Physiological thermal niches, elevational ranges and thermal stress in dendrobatid frogs: An integrated approach
Abstract
enAim
We investigated the relationship between thermal physiology, elevational distribution and thermal stress among nine closely related dendrobatid frogs during their aquatic stage by employing an integrated approach, combining thermal physiology, environmental temperature modelling and predictive assessments of current and future exposure to thermal variation.
Location
Ecuador.
Taxon
Amphibians; Anura, Dendrobatidae, Epipedobates, Hyloxalus.
Methods
We determined the thermal performance curves (TPCs) of larval growth for each species and modelled the thermal variation in contrasting aquatic larval environments for both present and future times. This allowed us to estimate the expected elevational distributions and forecast periods of exposure to stressful temperatures that inhibit larval growth due to elevation and global warming.
Results
We found significant correlations between optimum temperature (Topt), 50% maximum performance temperature (maxB50), 50% minimum performance temperature (minB50) and cold resistance (survival at 9°C) with the current elevational distributions. However, thermal physiology predicted lower than observed distributions for high-elevation dendrobatids and higher than observed maximum elevations for lowland species. Nonetheless, our models predicted that low thermal variability habitats (i.e. streams and deep permanent ponds) can buffer the future temperature increase for all taxa, even when considering the most extreme scenario. In contrast, all species within high thermal variation habitats (open forest temporary ponds) are expected to experience stressful temperatures under present conditions.
Main Conclusions
The findings indicate that thermal physiology may not be a limiting factor for dendrobatid frog species' ranges in this equatorial mountain gradient. Highland species may need to adapt to suboptimal performance, while some lowland species could occupy higher elevations. This study emphasizes the importance of habitat buffering to mitigate thermal stress in the face of climate change for amphibians in tropical mountains.
Resumen
esObjetivo
Empleando un enfoque integrado que combina la fisiología térmica, la modelización de la temperatura microambiental y evaluaciones predictivas de la exposición presente y futura a las variaciones térmicas, investigamos la relación entre la fisiología térmica, la distribución altitudinal y el estrés térmico en nueve ranas dendrobátidas estrechamente emparentadas.
Ubicación
Ecuador.
Taxón
Anfibios; Anura, Dendrobatidae, Epipedobates, Hyloxalus.
Métodos
Determinamos la sensibilidad térmica del crecimiento larval (TPCs) para cada especie y modelamos la variación térmica en diferentes ambientes larvales acuáticos tanto para el presente como para el futuro. Esto nos permitió estimar las distribuciones altitudinales esperadas y el porcentaje de tiempo de exposición a temperaturas estresantes que inhiben el crecimiento larvario, debido al calentamiento global, para cada especie y hábitat.
Resultados
Encontramos correlaciones significativas entre las temperaturas óptima (Topt), la temperatura máxima (maxB50) y mínima (minB50) de crecimiento superior al 50% del máximo de crecimiento larvario, y la resistencia al frío (supervivencia de las larvas a 9°C), con las distribuciones altitudinales actuales. Sin embargo, la fisiología térmica predijo distribuciones más bajas para las especies de altitud y elevaciones máximas más altas para las especies de tierras bajas, con respecto a la distribución observada. Por otro lado, nuestros modelos predijeron que los hábitats con baja variabilidad térmica (arroyos y charcas permanentes profundas), pueden amortiguar el aumento futuro de las temperaturas, incluso al considerar el escenario más extremo. En contraste, se espera que todas las especies en hábitats con alta variabilidad térmica (charcas temporales soleadas) experimenten temperaturas estresantes ya en el presente.
Conclusiones Principales
Los resultados indican que la fisiología térmica no parece ser un factor limitante para la distribución altitudinal de las especies de ranas dendrobátidas en un gradiente montañoso ecuatorial. Es posible que las especies de tierras altas necesiten adaptarse a un crecimiento subóptimo, mientras que algunas especies de tierras bajas podrían ocupar elevaciones más altas. Este estudio enfatiza la importancia del hábitat para amortiguar el estrés térmico en los anfibios de montañas tropicales debido al calentamiento global.
1 INTRODUCTION
The influence of temperature on distributional patterns has been a longstanding topic in ecology (e.g. Grinnell, 1917; Hutchinson, 1957; Kearney & Porter, 2009; Whittaker et al., 1973). The need to address this question has become of utmost importance recently due to the challenge that global warming represents for biodiversity (Deutsch et al., 2008; Tewksbury et al., 2008) including alteration in the distributional ranges of species worldwide (Pecl et al., 2017) and particularly in highly diverse tropical montane systems (Strangas et al., 2018). The threats posed by climate change have increased the use of physiological data to assess species distribution and inform conservation actions in recent years (e.g., Evans et al., 2015; Helmuth et al., 2014; Huey et al., 2012; Sunday et al., 2014).
