), local hotspots became particularly evident in southern England, the San Francisco Bay and Monterey Bay areas and southeastern Sweden in the Kattegat Sea. The species richness estimates standardised for sampling completeness (Figures 1 and 2) closely matched observed patterns, with the fluctuations in species richness aligning as anticipated based on the standardised percentile calculations.
3.3 Latitudinal Diversity Trends
The LOWESS curves for our finest-scale resolution at 200 km are the flattest, revealing the most noticeable peak in species richness between 40°–60° N and a slight increase around 40° S. This trend becomes more noticeable at increasing resolutions, while the peak shifts slightly south; our 400 km and 600 km resolutions both reveal more pronounced peaks at 40° North and South. Our coarsest resolution, 800 km grid, shows a peak in species richness between 30°–40° N and 40° S.
4 Discussion
The observed latitudinal patterns in species richness in sea anemones reveal a clear peak in temperate latitudes, although the latitude for the peak differs across scales. We find latitudinal diversity gradients more pronounced at coarser spatial resolutions than our finer resolutions (Figure 3). At a 200 km resolution, the LOWESS curve for species richness is relatively flat, showing a noticeable peak around 40°–60° N and a subtler increase at about 40° S. At intermediate resolutions (400 km and 600 km), these peaks become especially pronounced around 40° N and 40° S, while at the coarsest resolution (800 km), the peak occurs between 30°–40° N and near 40° S. Taken together, these results reinforce the notion of an asymmetric bimodality in sea anemone diversity with preference for temperate environments, emphasise the scale-dependence of such patterns, and underscore how uneven sampling efforts in certain regions may affect the visibility of these peaks.
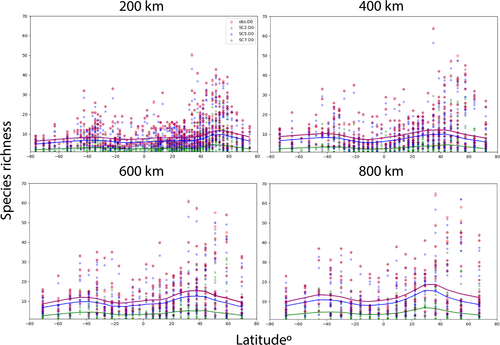
Although the temperate peaks appear robust, coverage-standardisation partly addresses—but cannot fully eliminate—biases introduced by more intensive sampling in certain temperate zones, especially present in the Northern Hemisphere. Sea anemones have long been interpreted to have mid-latitudinal increases in species richness, a result initially demonstrated from a smaller observance record dataset of around 11,662 (Fautin et al. 2013) and now reinforced by our study. Past studies have also noted that a higher proportion of sea anemone taxa follow bimodal distributions at family or genus levels (Rodríguez et al. 2009). More recent examinations of species richness of diverse marine lineages at a global scale found this bimodality to be asymmetric, with a noticeable dip between 10°–15° S (Chaudhary et al. 2016). Our findings are consistent with those observations, as we find asymmetric bimodality for sea anemones at most grid scales, although the exact latitudinal position of the peaks shifts slightly depending on resolution.
Viewed within the framework of the latitudinal diversity gradient (LDG), these results highlight a departure from the often-assumed tropical peak. Sea anemones show a pattern that more closely aligns with what has been observed for other marine invertebrates, such as spiny dogfish (Veríssimo et al. 2010) and copepods (Havermans et al. 2013), in which richness is higher in mid-latitudes and dips near the equator. Multiple factors could underlie this phenomenon. For instance, the high species richness we consistently observe in rocky coastal habitats could be owed to habitat heterogeneity (Sanciangco et al. 2013). It is also possible that tropical underestimation arises from historically incomplete sampling (especially when compared with densely sampled regions such as northern Europe and the southern California coast), an issue that may be especially pronounced off the coasts of western Africa, certain parts of Southeast Asia and in the deep sea. By merging smaller-scale habitat heterogeneity into large grid cells, coarser resolution can artificially inflate species counts and mask local hotspots, whereas finer grids can reveal nuanced peaks but fail to capture less-sampled regions accurately.
Biodiversity estimation is an amalgamation of occurrence records, which are inherently reflective of sampling effort, sampling completeness, true biodiversity and the reliability of the methods to estimate this biodiversity (Kusumoto et al. 2020). Consequently, it is challenging to discern how much of these identified patterns are artefacts of sampling biases and methodological limitations or reflect the actual distribution and diversity of their respective taxa. Menegotto and Rengel (2018) proposed that in some cases, this pattern may be an artefact of low sampling efforts in the tropics and could actually indicate a Wallacean Shortfall (sensu Lomolino 2004) rather than a latitudinal gradient of species absences. Previous research in scleractinian distribution and diversity has found that while taxonomic errors (Linnaean shortfall) are significant, genus-level diversity estimates showed consistent diversity patterns across the data, indicating that observed biogeographical patterns of diversity were stable and less afflicted by potential errors in species-level taxonomy. Both Wallacean and Linnean shortfalls are possible confounding issues for understanding actiniarian biogeography and diversity.
