So Young, So Rich: Habitat Shifts Combined With Trait Evolution Promoted Species Radiation in Senecio in the Andes
Funding: This work was supported by OP RDE project ‘International mobility of researchers at Charles University (MSCA-IF III)’ reg. n. CZ.02.2.69/0.0/0.0/19_074/0016231, awarded to L.S., and the Czech Science Foundation (GAČR) (grant 20-10878S to R.S. and F.K.). Financial support also came from the long-term research development project no. RVO 67985939 of the Czech Academy of Sciences.
ABSTRACT
Aim
The outstanding Andean biodiversity has been linked to the occurrence of evolutionary radiations that are common among high-elevation plant lineages. One of the most iconic examples is found in the species-rich genus Senecio, with an impressive variation in growth forms and habitat preference. Here, we use Hyb-Seq to overcome the lack of phylogenetic resolution found in previous studies with the aim of disentangling the processes shaping Senecio's hyper-diversity in the Andes, including the evolution of woodiness, growth form and habitat preference.
Location
Central and Northern Andes.
Taxon
Senecio ser. Culcitium.
Methods
Hyb-Seq data for 104 accessions of Senecio were newly generated and analysed using a data analysis workflow that utilises paralogs for phylogenetic reconstruction. The robustness of the species tree under different missing data treatments was investigated, and the phylogeny was dated. The role of hybridisation in the diversification of this lineage was addressed. The evolution of morphological key features and changes in habitat preferences were evaluated. In addition, the association of these features with diversification rate heterogeneity was tested.
Results
Senecio ser. Culcitium is a monophyletic lineage, likely of a Pleistocene origin. Hybridisation, possibly promoted by altitudinal range shifts during the Pleistocene climatic oscillations, played an important role in its evolution. We found evidence for several events of south-to-north migration from the puna on the Central Andes to the páramo on the Northern Andes. One of these migrations to the páramo resulted in a dramatic species diversification. Habitat changes from the páramo to the montane forest occurred multiple times and were associated with growth form shifts.
Main Conclusions
With a net diversification rate of 2 species per million years, high-elevation Andean Senecio is among the fastest diversifying lineages documented so far in the region. Frequent shifts in woodiness, growth form, and habitat played a crucial role in the diversification of this lineage.
1 Introduction
The Central and Northern Andes, spanning from northern Chile and Argentina to northern Colombia and Venezuela, are by far the most species-rich tropical mountains and an important biodiversity hotspot (Young et al. 2015). In terms of the flora, it holds one of the highest plant species diversity in the world, with at least 44% of the species being endemic (Myers et al. 2000). This endemism is even higher in the Northern Andes, reaching ca. 60% in the páramo with its tropical alpine flora (Luteyn et al. 1999), considered a ‘hotspot within a hotspot’ and the fastest evolving ecosystem in the world (Madriñán et al. 2013). Deciphering why the high Andes hold this exceptional plant diversity will thus shed light on the processes involved in the origin and patterning of plant diversity worldwide. The Andes are a very young mountain system that reached its current elevation in the last 10 million years (Gregory-Wodzicki 2000; Garzione et al. 2008), with rapid uplifts within the last 10–6 million years ago (Mya) in the Central Andes (Garzione et al. 2008) and ~2.7 Mya in the Northern Andes (Hoorn et al. 2010; Mulch et al. 2010). The Andean uplift created new open habitats emerging above the natural tree line, which were colonised by plant lineages of tropical and temperate origin (Bell and Donoghue 2005; Hughes and Eastwood 2006; Sklenář et al. 2011). With their arrival to the high Andean habitats, many of the temperate plant lineages experienced not only evolutionary radiations, i.e., the substantial increase in the number of taxa in a clade compared to its sister clade (Simões et al. 2016), but also a marked diversification of growth forms, involving the evolution of the woody habit, such as in Draba, Gentianella, Lupinus and Valeriana (Contreras-Ortiz et al. 2018; Cruz et al. 2023; Ramsay and Oxley 1997; Sklenář et al. 2011). While the woody habit is ancestral in angiosperms, with the herbaceous habit having evolved several times in different plant families (Klimeš et al. 2022; Lens et al. 2013), many herbaceous lineages have regained woodiness on islands (i.e., insular woodiness) or tropical mountain peaks (Carlquist 1974). Frequent transitions have recently been shown in the case of insular woodiness to correlate with drought and herbivory (Zizka et al. 2022). In tropical alpine ecosystems, woodiness is considered a key trait for plant radiations (Nürk et al. 2019).
New habitats not only became available as a result of the Andean uplift, but also as a consequence of Pleistocene glacial cycles causing altitudinal vegetation shifts in the tropical Andes (van der Hammen 1974; van der Hammen and Cleef 1986). During the cold periods, the alpine-like areas (including the equatorial páramo) moved downslope and potentially merged, comprising larger areas, while during interglacial periods, these areas were confined to small, scattered patches at higher altitude. As a consequence of these pulses of expansion/contraction, populations of plant species in the páramo became isolated on mountain tops during warmer periods and were potentially interconnected during colder periods (described as ‘flickering connectivity system’; Flantua et al. 2019). About 20 cycles of habitat fragmentation during the Pleistocene (Flantua et al. 2019) promoted both allopatric differentiation as well as hybridisation after secondary contact (e.g., Bartsia, Uribe-Convers and Tank 2016; Diplostephium, Vargas et al. 2017; Hypericum, Nürk et al. 2013; Lupinus, Contreras-Ortiz et al. 2018). Hybridisation may lead to speciation (reviewed in Schley et al. 2022 for Neotropical plants), although evidence for tropical alpine plants is scarce (e.g., Polylepis; Schmidt-Lebuhn et al. 2006).