However, concerns persist regarding the limitations of thermal physiology when assessing species distribution (Araújo et al., 2019; Bovo et al., 2018, 2023; Camacho et al., 2024; Strangas et al., 2018). The accuracy of physiological models largely depends on the extent to which observed species' distributions (realized niches) are influenced by organismal biological tolerances (fundamental niches). Furthermore, other non-climatic factors (e.g. dispersal ability and biotic interactions) may also contribute to shaping species' distributions (Araújo & Luoto, 2007; Soberón & Peterson, 2005; Wisz et al., 2013). Yet, only a few studies have investigated the role of thermal physiology in determining distribution limits at an interspecific level (Bovo et al., 2023; Moore et al., 2023; Overgaard et al., 2014; Sánchez-Fernández et al., 2012; Strangas et al., 2018; Sunday et al., 2019). Data are particularly scant in extremely species-rich regions, like the Neotropical Andes (Bennett et al., 2018; Myers et al., 2000, but see Bovo et al., 2023 for the Atlantic Forest mountain ranges). This aspect is particularly relevant for understanding the biogeography of tropical mountains, as physiology has been proposed as an important contributing factor for limiting species distribution (Janzen, 1967).
Tropical montane species are anticipated to exhibit sensitivity to climate change due to the presence of stable climatic zonations throughout the year. These climatic bands act as potentially important thermal barriers across different elevations, hindering vertical migration. This circumstance is likely to result in the development of highly specialized thermal niches, thereby promoting increased geographical isolation and constraints on both species' distributional shifts and range sizes (Janzen, 1967; Colwell et al., 2008; La Sorte & Jetz, 2010, but see Logan et al., 2013). Similarly, tropical lowland forest species are at risk from climate warming, given their exposure to temperatures near their physiological optimum (Deutsch et al., 2008; Huey et al., 2009) and thermal limits (Pintanel et al., 2019; Pintanel et al., 2022; von May et al., 2019). The predicted rise in environmental temperatures makes upslope shifts towards cooler habitats the most probable alteration in species distribution (Chen et al., 2011; Colwell et al., 2008; Parmesan & Yohe, 2003).
However, establishing a correspondence between physiological estimates and elevational ranges, both presently and in the future, presents several challenges. One significant challenge stems from the organisms' occupancy within critical horizontal microclimatic gradients, such as forested versus open habitats (González-del-Pliego et al., 2020; Montejo-Kovacevich et al., 2020; Pintanel et al., 2019) or ponds versus streams (Pintanel et al., 2022). Habitats with this low-level thermal variability can act as microclimatic thermal refugia that mitigate extreme temperature events and their occupancy may determine the elevational ranges of organisms (Pintanel et al., 2019). Additionally, utilizing thermal environment estimates at varying spatial resolutions may yield divergent predictions concerning current and future species distributions (Helmuth et al., 2014; Randin et al., 2009; Stickley & Fraterrigo, 2023). Moreover, it is essential to estimate microclimatic variation to determine whether species need to shift their range or can withstand excessively warm local temperatures by changing their habitat usage (Suggitt et al., 2018; Sunday et al., 2014). With estimates of maximum temperatures at protected habitats (Ex. Camacho et al., 2015), researchers can estimate how close they are to optimal or critical temperatures (thermal safety margin and warming tolerance, respectively; sensu Deutsch et al., 2008; Garcia et al., 2019) and/or to assess the duration of exposure to stressful temperatures (hours of restriction; Sinervo et al., 2010, but see Kearney, 2013).
Comparisons between environmental temperatures and traits representing organismal thermal sensitivity are essential. Despite their resource-intensive nature, thermal performance curves (TPCs) of growth rate have been proposed as effective proxies for fitness, aiding in predicting species' responses to changing environments (Overgaard et al., 2014). TPCs encompass both acute effects (exposure to minimum and maximum thermal limits) and chronic effects of temperature on individuals' growth, both of which can have implications for survival (Ex. Lutterschmidt & Hutchison, 1997; Rezende et al., 2020). Ectothermic animals often seek temperatures that maximize their fitness while avoiding temperatures exceeding their optimal range, which can be highly detrimental (Martin et al., 2008; Ruel & Ayres, 1999). TPCs hold particular importance for the larval stages of anurans, given their development in relatively uniform thermal habitats (e.g., small ponds) with limited thermoregulatory options and metamorphosis is necessary before habitat desiccation. Therefore, we contend that TPCs for tadpole growth rates can effectively predict species' tolerance to thermal stress. In this study, we integrated tadpole growth rate TPCs with micro and macro climatic information from their aquatic habitats, to investigate their association with current elevational ranges and potential future alterations. Specifically, we sought to ascertain whether different estimates of optimal ranges of growth rates can elucidate the limits of their elevational range in an equatorial mountain. Additionally, we projected how elevation and microenvironment might alter exposures to stressful temperatures in the future. We explored these relationships within dendrobatid frogs, a taxonomic group widely distributed across elevations from sea level to almost 4000 metres above sea level (Coloma, 1995; Graham et al., 2004; López-Hervas et al., 2024) resulting in distinct thermal conditions and selective pressures experienced by different species.