In addition to biases in geographic coverage, variation also exists in sampling effort across depth, with the largest ecosystem on Earth, the deep pelagic ocean, understandably undersampled (Webb et al. 2010). In the marine realm, depth and distance from shore add an additional element to patterns of diversity. Previous research into the global species richness of brittle stars found richness in the deep-sea peaking at mid-to-high latitudes, unlike shallower regions (Woolley et al. 2016). Across the Tree of Life, sampling events are most numerous in mid-latitudes, mostly the Northern hemisphere and the fewest in equatorial regions. The increased concentration of sampling at mid-latitudes in both hemispheres is likely reflective of higher funding and prioritising of marine research by these countries (Mora et al. 2008).
The study of global trends of sea anemone diversity is hindered by several factors, and the reliance on occurrence data poses specific challenges. In our analysis, we used the two major biological occurrence databases GBIF and OBIS; however, there are other occurrence data sources not yet digitised and included in larger databases (Page et al. 2015). Furthermore, data assembled from diverse sources may not be directly useful in elucidating distribution patterns, and errors could be attributable to the fact that sea anemones in some parts of the world are just poorly inventoried. Inventories in northern latitudes, particularly in the northern Atlantic and around the British Isles, are considerably more complete compared to those in southern regions. Inventory completeness is variable by region, attributable to the stringency of collecting laws and permits. For example, much of the mid-African continent and India lack comprehensive representation, which is not reflective of a lack of diversity in these areas. Further influencing the completeness of these inventories is the lack of representation for difficult-to-sample environments, such as the deep sea. Previous studies have demonstrated high levels of endemism for deep-sea, polar and chemosynthetic environments (Rodríguez et al. 2007; Rodríguez and Daly 2010). The deep sea is the largest inhabitable environment on Earth; however, our basic knowledge of its biodiversity remains limited due to the logistical challenges of exploration. The absence of these data, which are likely to be significant for global diversity assessments, complicates our understanding of holistic diversity patterns.
Additionally, the comprehensiveness of the sea anemone inventories along latitudinal gradients may be skewed by several factors. The number of species in each latitudinal band is not necessarily proportional to sea surface area and coastline length for that band. Respective sampling effort represents a significant potential bias, as expeditions and varied collection efforts are unavoidable when analysing such a diverse dataset. Uniformity in collections is further hindered by a predominance of intertidal occurrence data compared to open ocean data. Although sea anemones often dominate certain intertidal environments and many lineages require hard substratum for anchorage, this bias does not reflect their overall distribution.
However, previous studies have demonstrated that while some taxa have biases in sampling records towards larger, more conspicuous individuals with larger geographical ranges; this may not be the case for sea anemones. Fautin et al. (2013) found 60% of species are known from only a single 10° band, indicating Actiniaria is not biased in favour of species with large geographical ranges. They further found that conspicuousness seems not to be a factor in sea anemones, owed to the occurrence representation of many records for small burrowing species such as Triactis producta.
We mitigated much of this by converting raw occurrence data to incidence-based coverage (SC) and standardising our analyses at various sampling completeness thresholds. Following methodologies by Kusumoto et al. (2020) and others, we assessed incidence-based sample coverage to ensure fairer comparisons across differently sampled regions; however, there is no way to entirely eliminate the effect of relative abundance from diversity estimates (Roswell et al. 2021). Further, sea anemone taxonomy remains in flux; new species, synonymies and ongoing discoveries may continue to reshape the clade's overall diversity patterns. Standardisation in such a way allows for fairer comparisons of species diversity between areas with disparities in sampling effort. This reality underscores why the larger trend, rather than exact species counts, is often the most reliable signal for understanding global biogeographic patterns.
Overall, these results join a growing body of evidence that the once-canonical unimodal tropical peak does not encompass the diversity patterns of all marine taxa. Sea anemones, like other marine invertebrates, appear to show robust mid-latitude peaks that vary slightly with spatial scale, highlighting how a single global narrative for marine biodiversity can be incomplete. Ultimately, ongoing data collection and careful standardisation, coupled with deeper exploration of historically undersampled habitats, will be crucial in building a more precise understanding of sea anemone diversity and, by extension, overall marine biodiversity.
5 Conclusion
Species richness of sea anemones along latitudinal gradients demonstrates scale-dependence between coarse and fine resolutions, highlighting gaps in our knowledge and methodological shortcomings of biodiversity estimates. Continuing data collection and thoughtful standardisation are vital to resolving the full picture of sea anemone diversity, and by extension, marine biodiversity as a whole. Targeted efforts in identified undersampled regions will contribute to more robust diversity findings. As global ocean conditions continue to change through climate change, habitat destruction and pollution, a more nuanced understanding of global diversity gradients will be essential for understanding historical patterns of species diversity to better anticipate their changes.
Author Contributions
C.B. and M.D. were responsible for the conceptualization of the study. Methodology, dara curation, formal analysis, and investigation were conducted by C.B., M.N., and M.B. Validation and visualization were carried out by C.B. and M.N. The original draft of the manuscript was written by C.B. and M.D., with review and editing performed by both authors. M.D. provided supervision and project administration. Resources were provided by M.D. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the entire Daly lab for continued support during the development of this project, and the Department of Evolution, Ecology and Organismal Biology (EEOB) at The Ohio State University for additional support. No fieldwork permits were required for the completion of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in anemones at https://github.com/manukala6/anemones.




