Novel harmful recessive haplotypes for reproductive traits in pigs
Summary
Harmful recessive haplotypes for reproductive and fertility traits have previously been detected in cattle, but so far, no studies have been published for pigs. The aim of this study was to locate chromosomal regions with putative lethal haplotypes and estimate the effects of the identified haplotypes on reproductive traits in the Finnish Yorkshire pig breed. We used marker genotypes of 871 Finnish Yorkshire AI boars, genotyped with Illumina's PorcineSNP60 BeadChip. The analysed traits were number of stillborn piglets in first (NSB1) and later (NSB2) parities, total number of piglets born in first and later parities and piglet mortality between birth and weaning in first and later parities. A haplotype was claimed as a putative lethal if it was common in the population, but no homozygous animals were found. We detected altogether 26 putative lethal haplotypes, but only one haplotype on chromosome 8 (position 107.0–113.3 Mb) was significantly associated with traits NSB1 and NSB2. Three possible candidate genes were found in this chromosomal region: MAD2LI, FGF2 and ANXA5. Further analysis is needed to confirm the role of these genes on pig reproductive performance.
Introduction
Litter size and piglet mortality are among the most important reproductive traits in pigs. Modern pig breeding programmes have gradually adopted new genetic marker-based methods to improve these traits, including genomic selection (Meuwissen et al. 2001) and genomewide association studies (GWAS). Both genomic selection and GWAS rely on data created using single nucleotide polymorphism (SNP) chips (Ramos et al. 2009). After the market launch of the Illumina PorcineSNP60 BeadChip (Illumina Inc., San Diego, CA, USA), also several GWAS related to pig reproduction have been published (Duijvesteijn et al. 2010; Uimari et al. 2011; Onteru et al. 2012; Schneider et al. 2012; Verardo et al. 2016).
A useful genomic approach to identifying genes that are important for reproductive and fertility traits is to look for chromosomal regions harbouring recessive lethal alleles. In live animals, recessive lethal alleles occur only in heterozygote carriers and do not affect the animal's own fitness or fertility. Thus, a lethal allele may have been segregating in a population for generations before being inherited from a common ancestor at some point and causing the death of an embryo or a foetus. Such lethal defects have traditionally been difficult to reveal even with large phenotypic and pedigree data (VanRaden & Miller 2006). With genomic data, however, no information is needed from stillborn individuals; recessive lethals can be discovered from the haplotypes of live animals by searching for chromosomal regions where certain haplotypes are common in the population but absent in the homozygous state (VanRaden et al. 2011).
Harmful recessive haplotypes for reproduction and fertility traits have previously been identified in cattle. For example, VanRaden et al. (2011) found five recessive lethal haplotypes in three cattle breeds, Sahana et al. (2013) reported six genomic regions with an effect on fertility traits, Fritz et al. (2013) detected nine haplotypes with a significant negative effect on calving rate in the Holstein breed and Pausch et al. (2015) found two deleterious alleles that compromise pre- and postnatal survival in the homozygous state. No corresponding studies have been published for pigs so far. Thus, the objectives of this study were to locate chromosomal regions with putative lethal haplotypes in the Finnish Yorkshire pig breed and to estimate the effects of the identified haplotypes on pig reproductive traits.
Materials and methods
Animal material and genotypes
In this study, we used the marker genotypes of 871 Finnish Yorkshire AI boars born between 1992 and 2013, genotyped with the Illumina PorcineSNP60 BeadChip. All the animals had a genotype call rate greater than 90%. Only SNPs with call rate over 0.9 were used, but no limits were set to minor allele frequency or Hardy–Weinberg equilibrium. Missing marker genotypes were imputed and haplotype phases determined using beagle software version 3.3 (Browning & Browning 2009) with default parameter settings. Information on the relationships between individuals was omitted. The outputs used for further analysis were the most likely haplotypes. A total of 55 709 SNPs on 18 porcine autosomes were included in the analyses. The positions of the SNPs were based on the porcine genome build Sscrofa10.2.