2 MATERIALS AND METHODS
2.1 Selection of species
We sampled nine Ecuadorian frogs belonging to the family Dendrobatidae during their larval stage, covering a wide elevational range in Ecuador ranging from 38 to 1900 m, between latitudes 1° N—4° S. We focused on five out of the six species of Epipedobates found on the coastal side of Ecuador, as well as four species of Hyloxalus from the Amazonian slope out of the twenty-seven that can be found throughout the country (Ron et al., 2020; López-Hervas et al., 2024; for more information see Table S1 and Figure S6). One population of each species was collected from their natural aquatic habitats from December 2014 to April 2016 and transported to the experimental facilities in the Pontificia Universidad Católica del Ecuador (PUCE). However, for three species (Epipedobates machalilla, E. tricolour and Hyloxalus nexipus) we sourced specimens from the ‘Balsa de los Sapos’ initiative PUCE. It is important to note that the specimens from captive breeding were first- or second-generation breeds and we assumed that their physiological performance was not affected by captivity (see Pintanel et al., 2020).
2.2 Thermal performance curves (TPC) for growth and survival
Negative values were considered as non-growth and treated as zero (Overgaard et al., 2014). Given the non-linear growth pattern of anuran larvae, which approaches exponential decay close to metamorphosis (Harris, 1999; Werner 1986, Appendix; Richter-Boix, Tejedo & Rezende, 2011), we selected experimental tadpoles in early-mid-developmental stages (ranging from 25 to 34 Gosner stages) from either field or captive breeding sources that reached as most 38–39 Gosner stages at the end of the growth experiment.
To fit the TPCs, we built a nonlinear mixed-effect model to quantify growth performance for each species across all treatment temperatures using the nlme R-package (Pinheiro et al., 2018). We adjusted the TPCs according to Angilletta (2006) and employed two distinct models (Gaussian and Gaussian-Gompertz) to estimate the TPCs for each species' (Frazier et al., 2006; Martin et al., 2008). The model with the lowest Akaike information criterion (AIC) was chosen for subsequent analyses (Burnham & Anderson, 2002). TPC parameters were then estimated for the fitted curves of each species'. The optimum temperature (T opt) was determined as the temperature at which performance was maximized (Z max) and the performance breadth (B50) was defined as the range of temperatures at which performance is greater or equal the 50% of maximum performance.
2.3 Estimates of micro- and macro-climatic temperatures along elevation
We gathered macroclimate data, including maximum, minimum and mean temperatures (T MAX, T MIN and T MEAN), from current and projected future climate conditions using WorldClim, with a spatial resolution of 30 arc-seconds (Hijmans et al., 2005). To assess shifts in thermal suitability for each species under different climate warming scenarios, we considered future projections for two time frames (2050 and 2070) under both low (RCP 4.5) and high (RCP 8.5) emission scenarios as provided by the CCSM4, HadGEM2-ES and GFDL-CM3 global circulation models. We used the ‘extract’ function from the raster R-package (Hijmans, 2017a) to extract climatic data from WorldClim at each species' georeferenced sampling locations.
We obtained georeferenced locations for Ecuadorian Epipedobates and Amazonian-side Hyloxalus were obtained from the Museo de Zoología of the Pontificia Universidad Católica del Ecuador database (Ron, 2018; see Figure S6). To address sampling bias, we retained georeferenced points that were separated by more than 1 km, using the ‘distGeo’ function in the geosphere R-package (Hijmans, 2017b). We collected in situ microclimatic data from 38 breeding sites, spanning elevations from 23 to 3631 m, representing diverse aquatic habitats with contrasting temperature profiles (see below). We recorded water temperature using temperature dataloggers (HOBO UA-002-64) to obtain continuous records of habitat temperatures (every 15 min; see Table S2). At each collection site, data loggers were placed at the deepest bottom of the pond or stream, since it is the coolest region of the habitat and could be selected to avoid peak temperatures (Duarte et al., 2012). For each data logger, we obtained mean (t mean), maximum (t max) and minimum (t min) daily water temperatures. The number of sampling days varied, ranging from 2 to 546 days depending on the ephemerality and accessibility of the breeding site (see Table S2). Finally, we categorized the water bodies into three different thermal variability regimes according to their mean daily thermal range (dr = mean t max – t min): low (0.05–1°C; streams and permanent deep ponds), medium (1–2.25°C; forested temporary ponds) and high (>2.39°C; temporary opened ponds; Table S2). We selected dr as the discriminating variable for differentiating between thermally contrasting habitats, because it was independent of elevation for all locations and within any thermal range category (R 2 = 0.02, n = 38, p = 0.891), thus mitigating the confounding effect of decreasing temperatures with elevation due to the adiabatic lapse rate (Dillon et al., 2006; Sarmiento, 1986).
