Gender differences for growth in North American Atlantic salmon
Summary
An assumption in aquaculture of Atlantic salmon is that male and female growth within families is perfectly genetically correlated. That is, families would rank identically if based on male growth only or female growth only. Also, growth in freshwater and sea water is assumed to be highly correlated between males and females within families. However, structural analysis of the DNA of Atlantic salmon has found that the linkage maps of females differ significantly from that of males. Genetic variability for any trait measured on females could be greater or lesser than on males. Thus, male and female growth might be considered as separate traits giving rise to families ranking differently depending on gender. A multiple trait family model for weight and length at 3 years of age in Atlantic salmon according to gender was applied to data on North American Atlantic salmon obtained from the Oak Bay Hatchery in New Brunswick, Canada. Genetic correlations between male and female growth in both freshwater and sea water were estimated by Bayesian methods. The estimates support the possible existence of gender dimorphism in North American Atlantic salmon for growth traits.
1 INTRODUCTION
The Cooke Aquaculture Atlantic salmon breeding programme at the Oak Bay Hatchery in New Brunswick, Canada, rears candidate broodstock entirely in freshwater tanks. The trait of economic interest, however, is sea water growth in sea cages, where the production fish are reared. Three-year-old weights are taken in February and March, and spawning occurs in October to December that year. Genetic improvement programmes are used to increase growth rates, reduce feed consumption, improve carcass shape and colour and increase resistance to health challenges due to parasites, bacteria and viruses.
Statistical evaluation methods for fish rely on the ability to identify individual fish, which can only be done once the fish are large enough (8 g or better) to be tagged such that they can be readily scanned electronically. Alternatively, fish can be genotyped to determine parentage at the time they are weighed and measured, but this depends on the costs and speed of analysing DNA samples (Liu, Palti, Gao, & Rexroad, 2016). Families have been identified by fin clipping, or by raising them in separate tanks until they are large enough to be tagged. This generally limits the number of families that can be reared at one time. Fish may also be grown communally and identified to parentage at 3 years of age using microsatellite markers or a panel of SNPs, which would allow a larger number of families to be created. With a communal system, some families could potentially be eliminated because they were not competitive during feeding. Parentage was determined using 12 microsatellite markers.
A complication in the genetic analysis of data on Atlantic salmon is that male and female fish do not perform equally within a family as seen in masu salmon (Tamate & Maekawa, 2004). In tilapia, male and female growth was highly correlated between .78 and .97 (Bentsen et al., 2012), although tilapia may not have the same genetic architecture as salmon. The female and male linkage maps are drastically different in Atlantic salmon (Lien et al., 2011). The differences support the possibility that males and females from a family may have different overall genetic variability and might rank differently in their genetic evaluations. This may be important for many traits in salmon. If true, this conclusion would necessitate analysing male and female data as different traits.
A purpose of this study was to explore the differences in growth between males and females using an appropriate multiple trait family model and estimating the genetic correlations between male and female growth traits.
2 MATERIALS AND METHODS
2.1 Weight and length data
Atlantic salmon began populating the coast of North America after the retreat of the glaciers that covered all of Canada some 10,000 years ago. That represents about 2,500 generations of breeding in the wild. The Oak Bay Hatchery of Cooke Aquaculture, Inc accommodates up to 150 families of Atlantic salmon in each of four different year classes, at one time. The St John River strain was started around 1984 (Glebe, 1998; Quinton, McMillan, & Glebe, 2005). Spawning typically occurs in October, November and December, and eggs are kept in freshwater (8°C). Some eggs can be chilled to 3–4°C in order to match up their degree days with eggs fertilized later. Once the alevins have absorbed their yolk sac and are ready to take exogenous feed, the temperature is increased to 12°C. As the fish grow, they are moved to larger tanks. The tanks have been communal, that is, tanks house representatives of many families. At smolting age (1 year), individual fish may be tagged, and a sorting is made for potential broodfish. Smaller, poorer fish are moved to sea pens as production fish, and these never return to the Oak Bay Hatchery. Another movement of fish occurs at 18 months and 2 years of age, when they are scanned by ultrasound to determine sex. All early maturing fish are removed. About 2% of fish (mostly males) mature early. Gender assignment is about 67% accurate at young ages.
