Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy
Abstract
Objectives Bacteriophages are bacteria-specific viruses that infect and, in the case of obligately lytic phages, destroy their host bacteria. Phage therapy has been used therapeutically to combat bacterial infections since their discovery. This paper reviewed recent in-vivo phage therapy studies, with a distinct focus on the effect of delivery routes, phage concentration and timing of administration on the success of the therapy.
Key findings It was found that the most successful route of administration for the treatment of systemic infections was via the parenteral route. Oral delivery is mainly used to treat gastrointestinal infections. However, in some cases phages can also reach the systemic circulation. Local delivery (skin, ears, teeth) has proved extremely successful in the treatment of topical infections, as has the inhalation of phages for the treatment of lung infections. The ability of phages to prevent biofilm formation on medical devices has received much attention, mainly in the area of catheter coatings. This review also highlights areas in which phage therapy needs substantial development. Many papers were lacking in formulation details, with crude phage stocks being used in most cases. No phage stability data were included in any of the papers.
Summary The review concluded that although phage therapy is an excellent alternative for the treatment of bacterial infections, optimisation of formulations and long-term stability data is required before it can be widely used within a clinical setting.
Introduction
The application of bacteriophages (phages) as antibacterial agents first began in the early 1920s, following their discovery by English bacteriologist Fredrick Twort in 1915 and also by French Canadian scientist Felix D'Herelle in 1917. D'Herelle recorded the discovery of a microbe that was antagonistic to bacteria and resulted in lysis and bacterial cell death. Two years earlier, Fredrick Twort had recorded a similar discovery, but he never considered phage therapy. D'Herelle devoted the rest of his scientific life to the study of bacteriophages.[1,2] Phages have been used in clinical applications ever since.[3,4] The discovery of penicillin hailed the beginning of the antibiotic era and phage therapy was largely supplanted across the developed world, with the exception of a number of Eastern bloc countries. Recently, the increasing incidence of antibiotic-resistant bacterial strains has stimulated a resurgence in interest into these bacteria-specific viruses.[3,5,6]
Multi-drug-resistant bacteria pose a major threat to human health and the long-term usefulness of conventional antibiotics.[7,8] In the European Union alone, infections caused by these bacteria cause around 25 000 deaths per year. Two-thirds of these deaths are due to infection with Gram-negative bacteria, such as Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacteriaceae, including Escherichia coli and Klebsiella pneumonia.[9] A recent report from the European Centre for Disease Prevention and Control and the European Medicines Agency states that only two new antibiotic drugs are under development, both in the early stages.[10] There are a multitude of obstacles to pharmaceutical companies investing in the development of new antibiotics. Firstly, there are many generic antibiotics that are still effective in the treatment of bacterial infections. Secondly, antibiotics are less profitable than many drugs because they are curative treatments and the duration of antibiotic regimens is limited. Thirdly, the rapid growth of resistance could shorten their lifespan sufficiently to affect profitability.[9] This reduction in investment and the cautious approach of big pharmaceutical companies towards the development of new antibiotics has prompted a renewed interest in phage therapy.
Bacteriophages are viruses that only infect bacteria. Lytic phages, unlike temperate phages and filamentous phages, multiply in the bacterial cell and lyse the bacterial cell at the end of their life cycle to release newly formed phage particles. The phage virion adsorbs to the surface of a susceptible host cell and injects its genome, which takes over much of the host metabolism and sets up molecular machinery for the replication and assembly of more phages.[11,12] Bacteriophages are structurally diverse. Phage virons can be tailed, polyhedral, filamentous or pleomorphic. Most contain double-stranded DNA (dsDNA), with a smaller number containing single-stranded DNA (ssDNA), or single- or double-stranded RNA (ssRNA, dsRNA). Approximately 96% of all phages are tailed and they represent the predominant therapeutic phage type.[13]Figure 1 illustrates an example of such a lytic bacteriophage replication cycle (Figure 1).