Lethal haplotypes
Regions with putative lethal haplotypes were scanned using both 25 and 75 SNP marker windows, following the settings of a similar study in cattle breeds by Sahana et al. (2013). The expected number of animals with a homozygous haplotype within each window was calculated assuming random mating and determined as the number of genotyped animals divided by 4 and multiplied by the square of the carrier frequency of that particular haplotype (VanRaden et al. 2011). A haplotype was defined as a putative recessive lethal if the expected number of homozygotes was at least six and none were observed in the data. According to the Poisson distribution, if a haplotype is not lethal, the probability of observing no homozygotes when six are expected is 0.0025 (Sahana et al. 2013).
Association analysis
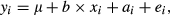
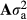 , where A is the pedigree-based additive relationship matrix and
, where A is the pedigree-based additive relationship matrix and 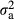 is the additive genetic variance; and ei is a random residual effect with mean 0 and variance structure of
is the additive genetic variance; and ei is a random residual effect with mean 0 and variance structure of 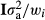 , where I is the identity matrix and
, where I is the identity matrix and 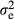 is the residual variance. Because the EBVs were based on different amounts of information, the observations were weighted (w) based on the accuracy of the EBVs of the animal itself and its parents (see Garrick et al. 2009 for more details). The analysed traits were number of stillborn piglets in first (NSB1) and later parities (NSB2), total number of piglets born in first (TNB1) and later parities (TNB2) and piglet mortality between birth and weaning in first (PM1) and later parities (PM2). The EBVs were provided by Figen Oy (Seinäjoki, Finland). More details on the statistical models used for breeding value estimation of these traits are in Uimari et al. (2011). The analysis was conducted with the AI-REML method in the dmu program package (Madsen et al. 2006). To account for multiple comparison, Bonferroni-corrected p-values were applied for statistical significance.
is the residual variance. Because the EBVs were based on different amounts of information, the observations were weighted (w) based on the accuracy of the EBVs of the animal itself and its parents (see Garrick et al. 2009 for more details). The analysed traits were number of stillborn piglets in first (NSB1) and later parities (NSB2), total number of piglets born in first (TNB1) and later parities (TNB2) and piglet mortality between birth and weaning in first (PM1) and later parities (PM2). The EBVs were provided by Figen Oy (Seinäjoki, Finland). More details on the statistical models used for breeding value estimation of these traits are in Uimari et al. (2011). The analysis was conducted with the AI-REML method in the dmu program package (Madsen et al. 2006). To account for multiple comparison, Bonferroni-corrected p-values were applied for statistical significance.Results
Putative lethal haplotypes
Twenty-six putative lethal haplotypes were identified on 12 chromosomes (Table 1). The naming of the haplotypes is based on the chromosome number followed by the order of the leftmost marker of the haplotype on that chromosome. The regions detected using 25- or 75-marker window scans overlapped; the regions found using a 75-marker window scan contained one or more regions found with a 25-marker window scan. Therefore, only the results from the 25-marker window scan are presented in Table 1, except for haplotype 1-3130 where the region found using the 75-marker window scan was not detected with the 25-marker window scan (for haplotype 1-3130 only four homozygotes were found when the expected number was 10 using the 25-marker window scan). In addition, the chromosomal regions found using the 25-marker window scan overlapped, so that the number of markers in each putative lethal chromosomal region ranged from 25 to 135 (Table 1). The number of expected homozygotes for the haplotypes varied from six to 20 and the probability of observing no homozygotes varied from 0.0025 to 2.06 × 10−09, respectively. The carrier frequency of a putative lethal was between 0.16 and 0.30, reflecting the limit of six animals set for the expected number of homozygotes and the number of genotyped animals in the data.