Three-year-old weights of broodstock and production fish are taken in February–March, and genetic evaluations are calculated in preparation for spawning in the fall. Weight and length data were collected over 9 years in freshwater, and on full-sibs and half-sibs in sea water. Parentage of sea water fish is determined by genotyping fin clips for 12 microsatellites, or more lately, a panel of SNPs at the time of weighing. Sea water evaluation at processing plants in December–January coincides with 3-year-old freshwater evaluations.
Fish with family identification were required. Table 1 contains the number of records by year class, type (freshwater, FW, or sea water, SW), and gender that were used in the study.
| Year class | Year weighed | Freshwater | Sea water | ||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| 2006 | 2009 | 0 | 1,392 | 0 | 1,566 |
| 2007 | 2010 | 404 | 1,196 | 1,551 | 1,371 |
| 2008 | 2011 | 1,171 | 1,106 | 2,325 | 1,380 |
| 2009 | 2012 | 424 | 0 | 2,095 | 0 |
| 2011 | 2014 | 0 | 1,179 | 0 | 2,417 |
| 2013 | 2016 | 250 | 81 | 449 | 56 |
| 2014 | 2017 | 0 | 562 | 0 | 3,091 |
The phenotypic means and standard deviations for weight and length are given in Table 2. Data were scanned for outliers within sex and within environment (sea water or freshwater), but none were found.
| Gender | Type-trait | Number | Mean | SD |
|---|---|---|---|---|
| Males | FW weight, kg | 2,249 | 4.86 | 1.51 |
| FW length, cm | 2,249 | 73.28 | 6.97 | |
| SW weight, kg | 5,516 | 5.50 | 1.53 | |
| SW length, cm | 5,516 | 78.76 | 5.98 | |
| Females | FW weight, kg | 6,420 | 5.52 | 1.85 |
| FW length, cm | 6,420 | 74.54 | 7.58 | |
| SW weight, kg | 9,881 | 5.63 | 1.53 | |
| SW length, cm | 9,881 | 78.01 | 5.68 |
- FW, fresh water; SW, sea water.
2.2 Gender differences
Male and female data from the Oak Bay Hatchery have been analysed collectively for four traits (FW weight, FW length, SW weight and SW length) in a multiple trait animal model over the last 3 years. Oak Bay employees observed the estimated breeding values by gender within families, and noticed that the variability of male and female EBVs differed.
Lien et al. (2011) noted significant differences in linkage maps of males and females, suggesting that males and females within families could possibly make families rank differently depending on which gender was considered. In essence, a gender dimorphism could exist as reported by Tamate and Maekawa (2004). Accounting for this phenomena can be accomplished by treating the four traits as different traits between genders, making a total of eight traits. One problem, however, is that at 3 years of age, gender is confirmed by visual assessment, but a few fish need to be reassigned at spawning. Because salmon do not have sex chromosomes, per se, work is underway to find SNP markers that are highly correlated with gender so that younger fish may be more accurately assigned to the correct gender. The latest gender assignments designated by Cooke Aquaculture for each fish were used in the analysis. The accuracy of gender assignment is an important concern for this type of study. For this study, the gender assignments made by the Cooke staff were assumed to be correct.
Only weights and lengths taken at 3 years of age were used in the analyses. There were a total of 24,066 fish measured at 3 years of age in this study. Any fish that were already sexually mature were removed.
2.3 Model for genetic evaluation
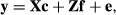 (1)
(1)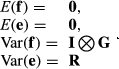
Matrices G and R, of order 8 by 8, were estimated by Bayesian methods using Gibbs sampling. The structure of R was such that it was block diagonal so that covariances between gender by environment subgroups were 0, because a fish can only belong to one gender and one environment. Ninety thousand Gibbs’ samples using the entire data set of 24,066 records were generated with a burn-in period of 45,000 samples. Standard errors on estimates of covariance components and on estimates of heritabilities and genetic correlations were obtained using the standard deviations of the Gibbs’ sample values after burn-in.