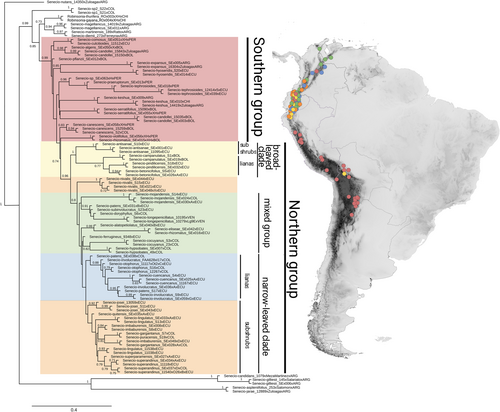
The drivers of tropical alpine plant diversification remain poorly understood, partly because of the methodological problems tackling rapid radiations (Carlsen et al. 2018; Fior et al. 2013; Nicholls et al. 2015). This applies in particular to most lineages within Asteraceae, which is one of the most species-rich families of vascular plants in the Andes with an intriguing morphological diversity (Pérez-Escobar et al. 2022). The cosmopolitan Senecio is the largest genus of the Asteraceae tribe Senecioneae, comprising ca. 1000 species in its narrow circumscription (Pelser et al. 2007). The genus is especially diverse in mountainous areas and deserts of the Americas, Africa and Asia (Cabrera et al. 1999). Although Senecio likely originated in western southern Africa about 10.7 Mya (Kandziora et al. 2017), very little in situ speciation occurred in tropical mountainous areas of Africa (Kandziora et al. 2016). In contrast, the tropical Andes present an incredible explosion of diversity in this genus. Because of the recent age of Senecio in the Americas (see Pelser et al. 2007), its high Andean diversity might have appeared surprisingly fast. Senecio ser. Culcitium is an exclusively Andean lineage recently defined based on a rigorous taxonomic revision (Salomón et al. 2018), including the former genera Lasiocephalus, Aetheolaena, Culcitium and some species originally described as Senecio. It comprises 43 species of herbs, subshrubs, shrubs and lianas. All species share a set of morphological features: discoid and nodding capitula usually with conspicuous calycular bracts, penicillate style arms with or without a tuft of long hairs, and microechinate pollen grains. Its distribution spans the Central and Northern Andes, from northern Argentina and Chile up to Venezuela (Figure 1), including the alpine-like ecosystems of puna and páramo (reaching up to 5200 m a.s.l.), and the montane forests (between 1800 and 3200(−3300) m a.s.l.). The large number of Senecio ser. Culcitium species, coupled with large morphological variation and low sequence divergence, resulted in a lack of phylogenetic resolution in previous studies (Dušková et al. 2017; Pelser et al. 2007; Salomón et al. 2018). This supports the idea of a recent, possibly adaptive radiation.
High-throughput sequencing (HTS) approaches, in particular genome skimming (Straub et al. 2012) and Hyb-Seq (Weitemier et al. 2014), are powerful tools in resolving phylogenies in Asteraceae (Gizaw et al. 2022; Siniscalchi et al. 2019), including Senecioneae (Ren et al. 2021), where traditional Sanger sequencing was not sufficiently informative in the past. But although HTS has been used to address the evolution of an increasing number of Andean lineages, including some Asteraceae (e.g., Kandziora et al. 2022; Nevado et al. 2018; Pouchon et al. 2018; Vargas et al. 2017), it has not been applied to Senecio. In particular, the species-rich and evolutionary young Andean Senecio remains poorly studied, and a robust phylogenetic hypothesis is still lacking for them.
In this study, we aimed to trace the evolutionary history of the Andean Senecio ser. Culcitium and to address the processes behind its radiation. We evaluated the role of woodiness, growth form and habitat shifts as well as hybridisation in the diversification of this plant lineage, in a stepwise manner: (1) First, based on a exhaustive sampling, we built a robust and dated phylogeny using Hyb-Seq data and different data analysis workflows; (2) we accounted for hybridisation in the evolution of Senecio ser. Culcitium with the hypothesis of frequent hybridisation due to the flickering habitat connectivity during the Pleistocene; (3) we hypothesised herbaceousness to be the ancestral state, from which woody growth forms evolved when colonising the páramo, and we tested this using ancestral state reconstructions; (4) we used trait-dependent diversification modelling to evaluate our hypothesis that the evolution of woody growth forms and the transition from the montane forest to the páramo increased net diversification.
2 Materials and Methods
2.1 Sampling
Our sampling included 36 out of the 43 species already assigned to Senecio ser. Culcitium, three described species now assigned to the group (Senecio algens, S. eliseae and S. quitensis) and additional putative new species to be described for this lineage. Most samples were collected across several field trips in the Andes over more than 10 years. Silica-dried material was collected for DNA extraction and herbarium material deposited in PRC, with duplicates in AAU, COL, LPB, QCA, QCNE and VEN. We included additional material from B, LPB, MA, SI and US. Herbaria acronyms follow Thiers (2016). The remaining seven species belonging to the series which we were unable to include in our sampling are very restricted endemics (except Senecio burkartii) and are only known from their type material (Senecio cajonensis, S. leucohorbius, S. piedrahitae, S. punasessilis, S. supremus and S. timidus). Nevertheless, each of their growth forms and habitat preferences is vastly represented in our sampling.