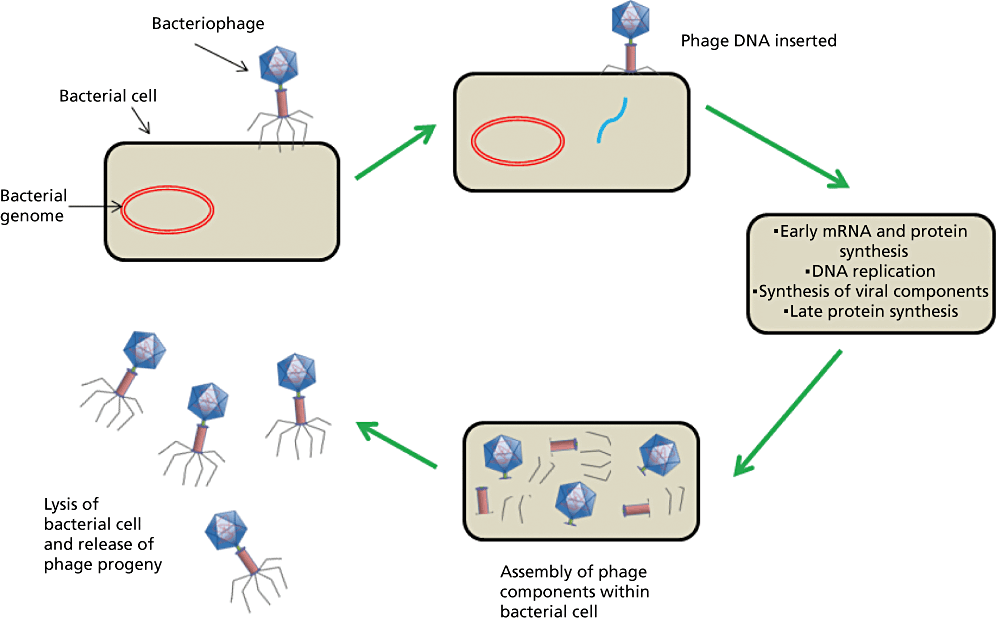
Lytic cycle of a bacteriophage. Adapted from Hanlon.[14]
Lytic bacteriophages possess a number of natural attributes that make them potentially good candidates for antibacterial therapy.[15,16] A recent review by Abedon et al. highlighted the advantages of phage therapy, noting the range of phage properties that contribute to phage therapeutic utility. These properties include the bactericidal effect of phages, auto-‘dosing’ because phages are capable of increasing in number specifically where hosts are located, low inherent toxicity, minimal disruption of normal flora, lack of cross-resistance with antibiotics, rapid recovery, formulation and application versatility, and biofilm clearance.[17] The ability of phages to replicate exponentially and kill pathogenic strains of bacteria in situ could play a vital role in the treatment of infectious diseases and permit reduced delivery time. Although bacteriophage therapy has many advantages, there are also a number of limitations to this approach. Abedon et al. also discussed the limitations of phage therapy due to the phenomenon of bacteriophage insensitive mutants and the development of phage resistance.[18] Phage resistance occurs at an equivalent rate to that of antibiotic resistance and it is difficult to isolate and develop lytic, virulent, broad-spectrum phages suitable for therapy. A precise bacteriological diagnosis is required before phage therapy can begin and there are questions regarding possible side effects and adverse immunological responses, especially following repeat exposure.
Reports of successful bacteriophage therapy have been reviewed extensively. Sulakvelidze et al.[5] published a comprehensive review of published literature relating to phage therapy in Eastern bloc countries from the early 1920s to the present time. This highlighted phage therapy successes and failures, including reasons for the failure of early phage therapy trials. This was mainly due to poor phage preparation and sterility protocols and also because of inaccuracies when matching phages to their host strains. Other papers covered successful experimental applications of phage therapy in vitro and in vivo. Slopek et al.[19] published an overview of bacteriophage treatments of suppurative bacterial infections (most of which were resistant to antibiotic treatment) between the years 1981 and 1986. Of 550 cases to which bacteriophage therapy was applied, positive results were obtained in 508 cases (92.4%). In 38 cases (6.9%), a transient improvement was observed and in four cases (0.7%) phage therapy proved ineffective. For a contemporaneous review of phage therapy the reader is directed to the special issue of the Journal of Current Pharmaceutical Biotechnology (2010), which contains excellent reviews of phage selection, isolation and preparation for phage therapy, design of phage therapeutics, phage therapy pharmacology, phage therapy for plant disease control, biocontrol of foodborne pathogens and the use of phages in clinical practice.[20]
Although the number of papers in the phage therapy field has increased substantially in recent years, there has been a distinct lack of focus on the effect of delivery routes and formulation type on the success of phage therapy. This review will attempt to address these lacunae in our current knowledge by examining the effects of routes of delivery on the success of bacteriophage therapy and by considering the limitations of each of the application routes. The effective concentrations of phage needed to eliminate bacterial infections and the timing of phage administration are also considered. This is, to our knowledge, the first review of bacteriophage therapy with a specific focus on formulation and delivery route.