A comparison run with the same data was made using ASREML 4.1 (Gilmour, Gogel, Cullis, Welham, & Thompson, 2015). The !AISING option had to be used with ASREML, which forces the estimated matrix to be non-singular and positive definite after each iteration. There were no such issues with the Bayesian method. Eighty likelihood evaluations were made to convergence. ASREML recognized the structure of R and acted accordingly. The estimated residual covariance matrix was very similar to that from the Bayesian analysis, but the G matrix gave much smaller genetic correlations among the eight traits than from the Bayesian estimates.
3 RESULTS
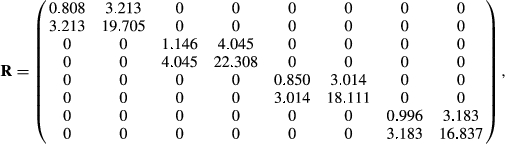
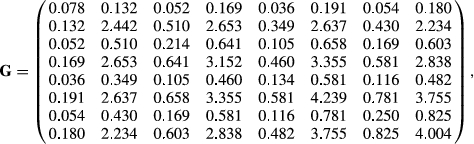
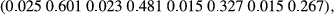
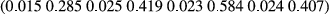
| Gender | Type | Weight | Length |
|---|---|---|---|
| Males | FW | 0.18 ± 0.03 | 0.22 ± 0.02 |
| SW | 0.31 ± 0.03 | 0.25 ± 0.03 | |
| Females | FW | 0.27 ± 0.04 | 0.38 ± 0.04 |
| SW | 0.40 ± 0.03 | 0.38 ± 0.03 |
- FW, fresh water; SW, sea water.
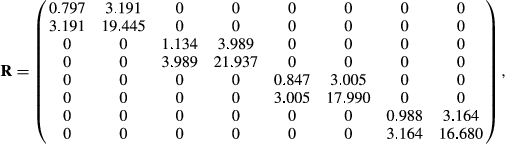
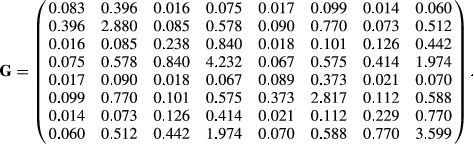
4 DISCUSSION
4.1 Variances
The genetic variances for each trait from ASREML and the Bayesian approach were very similar, but the covariances were greatly different. Keep in mind that the family genetic variances are one-half the total additive genetic variance. Assuming the Bayesian estimates are correct, genetic variances for female weight and length were greater than those for males within either FW or SW environments. For this reason alone, separate analysis of males versus females with an 8-trait model is warranted. The differences in variances would cause female EBVs to be more variable than male EBVs even when analysed together. The observations from Oak Bay Hatchery personnel on EBV variability were, therefore, confirmed by this analysis. Given that the genetic variances differ in both FW and SW environments, this cannot be due to unequal selection pressure on males versus females in being selected for broodstock or as production fish. The differences must be attributed to the differences in linkage maps between males and females in Atlantic salmon.
Residual variances were similar (i.e., not significantly different) for males and females, and slightly greater in SW than in FW environments. One would expect the residual effects would be the same on both genders of fish because males and females are reared in the same tanks with each other. Female SW lengths had a much higher residual variance than for the other length traits. The reason is unclear given that the phenotypic variances (Table 2) for length were similar for females in FW and SW.
4.2 Heritabilities
Growth is generally thought to have a moderate-to-high heritability in most species, and the results support that tendency. The heritabilities for weight and length in males were significantly lower than for females in both environments. The heritability results follow those for the variances.
4.3 Genetic correlations
Genetic correlation results and their standard errors for male traits are in Table 4, for female, traits are in Table 5, and between male and female traits in Table 6. A simple quick, conservative test is to subtract the correlation from 1, then see if that difference is greater than three times the standard error. Due to the small SE for all correlations, all genetic correlations were significantly different from unity (i.e., less than 1).
| FW wt | FW lg | SW wt | SW lg | |
|---|---|---|---|---|
| FW wt | 1.00 | |||
| FW lg | 0.30 ± 0.06a | 1.00 | ||
| SW wt | 0.40 ± 0.06a | 0.71 ± 0.03a | 1.00 | |
| SW lg | 0.34 ± 0.06a | 0.96 ± 0.01a | 0.78 ± 0.02a | 1.00 |
- FW, fresh water; SW, sea water.