| Haplotype | Position, Mb | Markers (n) | Starting marker | Ending marker | Carrier frequency | No. of homozygotes | Probabilityb | |
|---|---|---|---|---|---|---|---|---|
| Obs | Exp | |||||||
| 1-104 | 14.19–15.34 | 31 | ALGA0001039 | DRGA0000114 | 0.17 | 0 | 7 | 9.12 × 10−04 |
| 1-6510 | 294.47–298.12 | 80 | H3GA0004734 | ALGA0010388 | 0.21 | 0 | 20 | 2.06 × 10−09 |
| 1-6865 | 307.57–309.03 | 27 | ASGA0008231 | ALGA0106630 | 0.17 | 0 | 8 | 3.35 × 10−04 |
| 1-3130a | 123.3–129.18 | 99 | ASGA0004260 | ALGA0005666 | 0.18 | 0 | 7 | 9.12 × 10−04 |
| 2-354 | 13.5–15.27 | 25 | MARC0078073 | MARC0089443 | 0.16 | 0 | 6 | 2.48 × 10−03 |
| 2-761 | 28.86–31.06 | 46 | DIAS0001906 | MARC0050106 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 2-951 | 36.89–38.68 | 39 | MARC0096910 | ALGA0013027 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 2-1571 | 80.76–82.66 | 29 | ALGA0014011 | H3GA0006928 | 0.16 | 0 | 6 | 2.48 × 10−03 |
| 3-1392 | 63.5–66.11 | 49 | ASGA0099398 | ASGA0098479 | 0.23 | 0 | 12 | 6.14 × 10−06 |
| 4-1322 | 48.33–50.61 | 33 | ALGA0024930 | MARC0038905 | 0.25 | 0 | 14 | 8.32 × 10−07 |
| 6-1 | 0.01–1.94 | 25 | ALGA0109178 | ASGA0106041 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 6-1057 | 46.66–48.72 | 26 | MARC0033200 | ALGA0102689 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 6-1132 | 52.57–56.88 | 49 | MARC0106688 | H3GA0019376 | 0.20 | 0 | 9 | 1.23 × 10−04 |
| 6-1438 | 70.02–71.96 | 70 | ALGA0122547 | ASGA0095317 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 8-904 | 35.25–36.74 | 33 | DRGA0008541 | ASGA0038654 | 0.16 | 0 | 6 | 2.48 × 10−03 |
| 8-2026 | 107.04–113.32 | 135 | ALGA0048805 | ASGA0039546 | 0.17 | 0 | 7 | 9.12 × 10−04 |
| 8-2174 | 114.35–116.64 | 34 | MARC0049164 | ALGA0049101 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 10-139 | 5.11–6.22 | 27 | ALGA0056456 | ALGA0120272 | 0.16 | 0 | 6 | 2.48 × 10−03 |
| 10-617 | 23.35–24.33 | 26 | INRA0033530 | DRGA0010739 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 10-1732 | 69.62–70.35 | 29 | H3GA0030667 | ASGA0048942 | 0.16 | 0 | 6 | 2.48 × 10−03 |
| 11-587 | 24.06–25.31 | 45 | ALGA0061440 | ALGA0061535 | 0.19 | 0 | 6 | 2.48 × 10−03 |
| 12-1 | 0.08–1.29 | 28 | H3GA0055340 | H3GA0056177 | 0.25 | 0 | 13 | 2.26 × 10−06 |
| 13-1218 | 45.35–47.19 | 49 | ASGA0057380 | ASGA0099506 | 0.30 | 0 | 20 | 2.06 × 10−09 |
| 15-2072 | 111.32–113.19 | 37 | MARC0001668 | ALGA0086522 | 0.17 | 0 | 6 | 2.48 × 10−03 |
| 15-2672 | 139.16–140.54 | 44 | MARC0003947 | DRGA0015563 | 0.19 | 0 | 8 | 3.35 × 10−04 |
| 18-854 | 37.35–38.76 | 34 | ALGA0097917 | ASGA0079572 | 0.16 | 0 | 6 | 2.48 × 10−03 |
- Obs, observed; Exp, expected.
- a Haplotype from the 75-marker window scan, all other haplotypes are from the 25-marker window scan.
- b Probability of not observing any homozygotes.
Association with reproductive traits
Descriptive statistics of the studied reproductive traits are given in Table 2. For each trait, dEBVs with weight <1.0 were removed from the data. The reliability of the original EBVs varied from 0.65 to 0.76. In general, the highest reliabilities were obtained for total number of piglets born and the lowest for piglet mortality. The differences between first and later parities were not significant.