Therapeutic applications and delivery mechanisms of bacteriophages
The last few years have seen a large number of new bacteriophage research directions, encompassing many delivery routes, the most popular being oral and parenteral. However, a significant amount of work has been directed towards local phage delivery (topical, otic, oral) and inhalation. The use of lytic bacteriophages to prevent biofilm formation on indwelling medical devices is also considered.
Parenteral delivery of bacteriophages
Parenteral delivery of bacteriophages in experimental animal studies has proven to be one of the most popular and successful of all delivery methods for bacteriophages because of the immediate distribution of phages into the systemic circulation. However, recent studies have highlighted that the specific site of administration – intramuscular (IM), subcutaneous (SC) or intraperitoneal (IP) – has a significant influence on the success of phage therapy.
The use of lytic bacteriophages for controlling E. coli septicaemia in chickens and meningitis in calves was examined by Barrow et al. to demonstrate the value of bacteriophage R administration.[21]E. coli H247 was isolated from diseased chickens and subsequently used to inoculate healthy chickens intramuscularly with 50 µl of a suspension containing 106 CFU. This was followed by IM administration of 50 µl dilutions (106 PFU) of phage preparations. The phage formulation consisted simply of phages suspended in Luria-Bertani broth (LB broth), with no other excipients. In the absence of phage, the E. coli produced almost 100% mortality in both 3-week-old and newly hatched chickens by both routes of inoculation. When both E. coli and phage were given by the IM route (in different muscles) and equal numbers of both were administered, no morbidity or mortality was observed at all. The administration of 104 plaque-forming units (PFU) of phage also produced significant protection, whereas 102 PFU produced some protection. Similarly, calves were inoculated orally with 3 × 1010 colony-forming units (CFU) of E. coli strain H247 and dilutions of phage R by IM (upper thigh) administration in 2-ml volumes. This experiment was limited to 3 days, but it was observed that calves that had received phage showed delayed onset of symptoms of E. coli bacteraemia. This study illustrated the importance of phage concentration, since higher concentrations of phage resulted in the most significant protection against infection.
A similar study was conducted by Biswas et al.[22] Bacteraemia was induced in mice by IP injection of a strain of vancomycin-resistant Enterococcus faecium. The phages used in these experiments were purified using a caesium chloride step gradient and toxin levels in phage preparations were measured by the Limulus amebocyte lysate assay. The phage stock was terminally sterilised by filtration through a 0.22 µm filter. A concentration of 109 CFU was administered and the resulting bacteraemia was fatal within 48 h. Two different phage strains were examined for their ability to rescue mice from bacteraemia. A single IP injection of 3 × 108 PFU of phage strains ENB6 was administered 45 min after the bacterial challenge. Bacteriophage ENB6 rescued 100% of the animals. When treatment was delayed to the point where all animals were moribund, approximately 50% were rescued by a single injection of this phage preparation. In addition, lower multiplicities of infection (0.03 and 0.003) resulted in a reduced rescue of animals (60% and 40%). The multiplicity of infection (MOI) is the ratio of adsorbed or infecting phages to total bacteria.[23] The mean bacterial titre in the blood 20 h after bacterial inoculation for this group was 8.74 × 104 ± 6.03 × 104 CFU/ml. Phage therapy thus resulted in a 200-fold decrease (compared to the control group) in blood bacterial titres at 20 h.