For species exhibiting morphological and/or genetic variation (from Dušková et al. 2017), we included up to five individuals from different populations to capture the variability. For widely distributed species, we covered most of their geographic distribution. As an outgroup, we selected several species from the Senecio s. str. New World Clade-1 (Pelser et al. 2007), including former Robinsonia species. In total, we sequenced 104 individuals, including ingroup and outgroup (Tables S1 and S2).
2.2 DNA Preparation and Sequencing
Hyb-Seq data were generated as in Gizaw et al. (2022), using the myBaits Expert Compositae-1061 target capture kit (Arbor Biosciences, Ann Arbor, Michigan, USA). For capturing off-target reads for retrieving plastid data, enriched libraries were mixed with unenriched libraries in the ratio 2:1 (run2), 1:1(run3) and 1.5:1 (run5). Run1 comprised enriched libraries only. Samples were sequenced on an Illumina (San Diego, California, USA) MiSeq at BIOCEV (Vestec, Czech Republic; run1), NextSeq at the Genomics Core Facility of CEITEC (Brno, Czech Republic; run2), and NovaSeq 6000 at IAB (Olomouc, Czech Republic; run3 and 5), utilising kits to obtain 150 base pair (bp) paired-end reads (Table S1). To check the consistency between sequencing runs, three technical replicates were included, Senecio candollei (15035xBOL, SEx003xBOL), S. tephrosioides (12414x5xECU, SEx039xECU) S. gilliesii (145xSalariatoxARG, SEx006xARG).
2.3 Phylogenetic Analyses
Because of the paleopolyploid origin of the Asteraceae (Barker et al. 2016; Zhang et al. 2021), we considered it important to account for paralogy in our dataset, which has been shown to increase species tree support and the number of concordant gene trees for some plant groups with similar paleopolyploid origins (Ufimov et al. 2022). For that, we used the ParalogWizard pipeline (hereafter PW; Ufimov et al. 2022; available at https://github.com/rufimov/ParalogWizard), which allows us to detect and retrieve paralogs to include them as separate alignments and gene trees, respectively, using their information in the phylogenetic reconstruction. For detecting paralogous loci, we used a minimum and maximum pairwise sequence divergence of 7.78% and 19.96%, respectively. After paralog retrieval, we built the phylogenies using the HybPhyloMaker pipeline (hereafter HPM; Fér and Schmickl 2018; available at https://github. com/tomas-fer/HybPhyloMaker). Alignments were generated using MAFFT v.7.029 (Katoh and Toh 2008). To account for a possible effect of missing data in subsequent analyses, we used different missing data filtering, allowing ≤ 25%, ≤ 50% and ≤ 70% missing data per accession for each locus and < 25% missing accessions per locus as a threshold. Gene trees were generated using RAxML v.8.4.2 (Stamatakis 2014) for each locus individually. The species tree was then reconstructed under the multispecies coalescent model using ASTRAL-III v.5.7.4 (Zhang et al. 2018). In addition, a concatenated supermatrix was built, and a phylogenetic tree was inferred using ExaML v.3.0 (Kozlov et al. 2015). Off-target plastid data were analysed following the methodology in Gizaw et al. (2022). The complete chloroplast genome of Dendrosenecio kilimanjari (GenBank accession number NC_037957) was used as a reference for read mapping. Two concatenated plastid phylogenies were generated using RAxML-NG v.8 (Kozlov et al. 2019), one based on the full plastome (hereafter fullplastome_RAxML), and a second based on coding and non-coding regions (introns, intergenic spacers) with the best model for each partition and 500 bootstrap replicates (hereafter cp_RAxML).
2.4 Divergence Time Estimation
To infer divergence times, we employed a penalised likelihood approach using treePL (Smith and O'Meara 2012). The 95% confidence interval of the highest posterior density (HPD) values from the crown age of Senecio s. str. New World Clade-1 from Pelser et al. (2007), as dated by Kandziora et al. (2017), was used as a secondary maximum/minimum calibration point (95% HPD: 9–3.1 Mya). We dated our nuclear phylogeny, which included all accessions (up to five per species) except described hybrids (Senecio superparamensis, S. josei and S. quitensis), using the best ExaML tree (with ≤ 70% missing data filtering; not the ASTRAL tree, as it lacks terminal branch lengths). Then, to obtain the confidence intervals for the dating following the tutorial by Maurin (2020; available at https://arxiv.org/abs/2008.07054), we generated 300 bootstrap replicates using RAxML and employing the ExaML topology as constraint. The optimal parameters were found using the ExaML phylogeny and a treePL wrapper script (available at https://github.com/tongjial/treepl_wrapper) with 100 prime steps and a cross-validation to find the best smoothing value. The optimisation parameters (opt, optad and optcvad = 2) and a smoothing value of 1 × 10−12 were chosen to date the 300 replicates using treePL. We summarised the results using TreeAnnotator in BEAST v.2.6.7 (Bouckaert et al. 2019).