Although most analysed dendrobatid tadpoles are rarely found in open temporary ponds, we included the ‘high’ category to encompass the full range of environmental temperatures available for frogs and to account for the effects of human-modified tropical forests resulting from anthropic activities such as logging, fragmentation and habitat conversion (González-del-Pliego et al., 2020; Senior et al., 2017).
Throughout the article, we use capital letters to refer to Worldclim macroclimatic data (i.e. T MAX, T MIN and T MEAN, as the Bioclim 5, 6 and 1, respectively) and lowercase letters for the field-measured microclimatic data (t max, t min, t mean and dr).
2.4 Modelling microclimatic temperatures through species' ranges
To gauge the microclimatic temperatures across species distributional ranges, we employed various GLS models to estimate both maximum (t max) and minimum (t min) temperatures. These models incorporated T MAX, T MIN and T MEAN, along with their squared values to help us explore and capture possible quadratic or other nonlinear relationships that may exist in our data, as response variables, while also considering habitat (refer to Tables S6 and S7). Following this, we selected the optimal model based on AIC comparison (with a ΔAIC >3) and extracted coefficients for the intercept and each explanatory variable. These model parameters were then interpolated using current and future temperatures at studied species locations to obtain maximum and minimum mean modelled microenvironmental temperatures (see Supporting Information). Given the low latitude of our sampling locations, which range from 1.165 N to 4.164 S, temperatures exhibit minimal seasonal variation, remaining relatively constant throughout the year. The primary source of temperature variability at our study sites arises from microenvironmental thermal variation (see Figure S2). To estimate realistic daily thermal variation, we interpolated estimated macroclimatic temperatures with field-measured microclimatic temperatures. This interpolation was based on three representative microclimatic datasets with contrasting temperatures, obtained from stream, forested pond and open pond environments, each collected over 78–100 days (see Figures S3–S5).
2.5 Relationships of elevational distribution with thermal sensitivity (TPC) parameters
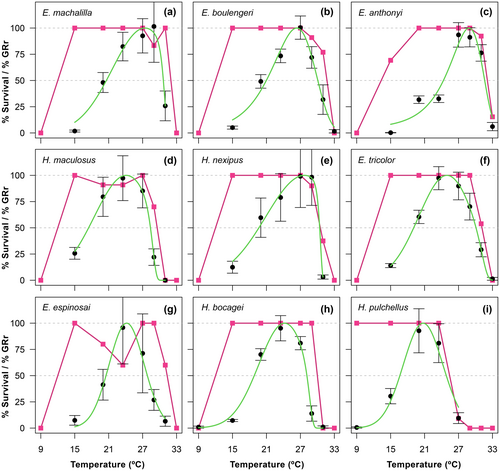
Our growth Temperature Performance Curves (TPCs) construction revealed that the warm extreme of B50 (maxB50), ranging from 24.8 and 31.6°C across species, represents a distinct thermal threshold. Beyond this range, mortality rates increased in TPC experimental treatments between 27 and 33°C (see Figure 1 and Results). Stressfully cold temperatures were challenging to estimate because most species, except H. pulchellus, could not survive the coldest treatment at 9°C over the ten-day experiment. However, the cold extreme of B50 (minB50), ranging between 16.6 and 24.3°C, also represented a critical cold threshold. The number of days tadpoles survived (1–10 days) exposed to 9 and 15°C significantly increased at lower minB50 (see Results).
2.6 Expected distributional ranges and chronic thermal stress across elevation
Expected elevational ranges per species were determined across habitats, defined as those elevations where potential hours of maximum performance (best growth) exceed 50% of the total hours (see Figures S7–S15). Potential hours of best growth per elevation were computed as hours when temperatures ≥ minB50 but ≤ T opt.
To quantify the impacts of climate change across habitats and throughout the elevational gradient, we projected the present and future percentages of total hours of heat stress (temperatures > maxB50) for each occurrence point. All analyses were conducted using R v3.4.3 (R Core Team, 2021).
2.7 Statistical analyses and phylogenetic reconstruction
We tested if the sample locality elevation correlated with tolerance to cold (log-transformed survival meantime at 9°C) and tolerance to heat (maxB50) by applying the Phylogenetic Generalized Least Squares (PGLS), accounting for phylogeny through maximum likelihood estimations of Pagel's λ using the R-package caper (Orme, 2013). For reconstructing the phylogenetic tree of our study species, we utilized the most comprehensive and recent amphibian phylogeny by Jetz and Pyron (2018), including all studied species, with the ape package in R (Paradis et al., 2004; Figure S16). Given polytomies in the phylogenetic tree, we utilized the multi2di function in ape to convert it into a dichotomous form. However, when employing a simpler GLS model, without phylogenetic control, the results did not differ.
Additionally, we employed a PGLS approach to test if the mean temperature (T MEAN; WorldClim; Hijmans et al., 2005) of the sample point, as an elevational proxy, correlates with T opt, given that T opt should align with the prevailing temperatures experienced by the species (e.g. Deutsch et al., 2008; Frazier et al., 2006; Huey et al., 2012).