Local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice was treated by Cerveny et al.[24] using phage therapy. A number of strains were isolated from oysters and seawater. To determine whether phage CK-2 could protect against V. vulnificus infection, mice were injected intraperitoneally with iron dextran, inoculated subcutaneously with 106 CFU (100 times the lethal dose) of MLT403 and immediately injected intravenously with 108 PFU of phage. Control mice were treated with iron-dextran and infected with MLT403, but received phosphate-buffered saline with 0.01% gelatin (BSG) instead of phage. Bacteriophages CK-2, 153A-5 and 153A-7 were suspended in BSG (0.1 ml) and this phage mixture was injected intravenously into the lateral tail vein at various times after bacterial inoculation. The control mice had a mean of 108 CFU/g of lesion tissue, and their livers contained a mean of nearly 105 CFU/g of tissue. Optimum protection required that the phages be administered within 3 h of bacterial inoculation at doses as high as 108 PFU/ml. One of the protective phages had a half-life in blood of over 2 h. These results demonstrate that bacteriophages have therapeutic potential for both localised and systemic infections caused by V. vulnificus in animals. The study concluded that phages administered intravenously can be effective at clearing local infection of the skin tissue and that IV administration is the most efficient method of delivering the phages throughout the body.
Bacteriophage ΦMR11 was used to protect mice against a lethal Staphylococcus aureus infection in a study described by Matsuzaki et al.[25] Bacterial strains were isolated from nasal swab samples of 162 individual patients. Staph. aureus , including methicillin-resistant bacteria, were injected intraperitoneally (8 × 108 CFU/ml) into mice. Varying inoculum densities of bacteria (suspended in 0.5 ml saline) were injected into the peritoneal cavities of mice through one side of the abdomen, and purified phage suspensions in a medium of 1 ml heart infusion broth supplemented with 20 mm each CaCl2 and MgCl2 (HIBMC) were injected through the other side. As controls, equal volumes of saline or HIBMC alone were injected intraperitoneally on all test occasions. The test animals were observed for between 1 week and 1 month. IP administration of purified ΦMR11 (MOI 0.1) suppressed S. aureus-induced lethality and ΦMR11 rapidly appeared in the circulation. Substantial levels of phage (up to 7.7 × 108 PFU/ml) remained in the blood until the bacteria were eradicated. These results suggest that ΦMR11 may be a potential prototype for gene-modified, advanced therapeutic S. aureus phages.
A prophylaxis study in which adult New Zealand white rabbits each received 8 × 107 CFU of S. aureus 2698 and either control suspension or 2 × 109 PFU of phage LS2a was conducted by Wills et al.[26] The phage suspension was prepared in nutrient broth then purified by filtration, ultracentrifugation, resuspension in saline and refiltration. The rabbits were injected subcutaneously with both bacteria and phage at the same site, simultaneously. After 4 days, all eight of the untreated rabbits had developed abscesses, compared with one of the phage-treated rabbits. A dose–response study was also carried out, in which rabbits each received 8 × 107 CFU of S. aureus 2698 and 6 × 107, 6 × 106 or 6 × 105 PFU of LS2a or control suspension, again via single IP injection. Abscesses were prevented in animals that received the highest titre of phage.
Mice compromised by a burn injury and subjected to a fatal injection with a strain of P. aeruginosa (PAO1) were administered a single dose of a P. aeruginosa phage cocktail by McVay et al.[27] A non-lethal full-thickness thermal injury to the skin was induced by placing the exposed back area in 90°C water for 10 s. Fluid replacement therapy consisting of an SC injection of 0.8 ml of a 9% NaCl solution was administered immediately following the burn. The phage cocktail consisted of Pa1 (ATCC 12175-B1), Pa2 (ATCC 14203-B1) and Pa11 (ATCC 14205-B1), and contained 1 × 108 PFU of each of the three different phages (3.0 × 108 PFU total). The cocktail was administered intraperitoneally, intramuscularly or subcutaneously to infected and uninfected wounded animals. No formulation details were given in the paper. In the absence of phage administration, there was a 94% rate of mortality in the wounded infected mice in the first 72 h. The phages administered intramuscularly or subcutaneously reduced the rates of mortality to 72% and 78%, respectively. In contrast, the rate of mortality was reduced to 12% when the phages were delivered by IP injection. This proved beyond reasonable doubt that IP administration is the most useful for phage therapy in this case. P. aeruginosa phages administered by the IP route reached a higher titre, were distributed to the tissues more rapidly and were delivered for a more sustained period of time to the examined tissues than phages delivered by the SC or IM route.