- a Significantly less than 1 at p < .01.
| FW wt | FW lg | SW wt | SW lg | |
|---|---|---|---|---|
| FW wt | 1.00 | |||
| FW lg | 0.77 ± 0.04a | 1.00 | ||
| SW wt | 0.64 ± 0.04a | 0.76 ± 0.03a | 1.00 | |
| SW lg | 0.66 ± 0.04a | 0.91 ± 0.02a | 0.82 ± 0.02a | 1.00 |
- FW, fresh water; SW, sea water.
- a Significantly less than 1 at p < .01.
| Male | Female | |||
|---|---|---|---|---|
| FW wt | FW lg | SW wt | SW lg | |
| FW wt | 0.35 ± 0.05a | 0.33 ± 0.06a | 0.39 ± 0.06a | 0.32 ± 0.06a |
| FW lg | 0.61 ± 0.05a | 0.82 ± 0.03a | 0.55 ± 0.04a | 0.72 ± 0.03a |
| SW wt | 0.62 ± 0.04a | 0.69 ± 0.03a | 0.73 ± 0.03a | 0.65 ± 0.03a |
| SW lg | 0.71 ± 0.04a | 0.92 ± 0.02a | 0.66 ± 0.03a | 0.80 ± 0.03a |
- FW, fresh water; SW, sea water.
- a Significantly less than 1 at p < .01.
The low genetic correlations between FW and SW growth could be due to a genotype by environment interaction, but also the SW environment offers more environmental variation in terms of temperature, hours of sunlight and pollution from sea cage wastes than in FW environments. Female traits were more strongly correlated with each other in both environments than male traits.
The estimated genetic correlations between male FW weights and all female traits were significantly less than unity. However, other male growth traits were positively correlated with female growth traits, but only moderately. The multiple trait animal model allowing separation of male and female growth as different traits does not cause any computational problems. There is not a significant increase in computational time to solve the model.
In the male traits, FW length was more highly correlated with SW growth (weight and length) than was FW weight. The same was true for female traits, but FW weight was more positively correlated with SW growth than in males. Length seems to be a better selection trait than weight, because length will simultaneously improve weight at the same time. Also, selection on length reduces the likelihood of having short, rounder body shapes which are undesirable to consumers. Female traits were highly correlated with male SW length. Male and female length in both FW and SW were more strongly correlated than weights.
4.4 Future spawning
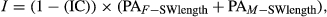
In a mating season, there can be up to 2,000 possible male candidates and up to 4,000 female candidates, resulting in 8 million possible matings. An index is calculated for all possible matings, and the results are sorted in descending order of index value. On a given day, only certain males and females are ripe for spawning, and a list is generated to identify the best matings for that day.
5 CONCLUSIONS
At the moment, growth in Atlantic salmon at the Oak Bay Hatchery appears to be different traits depending on the gender of the fish, given the differences in estimates of genetic variances, as well as the less than unity genetic correlations between male and female growth, and differences in linkage maps of males and females (Lien et al., 2011). The multiple trait genetic evaluation model is feasible. If, as data accumulate, the correlation between male and female growth increases close to unity, then the male and female data could be analysed together again.
A selection index can be constructed to combine male and female growth, preferably SW length. SW length seems to avoid morphological problems with weight. The index should probably be based mostly on length, but also on weight in SW. An index on traits only on females seems desirable because of their greater genetic variability for weight and length, but male growth should also be included because the genetic correlation is significantly less than one.
ACKNOWLEDGEMENTS
The authors acknowledge the financial assistance from the Genome Atlantic, Genome Canada, and Ontario Genomics GAPP Salmon and Chips Project, and the early assistance of Brian Glebe and Erica Harvey for help in collecting data, and Sarah Loker for analysing the early data.
CONFLICT OF INTEREST
The authors hereby declare that they have no conflict of interests with this study or paper. Schaeffer, Tosh and Boulding conducted analyses of the data as part of a grant in cooperation with Cooke Aquaculture. Ang, Elliot, Herlin and Powell are employees of Cooke Aquaculture and have collected and provided the data for the analysis. All authors have read the paper and approve its submission for publication.