3 RESULTS
3.1 Thermal performance of growth rates and survival across dendrobatids
The Gaussian–Gompertz function best fitted thermal sensitivity for larval growth. However, for H. pulchellus and E. espinosai, we fitted the data using a Gaussian function due to its lower AIC (Figure 1; Table S3). Survival of tadpoles was highest at intermediate temperatures (>90%) and decreased at the lowest (9°C) and highest temperatures (29, 31 and 33°C), generally resulting in an inverted ‘U' shape (Figure 1). All individuals maintained at 9°C died, except for the high-elevation Hyloxalus pulchellus. We observed significant differences in survival time between genera (χ 2 = 103.63, df = 70, p < 0.001) and species (χ 2 = 5.23, df = 63, p < 0.001) at 9°C. Tukey's post-hoc analysis showed that H. bocagei survived for a shorter time compared to H. pulchellus but longer than the rest of the species (see Table S4). Conversely, H. pulchellus did not survive treatments exceeding 27°C (Figure 1i).
3.2 Correlations of elevation with thermal tolerance traits
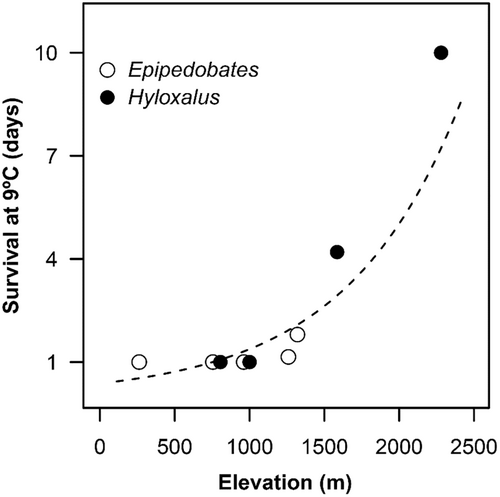
When we applied a simpler GLS model without phylogenetic control, the majority of the results did not show significant differences. Therefore, we chose to present the conventional GLS analysis. Survival time at 9°C (log-transformed survival) increased with the species sampling elevation (Figure 2). In turn, the capacity to tolerate heat stress (maxB50) was negatively correlated with elevation (t = −3.55, R 2 = 0.591, p = 0.009), with lowland species exhibiting higher tolerance to chronic heat stress. Optimal temperatures (T opt) were negatively correlated with elevation (for sample point: t = −5.44, R 2 = 0.782, p < 0.001) and mean temperature (Y = 10.66 + 0.69X, R 2 = 0.75, t = 4.97, p = 0.002).
3.3 Expected distributional ranges
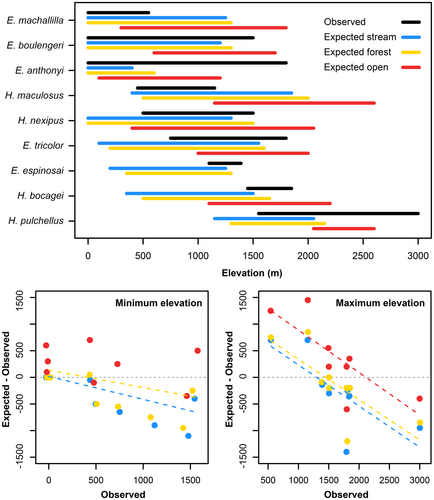
Expected fundamental niche models for high-elevation species showed lower distributions than the observed elevational ranges. Conversely, expected physiologically-based distributional ranges for lowland species exceeded their observed maximum elevation, except for Epipedobates anthonyi (Figure 3).
3.4 Chronic thermal stress across elevation at present and future (2050 and 2070)
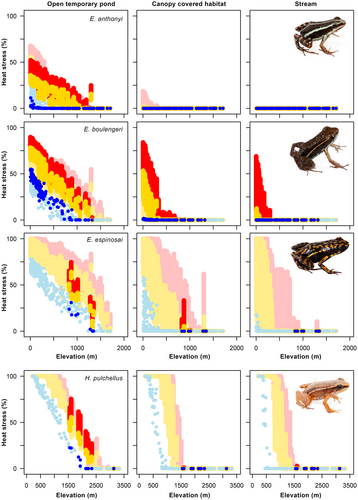
Habitats with high thermal variation, such as opened ponds, have temperatures that could be stressful in the present for low and even mid-elevation populations of some species (Figure 4). Conversely, none of the stream-like water environments (low thermal variation) within each species distribution showed temperatures higher than the species' maxB50 under current climates. Regarding predictions for the future, temperatures are not expected to exceed maxB50 in streams by 2070 for any population, even under the highest emission scenario (RCP 8.5), with the sole exception of the low-elevation populations of E. boulengeri (Figure 4). Instead, future predictions for high-variation environments, suggest that even upland species could be susceptible to thermal stress (Figure 4). In certain instances, particularly among lowland populations, microenvironmental temperature could exceed their physiological safety margins for over 50% of the daytime. In microenvironments with medium thermal variation, such as forested ponds, the occurrence of heat peaks of stressful temperatures could be mitigated by 2050. By 2070, it is projected that only lowland populations of some species will experience severe thermal stress. However, these species would not be subject to thermal stress for more than 20% of the daytime.