2.5 Analyses Addressing Reticulate Evolution and Hybridisation
We addressed reticulate evolution and tested for signatures of hybridisation using a suite of approaches after removing the described hybrid species (Senecio superparamensis and S. josei). We inferred cytonuclear discordance (i.e., incongruence between the nuclear and plastid phylogeny) comparing the nuclear and plastid topologies visually, and estimated introgression based on the D statistic (Patterson et al. 2012) using Dsuite v.0.5 (Malinsky et al. 2021). We relied on estimates of the D statistic and not the f-branch statistic (Malinsky et al. 2018) because the f-branch measure is better suited for population-based sampling, and our Hyb-Seq sampling might not be adequate for this estimate. We adjusted p-values using the False Discovery Rate (FDR) method (Jafari and Ansari-Pour 2019) available in the package ‘stats’ v.3.6.3 in R v.4.1 (R Core Team 2018). Dsuite gives evidence for introgressive hybridisation/introgression, i.e., the introduction of syntenic nucleotide variation from a donor species into the genome of a recipient species by means of hybridisation and backcrossing (Anderson and Hubricht 1938). We followed the methodology in Gorospe et al. (2025) to generate the input for the analysis in Dsuite. Five accessions (11117xOt2xCxECU, 11540xO26xBxECU, FAA628xI17xCOL, 10279xLg9ExVEN, 11095xECU) with less than 890 loci were excluded to avoid losing a large amount of single nucleotide polymorphisms (SNPs) due to missing data. A VCF file with a total of 1019 SNPs was obtained, after filtering using a 20% missing data threshold, keeping biallelic sites and retaining one random SNP per locus. The most external ingroup taxon, Senecio comosus, was used as outgroup because our original outgroup was too distantly related for inferring introgression. A heatmap was generated showing significant D statistic results. We also constructed a network from the nuclear concatenated loci using Neighbour-Net (Bryant and Moulton 2004) to visualise reticulation. Due to the large and complex dataset, other resource-intensive methods such as PhyloNet (Than et al. 2008) were not possible to implement. We also addressed gene tree discordance using Phyparts (Smith et al. 2015) and the ASTRAL-III-t 4 option.
2.6 Ancestral State Reconstruction
First, we built a matrix (Table S3) of one morphological, one anatomical and one ecological ‘feature’ and their respective (character) states: (i) growth form (narrow-leaved rosette herb, narrow-leaved ascending herb, broad-leaved rosette herb, acaulescent herb, erect subshrub, narrow-leaved ascending subshrub, broad-leaved subshrub, narrow-leaved liana, broad-leaved liana); (ii) habit (herbaceous, woody); (iii) habitat (páramo, puna, montane forest). Each individual sampled was classified according to these states, following morphological features recorded in the field and/or on the vouchers, herbarium label details or based on literature and personal observation. Features were coded as ambiguous if different individuals of the species presented different states for the same feature.
To infer ancestral states, we built a new ExaML tree with only one representative per species (exceptionally two), and we converted it to an ultrametric tree using the function ‘force.ultrametric’ of the package ‘phytools’ (Revell 2012) in R v.2021.09.2. We performed ancestral state reconstructions of the three features (growth form, habit, habitat; see Table S3) using a maximum likelihood approach implemented in the R package ‘corHMM’ (Beaulieu et al. 2017). Three models were evaluated: one-parameter equal rates (ER) model, in which a single rate is estimated for all possible transitions; symmetric (SYM) model, in which forward and reverse transitions between states are constrained to be equal; and all rates different (ARD) model, in which all possible transitions between states receive distinct values. The best model was selected using the Akaike information criterion corrected for small sample sizes (AICc).
2.7 State-Dependent Diversification Rate Shift Analyses
We calculated the net diversification rate for Senecio ser. Culcitium following the equation in Magallon and Sanderson (2001): ln(N) / t; N: number of species, t: crown age. As we observed a strong difference in the species number in the páramo compared to the puna or montane forest (24, 13 and 9 species, respectively), we tested if the woody habit or habitat shifts were associated with a shift in the net diversification rate. To do so, we applied the state-dependent speciation and extinction (SSE) models implemented in Binary State Speciation and Extinction (BiSSE; Maddison and FitzJohn 2015) and Hidden State Speciation and Extinction (HiSSE; Beaulieu and O'Meara 2016). The ultrametric ExaML tree with one representative per species was used for the analyses. For ‘habit’, we considered ‘woody’ and ‘herbaceous’ as the binary states, and for ‘habitat’, we considered ‘páramo’ and ‘out of the páramo’ as the binary states. Because of the polyphyly of some species, we implemented different sampling fractions (f = 1,1 and f = 0.85,1) in the analyses to account for the effect of cryptic species diversity in our tree. We selected the best-fitting model using AICc based on the log-likelihood and the number of free parameters for the model.
3 Results
3.1 Phylogenetic Analyses and Divergence Time Estimation
It has been shown for coalescent-based species tree estimation that a higher number of loci, although containing more missing data, gives more accurate results than a lower number of loci with less missing data (Molloy and Warnow 2018; Nute et al. 2018; Sayyari et al. 2017; Streicher et al. 2016). Given that our results comparing the different missing data thresholds did not substantially differ in species tree topology, but the more conservative thresholds gave lower support values (Figures 1 and S1–S3), and based on the above-mentioned empirical studies and simulations, we chose our most relaxed missing data filtering results (≤ 70% missing data per locus, 25% missing accessions per locus) to present here and base our discussion and conclusions on.
The nuclear dataset consisted of 1026 loci after paralogs were detected and included for phylogenetic reconstruction using PW (total 26,627 parsimony informative sites PIs; average 10% PIs per locus). In contrast, little variation was detected in the full plastome dataset consisting of 145 partitions (total 1558 PIs, average 1% PIs per partition). Phylogenetic analyses using nuclear as well as plastid data recovered Senecio ser. Culcitium as monophyletic with high support in both nuclear and plastid data (ASTRAL: 0.99 local posterior probability (LPP), ExaML: 100% bootstrap support (BS), cp_RAxML: 100% BS; fullplastome_RAxML: 100% BS; Figures 1 and S3–S5). All technical replicates were monophyletic, proving consistency between sequencing runs.