| Trait | n | Mean | SD | Min | Max | R a |
|---|---|---|---|---|---|---|
| TNB1 | 856 | −0.20 | 0.97 | −3.09 | 4.63 | 0.75 |
| NSB1 | 854 | 0.00 | 0.46 | −1.70 | 2.46 | 0.71 |
| PM1 | 854 | −0.12 | 0.52 | −1.98 | 2.69 | 0.65 |
| TNB2 | 855 | −0.09 | 1.00 | −3.18 | 4.62 | 0.76 |
| NSB2 | 852 | −0.01 | 0.45 | −1.77 | 2.03 | 0.72 |
| PM2 | 851 | −0.06 | 0.55 | −1.92 | 2.42 | 0.67 |
- TNB1, total number of piglets born in first parity; NSB1, total number of stillborn piglets in first parity; PM1, piglet mortality between birth and weaning in first parity; TNB2, total number of piglets born in later parities; NSB2, total number of stillborn piglets in later parities; PM1, piglet mortality between birth and weaning in later parities.
- a Average reliability of the estimated breeding values.
Although several putative lethals were observed, only haplotype 8-2026 showed a significant association with some of the studied reproductive traits. Compared to non-carrier sires, boars carrying one copy of haplotype 8-2026 had 0.15 ± 0.04 (p-value = 8.95 × 10−5) piglets higher dEBV for NSB1 and 0.13 ± 0.04 (p-value = 6.78 × 10−4) piglets higher dEBV for NSB2. Using the original EBVs, the difference in the average number of stillborn piglets between carriers and non-carriers was 0.13 ± 0.03 piglets (p-value = 7.78 × 10−7) for NSB1 and 0.12 ± 0.03 piglets (p-value = 1.62 × 10−6) for NSB2. These values correspond to 0.38 (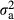 = 0.117) and 0.36 (
= 0.117) and 0.36 (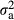 = 0.112) of the genetic standard deviations for NSB1 and NSB2, respectively. The original EBVs from the national evaluation were used; thus, a higher (positive) value is unfavourable for NSB1 and NSB2.
= 0.112) of the genetic standard deviations for NSB1 and NSB2, respectively. The original EBVs from the national evaluation were used; thus, a higher (positive) value is unfavourable for NSB1 and NSB2.
Discussion
Whole-genome SNP data from the Finnish Yorkshire pig breed were analysed with the Illumina PorcineSNP60 BeadChip to identify homozygous haplotypes which might be putative lethals. We found altogether 26 putative lethal haplotypes, but only one haplotype (8-2026) displayed a significant association with the studied reproductive traits. This haplotype was found on chromosome 8 (position 107.04–113.32 Mb) and was associated with the number of stillborn piglets in first and later parities (NSB1 and NSB2). Given the number of tested haplotypes (26), the Bonferroni-corrected p-value to claim a significant association is 1.92 × 10−3. The obtained p-values for the associations between haplotype 8-2026 and NSB1 or NSB2 were considerably smaller than that. Thus, even though only one haplotype showed significant association with the reproductive traits, the probability that this association is a false positive is relatively low. There could be multiple reasons why the other haplotypes given in Table 1 did not show significant association with the studied reproductive traits. The simple explanation could be that some of them are false-positive findings. In addition, there might not have been enough observations in the association study given the relative small effect a lethal haplotype has on average litter size of the daughters of any particular carrier boar.
The differences in EBVs between boars reflect the reproduction of their daughters. If a carrier boar is mated with random sows, the probability that the daughter of the carrier boar is also a carrier is (1-H) × 0.5 + H × 0.67 (probability that a sow is a non-carrier × probability of a heterozygous daughter from this mating + probability that a sow is a carrier × probability of a heterozygous daughter from this mating, given that 25% of piglets die during pregnancy). The probability that these daughters will be mated with a carrier boar is again H, and the probability of lethal homozygotes produced from this mating is 0.25. For example, if the carrier frequency H is 17%, approximately 53% of the daughters of the carrier boar are carriers as well. The average litter size of a carrier boar's daughters is 2.3% [p(daughter is carrier) × p(daughter mated with a carrier boar) × p(piglet is a homozygous) = 0.53 × 0.17 × 0.25 = 0.023] smaller than if no lethal haplotype was segregating in the population. The average litter size of the non-carrier boar is also reduced by 0.4% because the mated sow could be a carrier [p(mated with a carrier sow) × p(daughter is carrier) × p(daughter mated with a carrier boar) × p(piglet is a homozygous) = 0.17 × 0.5 × 0.17 × 0.25 = 0.0036]. Thus, the differences between average litter sizes of the daughters of the carrier and non-carrier boars are only 1.9%. Given an average number of 50 daughters, 0.1 heritability and average litter size of 13 piglets, the difference in EBVs for the number of stillborn piglets between carrier and non-carrier boars is approximately 0.3 piglets (using the selection index theory). This estimate is more than twice that found here for haplotype 8-2026 and number of stillborn piglets. Naturally, the possible lethal haplotype may affect both the total number of piglets born (TNB1 and TNB2) and the number of stillborn piglets (NSB1 and NSB2), because the time of death of the foetus varies (e.g. Van der Lende & Van Rens 2003) and not all homozygous piglets are stillborn.