4 DISCUSSION
Addressing the seminal question of how temperature influences distributional patterns is a longstanding topic in ecology (e.g. Hutchinson, 1957; Kearney & Porter, 2009; Whittaker et al., 1973). However, our results, as also observed in lizards (Strangas et al., 2018), suggest that physiologically informed approaches may still fall short in accurately predicting species' elevational ranges possibly due to factors other than temperature exerting stronger restrictions on vertical dispersion.
The predicted elevational ranges align partially with the current distribution of lowland species and, in certain cases (e.g., E. machalilla, E. boulengeri, H. maculosus), may imply an upward shift in expected ranges. However, the anticipated elevational ranges do not correspond to the current distribution of high-elevation species (E. espinosai, H. bocagei, H. pulchellus). In other words, fundamental niche models based on optimum growth performance might inadequately explain how medium–high elevation dendrobatid frogs have colonized mountaintops. Our models suggest that these species should be distributed at lower elevations based on their physiological optimal growth conditions. Instead, their high tolerance for cold temperatures, rather than optimizing their performances to local temperatures, has likely made them suitable for high elevations.
Aquatic environments exhibit considerable thermal homogeneity, particularly in streams and forested ponds (Pintanel et al., 2022) and body temperatures of small aquatic ectotherms, such as tadpoles, often closely track environmental temperatures (Lutterschmidt & Hutchison, 1997). Consequently, behavioural thermoregulatory compensation is more limited for amphibian larvae inhabiting cold mountaintops. The absence of seasonality in the tropics may further constrain mountaintop species from exploiting warmer temporal windows, contrary to predictions for high-latitude species (Enriquez-Urzelai et al., 2018; Overgaard et al., 2014; Yamahira et al., 2007). Nevertheless, we observed thermal heterogeneity between habitats, with open ponds exhibiting higher and more variable temperatures, allowing habitat selection as an alternative to exploiting warmer environments. Despite this, our results suggest that even when occupying open ponds, the expected elevational range for H. pulchellus falls 400 m below the maximum observed elevation (2970 m; Coloma, 1995), indicating how mountaintop species must perform in suboptimal environments. On the contrary, other traits (i.e. locomotor performance, vocal behaviour) of high-elevation anurans appear to be minimally sensitive to broad thermal ranges (Enriquez-Urzelai et al., 2018; 2019; Lüddecke & Sánchez, 2002; Navas, 1996).
Considering that temperatures for optimal growth occur at lower elevations than observed species' ranges, two questions arise: why are highland species not distributed at lower elevations? and, how can they inhabit environments with suboptimal temperatures? The mismatch between predicted and observed elevational ranges could be explained by various factors unrelated to the current climate, such as evolutionary history, dispersal ability, biotic interactions, or stochastic extinction events (Pearson & Dawson, 2003; Rapp et al., 2012). Elevational gradients encompass significant changes beyond temperature, including ultraviolet radiation, oxygen pressure and the intensity of biotic interactions (i.e. diversity and density of predators, parasites and competitors; Navas, 2006), which could synergistically and/or inversely affect distributions. Given the greater biodiversity in the lowland tropics (Lomolino, 2001; McCain & Grytnes, 2010), lowland species might experience heightened biotic interactions compared to those expected at higher elevations due to the reduced presence of predators, pathogens and competitors at the summits (Jankowski et al., 2013; Wisz et al., 2013). This competitive and predatory release might favour the occupation of higher-elevation environments, even at the expense of reduced growth performance. Another possibility is that current environmental temperatures differ from paleoclimatic conditions. The discrepancy between fundamental niche models and current distribution might be partially explained by the colder climatic conditions that occurred in the Andes during the Pleistocene (200.000–14.000 years ago), where temperature drops were more pronounced in the highlands than in the lowlands (Haffer, 1979; Hooghiemstra et al., 2006). This could have promoted cold tolerance adaptations in current lowland dendrobatids, enabling them to physiologically occupy higher elevations but not yet uplifted due to a myriad of collateral factors, as mentioned above. Conversely, in upland dendrobatids such as H. pulchellus, the mismatch between predicted (fundamental niche) and realized distribution may stem from a distributional shift promoted by recent Quaternary mountaintop uplifts of the northern Andes (van der Hammen, 1974).