In the ASTRAL (Figure 1) as well as the ExaML tree (Figure S3), internal branches within Senecio ser. Culcitium usually exhibited low support, with some notable exceptions. Several small, supported clades (support ranging from 0.83–1 LPP and 76%–100% BS) formed a basal paraphyletic group within Senecio ser. Culcitium composed of species mostly distributed in the southern part of the series' range, namely the southern Central Andes (Argentina, Chile and Bolivia) as well as the northern Central Andes in Peru, rarely extending to Colombia (hereafter ‘Southern group’, corresponding to ‘group A' in the network; Figure 2). This group consists exclusively of herbs, with the exception of one new species that appears to be shrubby (to be described based on morphological and molecular data, referred here as Senecio-sp_SEx063xHxPER). Within the ‘Southern group’, we consistently recovered a morphologically well-delimited group comprising small acaulescent herbaceous species (0.85 LPP, 100% BS; Figures 1 and S3), and two clades of herbs (mainly narrow-leaved rosette herbs) that showed different placements depending on the phylogenetic tree.

The remaining species of Senecio ser. Culcitium clustered in a strongly supported clade by the nuclear markers (0.96 LPP, 100% BS). This clade is composed almost entirely of species from the northern part of the series' distribution, in Ecuador, Colombia and Venezuela, but also a few species appearing in Bolivia (hereafter ‘Northern group’, corresponding with groups B–E in the network; Figure 2). Within this clade, we recovered several groups that share morphological traits. First, a moderately supported clade (1 LPP, 89% BS; Figures 1 and S3) comprising broad-leaved woody species from Ecuador (and rarely also Bolivia) was recovered at the base of the ‘Northern group’ (hereafter ‘broad-leaved clade’, group E in the network, divided into the subclades ‘broad-leaved subshrubs’ and ‘broad-leaved lianas’; Figure 2). The sister clade to the ‘broad-leaved clade’ was recovered with moderate support with ASTRAL (0.8 LPP; Figure 1) but not ExaML (30% BS; Figure S3). This clade included Senecio nivalis (high support: 1 LPP, 100% BS; coloured in Figures 1 and S3 as group C to match with Figure 2), several species of variable morphology (herbs, shrubs and lianas) for practical reasons called ‘mixed group’, and a so-called ‘narrow-leaved clade’ (unsupported). The ‘narrow-leaved clade’, corresponding to groups C and D in the network (Figure 2), comprised mostly subshrubs and narrow-leaved lianas from páramos and Andean forests of Ecuador, Colombia and Venezuela. Within this clade, two subclades distinct in growth forms, but both with weak support, were revealed (‘narrow-leaved subshrubs’, group C in the network and ‘narrow-leaved lianas’, group D in the network; Figures 1 and 2). The placement of the herb Senecio nivalis was ambiguous: in the ASTRAL tree, it appeared sister to the ‘mixed group’, in the ExaML tree, it was placed as sister to a subclade of the ‘mixed group’ (Figure S3), and in the network, it appeared within group C. In the network, the ‘mixed group’ (group B) comprised a subgroup with herbs, shrubs and lianas (B1), and a second subgroup of herbaceous species (B2; Figure 2).
Most species were supported to be monophyletic in the ASTRAL tree, with the exception of five species (Senecio candollei, S. imbaburensis, S. involucratus, S. patens and S. otophorus; Figure 1).
The plastid cp_RAxML and the fullplastome_RAxML phylogenies showed lower support and resolution within Senecio ser. Culcitium (Figure S4) compared to the nuclear trees. Nevertheless, some supported incongruences between both data sources were detected, especially regarding the basal group of Senecio ser. Culcitium, which in the cp_RAxML, and fullplastome_RAxML phylogenies did not correspond with the ‘Southern group’ recovered based on nuclear data (Figures 1 and S3). Instead, both northerly and southerly distributed species were found as the basal clade for the series. The cp_RAxML and fullplastome_RAxML trees were not resolved, with some polytomies at the base, most species being recovered as non-monophyletic, and most clades lacking support (Figures S4 and S5). By using the Compositae1061 probe set, we targeted nuclear exons, while plastid data were obtained as part of the off-target reads, and although we aimed at increasing the proportion of plastid reads by adding unenriched libraries, there were differences in the ratio of enriched to unenriched libraries between sequencing runs (see Methods). As a result, plastid data presented a higher amount of missing data per accession than nuclear data. Around 15 accessions of the ingroup displayed between 30% and 35% missing data, which could have affected our results, although the overall tree support did not improve significantly after removal of those accessions (data not shown).
Our divergence time estimation using penalised likelihood revealed that Senecio ser. Culcitium originated during the Pleistocene, around 1.9 Mya (95% HPD: 1.85–2.01 Mya), while the Northern group originated 1.67 Mya (95% HPD: 1.62–1.72 Mya). The estimations using different missing data thresholds (≤ 25%, ≤ 50% missing data per locus instead of ≤ 70%, < 25% missing accessions per locus) gave comparable results, placing the origin of this lineage also in the Pleistocene (data not shown).
3.2 Reticulate Evolution and Hybridisation
We encountered a high degree of gene tree incongruence using Phyparts (Figure S6), and when accounting for quartet support using the ASTRAL-t 4 option, some nodes showed support for alternative topologies (Figure S7). We obtained a star-shaped Neighbour-Net network (Figure 2), which corresponds with the pattern of a recently diverged lineage. In this network, we recovered several groups showing some basal reticulation, described as the groups A–E detailed. The D statistic estimated using Dsuite revealed multiple evidence for introgression between species of different growth forms (Figure S8 and Table S4). Several accessions from different species within the ‘Southern group’ showed introgression with species that belong to clades and groups from the ‘Northern group’ (broad-leaved clade, mixed group and narrow-leaved clade). Within the ‘Northern group’, introgression signals were obtained among all groups and clades, with most of the signals appearing between the mixed group and both the broad-leaved clade and the narrow-leaved clade. Introgression between lianas and subshrubs within the narrow-leaved clade was also detected. We did not omit accessions with significant introgression values (p-value < 0.01) from our phylogenetic results, as less than half of the accessions would have been retained, and such a reduced dataset would not reflect the true diversity of Senecio ser. Culcitium.