We used the same approach for the expected number of homozygotes as described in VanRaden et al. (2011) and Sahana et al. (2013). The expected number of homozygotes may be overestimated if inbreeding has been avoided and underestimated if allele frequencies have changed in the past (VanRaden et al. 2011). Previous studies have reported average inbreeding rates of 3.7% (VanRaden et al. 2011) and 5.5% (Sahana et al. 2013) for dairy cattle breeds. Given the average inbreeding coefficient for Finnish Yorkshire of approximately 10% (Uimari et al. 2010), the method used in the above two studies was deemed appropriate for our study as well. As in Sahana et al. (2013), we defined a haplotype as a putative recessive lethal if the expected number of homozygotes was at least six, but none were observed in the data. Sahana et al. (2013) allowed one homozygote for haplotypes that had been reported as putative lethals in earlier studies, to account for possible errors in imputing and haplotype phase reconstruction. We examined all the haplotypes detected in our study with a similar criterion, but none were associated with the studied reproductive traits.
The most interesting chromosomal region detected in this study is on chromosome 8 from 107.0–113.3 Mb (position of haplotype 8-2026). In a previous study, Li et al. (2009) identified one suggestive QTL at linkage map position 84 cM on chromosome 8 connected with the number of stillborn piglets in a White Duroc × Chinese Erhualian intercross resource population. Although the significant region observed in our study is not the same, it may reflect the same finding, given the typically long confidence intervals in linkage analyses (dozens of megabases). Some other reproductive traits have also been associated with chromosome 8. For example, King et al. (2003) found two significant locations (125 cM and 127 cM) linked to prenatal survival and litter size, and Li et al. (2009) reported two candidate genes OPN and GNRH on chromosome 8. However, these genes are not located in the same chromosomal region as haplotype 8-2026.
Based on the NCBI database (http://www.ncbi.nlm.nih.gov/gene), we mapped 26 genes to the haplotype 8-2026 region (Table 3). One possible candidate gene for reproduction in this region is MAD2 mitotic arrest deficient-like 1 (yeast) (spindle checkpoint gene MAD2L1). In mammalian females, the spindle checkpoint system is involved in the regulation of meiosis and prevention of aneuploidy, an abnormal number of chromosomes (Wang & Sun 2006). The spindle checkpoint protein MAD2 is known to prevent aneuploidy during mitosis (Homer et al. 2005). In humans, errors in meiosis 1 are, in fact, the most common genetic reasons for stillbirth and mental retardation (Hassold & Hunt 2001).