In a similar study, a systemic murine model of S. aureus infection was challenged using phage therapy.[28]S. aureus stains were isolated from hospitalised patients at the Medical School of the University of Naples. A control group was set up in which 108 CFU/mouse of S. aureus A170 was injected intravenously. Three other groups were intravenously treated with phage MSA at final concentrations of 107, 108 and 109 PFU/mouse respectively. Phages were administered immediately after infection. No formulation details were given in this paper. All mice in the control group and the lowest titre group (107) died within 4 days. The survival rate for the 108 group was 40% and the mice treated with the highest concentration (109) all survived. In keeping with other studies described, all S. aureus A170-infected mice that were not administered with phage displayed a high bacterial load, while no bacteria were isolated from phage-treated mice. Phage treatment was also deemed to drastically reduce inflammation caused by S. aureus infection. Phage MSA showed antimicrobial behaviour against MRSA and phage treatment was also successful when delayed until 10 days after infection. Phages were also administered subcutaneously for the treatment of local infections, as S. aureus accounts for a large proportion or morbidity and mortality due to surgical wound infections. Phage (109 PFU/mouse) was administered concurrently with S. aureus and 4 days after infection. Both phage and bacteria were administered subcutaneously on both sides on the abdomen. Given concurrently with bacteria, phage MSa inhibited abscess development and a single dose given 4 days after infection reduced abscess size but did not prevent their formation.
Oral delivery of phages
Oral delivery of bacteriophages has proven successful in the treatment of gastrointestinal infections and, in some cases, systemic infections. The main issue with delivery of bacteriophages via the oral route is phage stability in the highly acidic and proteolytically active environment of the stomach. Protection of phages from gastric acidity by methods such as polymer microencapsulation may enhance the efficacy of orally administered phages.[29] Deactivation of bacteriophages may occur, dependent on the acid sensitivity of the individual phage, with the necessity for each bacteriophage to be characterised independently. A substantial body of knowledge has been accumulated on E. coli and its phages.[30] The pathogenic target bacteria are located in the gut and are thus principally accessible to orally applied phages.[31] Recent research has also illustrated the ability of some orally applied phages to be absorbed into the systemic circulation.[32] This phenomenon was reviewed recently by Gorski et al.,[33] who concluded that some phages may not only reside within the gut lumen but also pass the intestinal wall in a process similar to bacterial translocation. Although the precise processes controlling the viral translocation remain obscure, it was suggested that phage passage is determined by a number of factors, including phage concentration, specific sequences within the phage capsid proteins interacting with enterocyte receptors, and phage interactions with gut immune cells.
The threshold for an in-vivo lytic effect of orally administered phages (JS4, JS94.1, JSD.1 and JSL.6) on the intestinal E. coli population in laboratory mice was determined by Chibani-Chennoufi et al.[31]E. coli strains were isolated from pediatric diarrhea patients. The phage cocktail was added to the drinking water of 10 mice in increasing doses (103 PFU/ml, 105 PFU/ml and 107 PFU/ml), separated by 3 days of phage-free drinking water. The effect on faecal counts was negligible and two hypotheses were proposed for these findings. Firstly, without protection of the phages by an antacid or microencapsulation, they might not survive the gastric passage and thus not be available in the intestine. Secondly, phages might be present in the gut, but for physiological reasons the endogenous intestinal E. coli cell population resists phage infection. The second hypothesis was challenged by examining the gastrointestinal passage of orally administered phages and the determination of the lowest phage concentration leading to stable faecal phage excretion. It was found that with the lowest phage concentration of 103 PFU/ml in the drinking water, only low faecal phage titres over short time periods were observed, while exposure to 104 PFU/ml resulted in faecal phage detection over the entire exposure period. Unprotected T4-like phage thus has the capacity to transit the entire gastrointestinal tract without appreciable infectivity loss.
Phage therapy has also been employed to combat gastrointestinal E. coli infection in both in-vitro and in-vivo settings.[34]E. coli O157:H7 ATCC43888 was used as the host strain, and a phage cocktail containing SP15, SP21 and SP22 bacteriophages was prepared. Preliminary in-vitro experiments exhibited 5 log (99.999%) reductions of the E. coli strain using a 109 PFU/ml bacteriophage cocktail stock suspended in SM buffer containing 0.25% CaCO3 (100 mm NaCl, 8 mm MgSO4•7H2O, 50 mm tris-Cl made up to 1 l with H2O). This formulation was then used in in-vivo experiments and was orally administered to mice through a plastic sonde (a type of plastic tubing) into the stomach. The phage cocktail was administered in a single dose at 108 PFU/ml concentration, single dose at 1010 PFU/ml concentration and a daily dose of 1010 PFU/ml. The E. coli O157:H7 inoculum had been administered 2 days in advance. Phage and E. coli concentrations were monitored for 9 days after phage administration and it was found that high titres of bacteriophage were recovered from the faeces of the animals (104–106 PFU) and titres of E. coli were significantly reduced. The most successful administration, as determined by reduction in E. coli viable counts, was daily oral administration of phages.