Apart from the mechanisms previously discussed to explain differences between observed and predicted distributional ranges, various sources of variation in experimental methodology may yield contrasting estimates of fundamental niche models and thereby influence our results on predicted distribution (Navas et al., 2021; Sinclair et al., 2016). For instance, most species consist of an array of locally adapted populations and hence, their thermal sensitivity could vary across their geographical range (e.g. Khelifa et al., 2019; Lindgren & Laurila, 2009; Richter-Boix et al., 2015; Zani et al., 2005). Notably, interpopulation adaptive divergence in thermal tolerance traits along elevational transects, tracking temperature changes, has been observed, especially in cold tolerance, in Epipedobates anthonyi (Páez-Vacas & Funk, 2024) and other amphibian tadpole species across the Ecuadorian Andes (Pintanel et al., 2022). Another possibility is that high-elevation species may better exploit high temperatures in cold climates compared to species at lower elevations or temperate latitudes (the countergradient effect; Buckley & Nufio, 2014; Yamahira et al., 2007). A recent study with reptiles showed a similar pattern, with high-elevation species adapted to hotter temperatures than those currently exposed (Strangas et al., 2018).
Relying solely on thermal sensitivity within the optimal range of growth performance may prove insufficient for a comprehensive explanation of ectotherm high-elevation distributions. The evolution of physiological traits related to tolerance of extreme thermal events may better define organisms' realized niches, at least in extreme thermal environments (e.g. Bozinovic et al., 2011; Clusella-Trullas et al., 2011; Gutiérrez-Pesquera et al., 2016; Kingsolver et al., 2011; Overgaard et al., 2014). Indeed, survival at low temperatures (9°C) increased with the species' elevational range, while their growth performance never exceeded 5% of the total (also see Zani et al., 2005). A similar mismatch in TPC for growth in other high-elevation amphibian lineages (Hyloscirtus, Gastrotheca), revealed that their expected maximum growth temperature corresponded to lower locations than they currently occupy (Pintanel, Tejedo, Merino-Viteri, unpublished). Tolerance to colder temperatures appears to be a primary factor driving successful colonization for amphibians in high Andean tropical elevations (Navas, 2006) and, more broadly, for ectotherms colonizing colder regions, both latitudinally and elevationally (Wiens et al., 2006). As expected, the highest elevation species analysed, H. pulchellus, exhibits the highest cold tolerance of all dendrobatids in this study (CTmin = 1.67°C), approximately 5°C colder tolerance than the average of the other species (Pintanel et al., 2022). Therefore, cold tolerance, rather than optimum performance, significantly shapes species' elevational distribution.
Our results reveal that ongoing global warming will likely have distinct effects on lowland and upland dendrobatid species. Tropical lowland species might be more vulnerable to warmer temperatures (e.g. Deutsch et al., 2008; Sunday et al., 2014). Our results confirm that lowland tropical amphibian larvae are already exposed to stressful temperatures, particularly in aquatic environments with high thermal variability (e.g. open ponds; Figure 4). However, this group of amphibians is widely distributed across different breeding environments (e.g., streams, ponds and bromeliads; see Coloma, 1995), which may enable these species to mitigate heat impacts by utilizing cooler breeding environments. Specifically, our fundamental niche models in low thermal variability microenvironments (i.e. streams and deep permanent ponds) predict that all taxa can physiologically tolerate the future increase in temperatures, even when considering the most extreme scenario (Figure 4). In contrast, all species within high thermal variation habitats (open temporary ponds) are expected to eventually experience stressful temperatures.
As warming continues, amphibians will increasingly rely on colder breeding microclimates to avoid heat stress and local extinctions (Potter et al., 2013; Suggitt et al., 2018; Sunday et al., 2014). This will be particularly critical for lowland species. However, the availability of habitats that could protect them from an increase in temperature may be limited in the future. Firstly, human environmental impacts, particularly deforestation, will have drastic consequences on the availability of suitable environments for organisms (Cushman, 2006; Dirzo et al., 2014; Mendenhall et al., 2014) and may result in drastic thermal shifts in the altered habitats left (Brusch et al., 2016; Frishkoff et al., 2015; Nowakowski et al., 2017). Secondly, the availability of aquatic microclimates is dependent on precipitation and thus, in a climate change scenario, alterations in precipitation regimes could diminish the availability of suitable aquatic environments, as has already been reported in temperate amphibian communities (McMenamin et al., 2008). Even if ponds do not completely dry out, the loss of water will reduce the availability of colder microclimates since deep ponds exhibit lower and more constant temperatures than shallower ponds (Pearman, 1995; also see Figure S2). Lastly, the increase in temperatures could compel amphibians to shift their ranges upwards (Chen et al., 2011; Parmesan, 2006; Raxworthy et al., 2008). Particularly, lowland dendrobatids such as E. machalilla, currently restricted to elevations below 500 m, might be forced to track predicted warming by uplifting to higher elevations. Conversely, medium and high-elevation species, Hyloxalus pulchellus, H. bocagei and E. espinosai, which could thrive at lower elevations (between 700 and 1000 m lower), might have their downward shift compromised by the expected increase in the thermal stress levels that will curtail their growth potential in the coming decades (Figure 4).