| Namea | Starting position | Function |
|---|---|---|
| SPRY1 | 108191806 | Sproyty signalling antagonist |
| SPATA 5 | 108342976 | Spermatogenesis-associated factor protein |
| NUDT6 | 108446197 | Fibroblast growth factor |
| FGF2 | 108489441 | Fibroblast growth factor |
| IL21 | 108688771 | Interleukin |
| IL2 | 108797436 | Interleukin, T-cell growth factor |
| ADAD1 | 108800560 | Testis nuclear RNA-binding protein |
| KIAA1109 | 108893577 | Fragile site-associated protein |
| TRPC3 | 109324966 | Transient receptor potential cation channel, among its related pathways are signalling by GPCR and developmental biology. |
| BBS7 | 109408946 | Protein-coding gene, mutations are implicated in Bardet–Biedl syndrome |
| CCNA2 | 109455844 | Cyclin-a protein-coding gene |
| EXOSC9 | 109461157 | Exosome component, overlap syndrome-associated autoantigen |
| ANXA5 | 109609048 | Placental anticoagulant protein |
| QRFPR | 110011557 | Pyroglutamylated RFamide peptide receptor |
| PRDM5 | 110658855 | PR domain-containing protein, associated with PRDM5, includes brittle cornea syndrome 2 and ehlers–danlos syndrome (VI). |
| MAD2L1 | 111496848 | Mitotic arrest deficient-like 1 (Yeast) protein-coding gene |
| PDE5A | 111948577 | Phosphodiesterase, associated with PDE5A, includes priapism and transient global amnesia. Among its related pathways are platelet homoeostasis and haemostasis. |
| FABP2 | 112120921 | Intestinal-type fatty acid-binding protein, associated with FABP2, includes acute vascular insufficiency of intestine and perinatal necrotizing enterocolitis. |
| C8H4orf3 | 112150838 | Open reading frame |
| USP53 | 112155286 | Inactive ubiquitin-specific peptidase |
| MYOZ2 | 112235079 | Myozenin, calcineurin-binding protein calsarcin, associated with MYOZ2 include myoz2-related familial hypertrophic cardiomyopathy and cardiomyopathy, hypertrophic, 16. |
| SYNPO2 | 112412100 | Synaptopodin, associated with SYNPO2, includes duchenne muscular dystrophy. |
| METTL14 | 112710189 | Methyltransferase-like protein. Acts as a regulator of the circadian clock and differentiation of embryonic stem cells. |
| SEC24D | 112778266 | Protein-coding gene (vesicle trafficking), associated with SEC24D, includes Cole–Carpenter syndrome. Among its related pathways are immune system, transport to the golgi and subsequent modification. |
| PRSS12 | 112836261 | Brain-specific serine protease. Associated with PRSS12 include autosomal recessive non-syndromic intellectual disability and Axenfeld–Rieger syndrome (3). |
| NDST3 | 112936355 | N-deacetylase/N-sulfotransferase (heparan glucosaminyl). Among its related pathways are metabolism and glycosaminoglycan metabolism. |
- a Only genes with known function are presented. Genes given in italics are also found in the NCBI RNA reference sequences collection (https://www.ncbi.nlm.nih.gov/refseq).
The fibroblast growth factor 2 (FGF2), which is a multifunctional protein, was also found in the chromosomal region of haplotype 8-2026. During pregnancy, fibroblast growth factors are involved with the structural reorganization of uterine and placental vascular beds (Chrusciel et al. 2010). The normal function of the umbilical cord is essential for embryo development during pregnancy, and although the development of the umbilical cord is not fully known, fibroblast growth factors seem to control its dynamic growth and formation, among other factors (Chrusciel et al. 2010). The placental anticoagulant protein annexin A5 (ANXA5) was also located in the haplotype 8-2026 region. Bogdanova et al. (2007) found that a common haplotype of ANXA5 is associated with recurrent pregnancy losses in human patients.
Exclusion of all carriers of genetic disorders from mating would lead to a smaller number of animals available for breeding programmes. Doing this without considering the population frequency of the disorder and the genetic merit of the animals would result in a decrease in genetic gain (Van Eenennaam & Kinghorn 2014). Segelke et al. (2016) suggested a genetic index approach which would allow to take into account the differences in economic value and frequency of the known alleles and simultaneously decrease harmful and increasing beneficial alleles. Even though the litter size of commercial F1 sows, mated with boars of a third breed, should not be affected by lethal haplotypes, the genetic index approach could be a useful tool in improving the reproductive performance of purebred mother-line sows.
Conclusions
Of the 26 putative lethal haplotypes identified in this study, one (8-2026) was associated with the number of stillborn piglets in first and later parities in Finnish Yorkshire. Three possible candidate genes (MAD2LI, FGF2 and ANXA5) were located in this chromosomal region. However, further analysis of the haplotype 8-2026 region is needed to confirm the candidate genes and to characterize the mutation(s) causing the lethality.
Acknowledgements
The authors thank Figen Oy for providing the genotypes and EBVs used in this study. We are grateful to Marja-Liisa Sevón-Aimonen for estimating the breeding values and to Hanni Kärkkäinen for estimating the haplotypes. Financial support is acknowledged from the Finnish Culture Foundation.