3.3 Evolution of Woodiness, Growth Form and Habitat
The reconstruction of the ancestral growth form showed the ancestor of Senecio ser. Culcitium as a narrow-leaved rosette herb (ER best supported model), which gave origin to other herbaceous forms (i.e., acaulescent rosette herbs and broad-leaved rosette herbs of the ‘Southern group’), still in the southern part of the distribution range (Figure 3). Also, this ancestral state gave rise to woody forms twice in this lineage (ER and SYM equally best supported models). Interestingly, the major transition to woodiness corresponded with the only páramo colonisation event that led to a massive species and growth form diversification (SYM and ARD equally best supported models): the formation of the well-supported ‘Northern group’ in the Northern Andes (Figure 3). During the diversification within the ‘Northern group’, there were likely four reversals from woodiness to the herbaceous state, all in páramo species. However, secondary woodiness or its reversal were not linked with shifts in net diversification rates, as the BiSSE and HiSSE results gave a better fit for the null model over the trait-dependent hypothesis based on AICc values (Table S5).
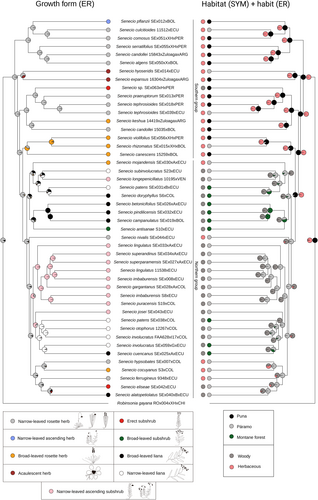
The reconstruction of the ancestral habitat, considering puna, montane forest and páramo (including its highest-elevation parts called super-páramo) showed the puna as the ancestral habitat of Senecio ser. Culcitium (Figure 3). From there, the páramo was colonised at least five times by Senecio ser. Culcitium. One of these colonisations, which gave rise to the ‘Northern group’, resulted in a high diversification within the páramo. From the páramo, the montane forest was colonised at least four times in the Northern Andes (Figure 3). However, páramo colonisation or out-of-the-páramo colonisation was not accompanied by pronounced shifts in net diversification rates, as also in this case the BiSSE and HiSSE results gave a better fit for the null model over the trait-dependent hypothesis based on AICc values (Table S5). Downslope colonisation of the montane forest came along with a growth form shift to lianas (Figure 3). From the montane forest in the Northern Andes, a dispersal event to the montane forest in Bolivia might have occurred. The calculation of the overall net diversification rate resulted in two species per million years [(3.81)/1.9 = 2].
4 Discussion
Our study is the first to reconstruct the evolutionary history of Senecio ser. Culcitium employing high throughput sequencing, addressing the impact of woodiness, growth form and habitat shifts as well as hybridisation on its radiation in the high Andes. We included about 85% of the species belonging to this lineage, i.e., most species from the former genera Lasiocephalus, Aetheolaena, and Culcitium, some originally described as Senecio species belonging to the series Culcitium, plus some newly assigned species and possibly new species. Hyb-Seq data have been shown to be powerful in reconstructing several key points in the evolutionary history of this very recent plant lineage, whose complicated taxonomic history corresponds with an intricate evolutionary history.
4.1 Pleistocene Origin of Senecio Ser. Culcitium in the Central Andes of Argentina, Chile, Bolivia and Peru and Northward Migration
Our results showed Senecio ser. Culcitium is a monophyletic lineage that originated about 1.9 Mya (95% HPD: 1.85–1.97 Mya). This estimate is congruent with the Pleistocene origin of this lineage proposed by Pelser et al. (2010), who included only a few species of the former Lasiocephalus, now considered to be part of Senecio ser. Culcitium. Such an evolutionarily young age of Senecio ser. Culcitium can explain the low sequence variability found in plastid regions, considering its conserved evolution, which resulted in a lack of resolution in our plastid tree reconstructions. The estimated net diversification rate for Senecio ser. Culcitium of two species per million years gave an even higher diversification than that estimated for most páramo lineages (average ~1.36 species/My; Madriñán et al. 2013). This makes Senecio ser. Culcitium one of the lineages with the highest net diversification rates known to date, although the rate heterogeneity within this lineage needs to be further explored.