In contrast, as the climate warms, environmental temperatures would approach the upland tadpoles' optimum temperatures benefiting them (for latitudinal patterns, see Deutsch et al., 2008; Tewksbury et al., 2008; but see Sunday et al., 2014). However, currently living at suboptimal temperatures may be offset by the benefits of reduced negative biotic interactions such as predation, competition or parasitism. An increase in mean and maximum temperatures at higher elevations might prompt a migration of lowland species upwards, potentially imposing negative interaction effects on upland species (Huey et al., 2009). Thus, unravelling the mechanisms involved in mountaintop species distribution is key to understanding the forthcoming effects of climate change.
In conclusion, this study suggests that larval growth performance partially predicts the current elevational distribution in dendrobatid species. Optimal growth performance is quite similar in all studied dendrobatid tadpole species. Therefore, while lowland species inhabit thermally optimal environments, highland species live in suboptimal environments. Cold tolerance could instead be a superior factor explaining the distribution of highland species. From a physiological standpoint, high-elevation species may benefit from warmer mean temperatures, but this may be detrimental for lowland species with T opt already close to mean temperatures, potentially compelling them to shift upwards to avoid stressful thermal conditions at their current elevation. However, we propose that living at suboptimal temperatures at high elevations may be compensated by a reduction in negative biotic interactions. Although analyses of predator–prey thermal mismatches in dragonfly naiads and amphibian tadpoles in the studied mountain range reveal that these mismatches remain constant across elevations and habitat ranges (Pintanel et al., 2022). It is important to note that warmer temperatures may alter current biotic relationships, exerting a stronger detrimental effect than warming. Warming seems weakly linked to population declines and extinctions (Cahill et al., 2012), except at the edges of the species distributional range (Cahill et al., 2014). We propose prioritizing research at the edges of ectotherms' distribution (Camacho et al., 2024) to further comprehend the factors that limit distribution across elevation and enhance our understanding of biogeographic patterns providing a more accurate forecast of climate-related impacts.
ACKNOWLEDGEMENTS
We thank Mayra Castro, David Jácome, Freddy Almeida, Flor and Javier Rosero for assistance in the field and laboratory. For constructive comments, we thank S. Salinas-Ivanenko, L.M. Gutiérrez-Pesquera and P. Jervis. We thank Santiago R. Ron for the photo credits of Figure 4. Ex situ frog management was funded by the General Academic Board of PUCE through research grant L13227 to AMV. This research was supported by AECID (AP/038788/11) and MINECO (CGL2012-40246-C02-01) grants to MT and AMV and Severo Ochoa (SEV-69) funds to MT. PP was supported by an MAE-AECID grant (BOE-A-2015-12270). Ministerio del Ambiente of Ecuador provided the permits to conduct this research (003-15/012-015/002-16 IC-FAU-DNB/MA). AC was supported by an MSCAH2020: 897901 fellowship.
FUNDING INFORMATION
Ex situ frog management was funded by the General Academic Board of PUCE through research grant L13227 to AMV. This research was supported by AECID (AP/038788/11) and MINECO (CGL2012-40246-C02-01) grants to MT and AMV and Severo Ochoa (SEV-69) funds to MT. PP was supported by an MAE-AECID grant (BOE-A-2015-12270). Ministerio del Ambiente of Ecuador provided the permits to conduct this research (003-15/012-015/002-16 IC-FAU-DNB/MA). AC was supported by a MSCAH2020: 897901 fellowship.
CONFLICT OF INTEREST STATEMENT
None of the authors have a conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Data on experimental, distributional and microclimatic data that support our findings are deposited on Dryad DOI: 10.5061/dryad.d51c5b094
REFERENCES
BIOSKETCH
Pol Pintanel received his doctorate from the University of Barcelona. His research is currently focused on the thermal adaptation of amphibians. The research group's focus is the evolutionary thermal ecology of amphibians in the context of global change.
Miguel Tejedo's research interests lie in analysing the thermal adaptations of amphibians to their local climatic environments. He aims to provide a physiological basis for understanding the vulnerability of tropical and temperate frogs to global change.
Agustín Camacho's primary interest is in understanding how the environment interacts with evolving phenotypes to determine where species can live and using that for developing a new approach for forecasting species' vulnerability to climate change.
Urtzi Enriquez-Urzelai is broadly interested in understanding the factors influencing species distributions and diversity patterns, from phenotypic adaptations (e.g., physiological, morphological and behavioural) to biogeographical aspects.
Gustavo A. Llorente focuses his research on the conservation of amphibian and reptiles within the context of global change.
Andrés Merino-Viteri research interests are centred around the ecology, evolution and conservation of tropical amphibians. He particularly focuses on scenarios where natural populations are scarce or at risk of extinction.
Author contributions: PP, MT and AMV conceived the idea; PP, MT, AMV, UEU, GAL and AC designed the research; PP, MT and AMV sampled tadpoles and dataloggers at the different Ecuadorian locations; PP, MT and AMV performed the experiments; PP, MT and AC wrote the manuscript with significant contributions from AMV, UEU and GAL.