Within Senecio ser. Culcitium, species in baselmost placements formed the ‘Southern group’, and those species were mostly from the Central Andes in Argentina, Chile, Bolivia and Peru. All species outside this early divergent ‘Southern group’ occur in the Northern Andes, indicating an origin of Senecio ser. Culcitium in the Central Andes and subsequent northward migration and colonisation of the Northern Andes. Both tropical and temperate areas acted as sources of the tropical high-elevation Andean flora (Sklenář et al. 2011). Several plant lineages (e.g., Cerastium, Draba, Gentianella, Halenia, Lupinus, Valeriana) reached the Andes from the northern temperate areas (Bell and Donoghue 2005; Hagen and Kadereit 2003; Hughes and Eastwood 2006; Koch and Al-Shehbaz 2002; Scheen et al. 2004). In contrast, immigration to the Northern Andes from the Southern Andes has been less frequently documented, for Azorella (Andersson et al. 2006), Calceolaria (Cosacov et al. 2009), Oreobolus (Chacón et al. 2006) and Ourisia (Meudt and Simpson 2006). From the plant lineages of a Central Andean origin, south-to-north migration, following the final uplift of the Andes, has been previously suggested for Puya (Jabaily and Sytsma 2013), Oritrophium (Salomón et al. 2022) and the ‘Oxalis tuberosa alliance’ (Emshwiller and Doyle 2002). Because of the lack of population-level sampling, we can not conclude if Senecio ser. Culcitium colonised the Northern Andes via a long-distance dispersal or if a stepwise migration was involved, such as proposed for the Andean species of Halenia (Hagen and Kadereit 2003) and Lupinus (Drummond et al. 2012), and for some Asteraceae, such as Oritrophium (Salomón et al. 2022) or a clade within Espeletia (Pouchon et al. 2021).
Despite the monophyly of Senecio ser. Culcitium and the monophyly of most species, some species appeared non-monophyletic in the nuclear tree (Senecio candollei, S. imbaburensis, S. involucratus, S. patens and S. otophorus), showing genetic differentiation according to geography. This was partly found in Dušková et al. (2017), who analysed some of the same individuals in an amplified fragment length polymorphism (AFLP) study. Due to this genetic differentiation, partly coupled with morphological variation (data not shown), we consider them as different entities awaiting taxonomic revision.
Some clades in the core Senecio ser. Culcitium lacked resolution even though we used more than 1000 nuclear loci for phylogenetic reconstruction, an approach that has been applied to other young lineages from the Asteraceae, including those from high elevation habitats in tropical mountains (Dendrosenecio, Gizaw et al. 2022; Loricaria, Kandziora et al. 2022). This, as well as the star-shaped Neighbour-Net network, denotes that Senecio ser. Culcitium radiated recently, but also hints that there may be some processes involved in its evolution that could partially mask the phylogenetic signal. The very high degree of gene tree incongruence we observed for our dataset can partially be explained by hybridisation, especially in a recently diversified lineage (Degnan and Rosenberg 2009) such as Senecio ser. Culcitium. When addressing reticulation, the Neighbour-Net network showed five main groups, all of them with basal reticulation. The D statistic gave a rather strong signal of introgression, often between species from distant clades and of diverse growth forms, even though we had already removed species of assumed hybrid origin based on morphological evidence before estimating introgression.
Strong hybridisation signal was found in other recent lineages from the Andes (Diplostephium, Vargas et al. 2017; Draba, Koch and Al-Shehbaz 2002; Espeletia, Cortés et al. 2018; Loricaria, Kandziora et al. 2022; Lupinus, Nevado et al. 2018), and hybridisation seems to have played a crucial role in their diversification. Hybridisation as a consequence of secondary contact of populations has been related to changes in the connectivity of populations in the Northern Andes during Pleistocene climatic cycles (Flantua et al. 2019). Since our divergence time estimation indicated a Pleistocene origin of Senecio ser. Culcitium, climatic oscillations and high habitat dynamics might have been important throughout the diversification of Senecio ser. Culcitium, where lineages could even have experienced multiple rounds of introgression.
Although Senecio ser. Culcitium showed substantial signals of introgression, a genetic clustering analysis of our Hyb-Seq dataset using STRUCTURE was not informative to detect admixture (data not shown). Because of the limitation of our data using this type of analysis due to the small number of unlinked SNPs, employing SNP-based analyses in combination with population sampling can be a direction to follow in the future in order to discriminate between ongoing vs. historical hybridisation in this lineage.
4.2 Frequent Shifts in Woodiness, Growth Form and Habitat
The woody habit is considered the ancestral state in flowering plants from which herbs frequently evolved in distant lineages. Repeated transitions to secondary woodiness (Judd et al. 1994; Smith and Donoghue 2008), and then back to the herbaceous habit (García-Maroto et al. 2009) are known, documenting the evolutionary flexibility of this trait. In Senecio ser. Culcitium, we found evidence for secondary woodiness, as the group had a herbaceous ancestor. Interestingly, multiple clades in the group showed shifts to woodiness, suggesting a repeated origin of this trait, in particular when colonising the páramo. In fact, shifts between herbaceousness and woodiness may have been more frequent than assumed throughout the evolution of Senecio ser. Culcitium. The basal Asteraceae lineages are woody, while basal Asteroideae are herbaceous (Panero et al. 2014); thus, woodiness of páramo Senecio ser. Culcitium may even be higher ranked than secondary. A shift from herbaceous basal rosettes (or semirosettes) to different woody growth forms (lianas, (sub)shrubs) has also been shown for Andean species of Valeriana (Caprifoliaceae; Cruz et al. 2023), and hypothesised for Loricaria (Asteraceae; Kolář et al. 2016). In addition, secondary woodiness has been found for Andean Arcytophyllum (Rubiaceae; Neupane et al. 2017).
Woody and herbaceous life forms differ in frost, drought and shade tolerance (Klimeš et al. 2022), among others. In Senecio ser. Culcitium, the herbaceous habit dominates in the dry puna in the southern Central Andes, which is a seasonal high-elevation grassland, whereas woody growth forms ((sub)shrubs, lianas) prevail in the more humid páramo and montane forest of the Northern Andes. The herbaceous members of Senecio ser. Culcitium from the puna, with its relatively small stature and fast growth, may have a clear adaptive advantage in this seasonal high-elevation environment compared to woody plants (Klimeš et al. 2022). Consequently, the lack of seasonality in the páramo might have promoted the explosion of woody growth forms after colonisation from the puna, following the south–north Andean orogenesis (Gregory-Wodzicki 2000).
Interestingly, species of high elevation in Valeriana from the Andes already acquired woodiness before colonising the páramo (Cruz et al. 2023), questioning the role of woodiness as a key trait for plant diversification in the páramo. In accordance, our results for Senecio ser. Culcitium did not show any correlation between habitat type occupied and an increase in the net diversification rate, which indicates a limited role of woodiness and associated habitat shifts in its diversification. Because state-dependent speciation and extinction (SSE) models, like those implemented in BiSSE and HiSSE, have been developed for a much higher number of taxa, the resolution of the method is low if the tree size is below 300 tips (Davis et al. 2013). Also, root age influences the robustness of SSE models, and trait-dependent diversification has been more frequently found for deeper phylogenies (Helmstetter et al. 2023). Although our data completeness is very high (ca. 85% of known taxa plus putative new species), Senecio ser. Culcitium comprises less than 50 species and is of a relatively young age, which sets limits on the detection power of the method, and our results should be taken with caution.
The montane forest was colonised at least four times from the páramo by Senecio ser. Culcitium, and subsequently one species reached the southern Central Andes in the Bolivian montane forest from a montane forest ancestor. In contrast, Cuatrecasas (1978) proposed for this lineage an ancestor from the montane forest that colonised the páramo, as was shown for Chusquea (Fisher et al. 2009), Disterigma (Pedraza-Peñalosa 2009), Huperzia (Wikström et al. 1999) and Lobeliaceae (Knox et al. 2008). Our result contradicts what Cuatrecasas expected, but it is in agreement with Dušková et al. (2017), who suggested a páramo ancestor for Lasiocephalus (former name for some species within Senecio ser. Culcitium) and further migration to the montane forest, although their results were not conclusive due to the unresolved phylogeny and limited sampling. A downwards colonisation of the montane forest from the páramo was also documented for Andean Chaetanthera (Hershkovitz et al. 2006) and Puya (Jabaily and Sytsma 2013).
Our character state reconstruction suggested that the montane forests were colonised by woody ancestors from the páramo, rejecting a herbaceous ancestor suggested by Dušková et al. (2017). Each time the montane forest was colonised, the resulting growth form was a liana, with certain morphological traits: all the liana species have much smaller capitula, with a lower number of flowers per capitulum and less conspicuous colours than páramo species. Thus, the morphological similarity among montane forest species may represent convergent adaptive evolution. Convergent evolution of growth forms may be common in the high Andes; Contreras-Ortiz et al. (2018) recently demonstrated it for the rosette growth form in Northern Andean Lupinus. In Senecio ser. Culcitium, the liana growth form may have been favoured over shrubs to reach the upper strata of the montane forest, competing for light, and the decrease in the number of flowers and brightness of the capitulum colour could be related to a different pollinator spectrum at lower elevation. Another possible key trait for the transition from páramo (sub)shrubs to forest lianas could be clonal reproduction. Belowground plant organs are still understudied, yet crucial for plant ecology and evolution (Klimešová et al. 2018), also in the high Andes (Cruz et al. 2023). For Senecio ser. Culcitium, we only know of rhizomes in rosette species up to now. Future research is needed to study the growth form evolution of Senecio ser. Culcitium from a whole-plant perspective.
Author Contributions
L.S. and P.S. conceived the initial idea. L.S. and R.S. designed the study. P.S. and L.S. collected the majority of plant material and gathered the trait data. L.S. conducted laboratory work. L.S. and J.M.G. analysed the data. T.F. and R.S. provided training and advice on data analyses. L.S. wrote the initial draft of the manuscript. L.S., R.S. and P.S. facilitated the project by logistic and infrastructure support. F.K. contributed with financial support. All authors contributed to the final version of the manuscript.
Acknowledgements
The authors thank Lenka Flašková for laboratory help, Diana L. Aparicio Vásquez for helpful comments, and two anonymous reviewers for their contribution. We also thank the herbaria B, LPB, MA, SI and US, which contributed material for this study, and Stephen Beck (LPB) for some additional material provided. Special thanks to Daniel Montesinos (B) for providing some material, helping organizing the fieldwork and managing the research permits in Peru. The research permits were issued by the Ministerio del Ambiente, Ecuador (number MAE-DNB-CM-2018-0082), the Ministerio de Medio Ambiente y Agua, Bolivia (number 026/09), the Administración de Parque Nacionales, Argentina (number 1621) and Servicio Nacional Forestal y de Fauna Silvestre in Peru (N° AUT-IFL-2020-024, RDG N D000081-2020-MINAGRI-SERFOR-DGGSPFFS). Computational resources were supplied by the project ‘e-Infrastruktura CZ’ (e-INFRA LM2018140) provided within the program Projects of Large Research, Development and Innovations Infrastructures. This research was funded by the OP RDE project ‘International mobility of researchers at Charles University (MSCA-IF III)’ reg. n. CZ.02.2.69/0.0/0.0/19_074/0016231, awarded to L.S., and the Czech Science Foundation (GAČR) (grant 20-10878S to R.S. and F.K.). Financial support also came from the long-term research development project no. RVO 67985939 of the Czech Academy of Sciences. Open access publishing facilitated by Univerzita Karlova, as part of the Wiley - CzechELib agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Raw reads are available under NCBI SRA BioProject PRJNA1109546.