A study in which the effect of phage therapy in the control of Campylobacter jejuni colonisation in young broiler chickens was conducted by Waganaar et al.[35] The C. jejuni challenge strain C356 used in this study was originally isolated from a commercial broiler in The Netherlands. Both the preventative and therapeutic effectiveness of orally delivered phages was examined. No details were given on the phage formulation. To examine preventative treatment, birds received phage by oral gavage. Oral gavage is a common and convenient approach for delivery of drugs to experimental animals. It mimics human oral consumption of drugs and is carried out using an oral gavage needle. Each day from day 7 to 16, chickens received an oral phage dose (phage strain 71) varying from 4 × 109 to 2 × 1010 PFU and, at day 10, an oral C. jejuni challenge of 1 × 105 CFU. To examine therapeutic treatment, birds received an oral dose of 1 × 105 CFU C. jejuni on day 10, followed by inoculation with phage strain 71 for six successive days (varying from 9 × 109 to 1 × 1010 PFU) for days 15–20, starting 5 days after the C. jejuni administration. Positive and negative controls were also examined. A 3 log decline in C. jejuni viable count was initially observed in the therapeutic group, but after 5 days bacterial counts stabilised at a level 1 log lower than that of the control group. Colonisation of C. jejuni in the prevention group was delayed by the treatment and after an initial 2-log reduction, colonisation stabilised within a week at levels comparable to the therapeutic group. In conclusion, the results described here show that it is possible to significantly decrease the number of Campylobacter in already-colonised chicken caeca by means of phage therapy. However, after an initially significant reduction of bacterial counts, phage and bacteria eventually reached equilibrium with bacterial colonisation levels 10 times lower than those of the control group. It would be expected that a resistant subpopulation might develop during treatment and this could be combated by using a phage cocktail rather than a single phage.
The application of phage therapy to the control of a P. aeruginosa induced gut-derived septicaemia in a murine model has been described.[36]P. aeruginosa strain D4 was isolated from the blood of a neutropenic mouse with bacteremia. Strain D4 was added to the drinking water of the animals, which induced septicaemia. Bacteriophage KPP10 was used to combat the bacterial infection. To evaluate the effect of timing on the phage host, a total of 0.1 ml of SM buffer with 1.0 × 1010 PFU of KPP10 was orally administered to each mouse 1 day before (group 1), 1 day after (group 2) or 6 days after (group 3) oral inoculation of the Pseudomonas. A significant protective effect of KPP10 was noted only in group 2, where phage had been administered 1 day after the bacteria (66.7% versus 0% (control group). There was no significant difference between groups 1 and 3, which suggests that the timing of phage administration is extremely important for a successful outcome. Another small study on the timing of phage administration was carried out, this time injecting the bacteria and phage intraperitoneally. To induce acute IP infection, each mouse was injected intraperitoneally with from 2.4 × 106 to 300 × 106 CFU of P. aeruginosa strain D4 suspended in sterile saline on one side of the abdomen. Purified phage strain KPP10 was suspended in SM buffer. A total of 0.1 ml of KPP10 suspension containing 1.0 × 1010 PFU of the phage was injected intraperitoneally into the contralateral side of the abdomen of each animal. Deaths among each group were enumerated every 24 h following bacterial challenge. Similar results were obtained when bacteria and phages were administered simultaneously. Phage strain KPP10 was simultaneously injected intraperitoneally with different doses of P. aeruginosa strain D4 (MOI of 100 to 10 000). It was found that treatment with phage provided significant protection against mortality in mice at a concentration of 19 × 106 CFU/mouse of P. aeruginosa strain D4. Finally, 12 of 13 (92.3%) phage-treated mice survived, whereas only 5 of 12 (41.7%) phage-untreated mice survived. In addition, the effect of the timing of phage administration on survival was studied. Administration of phage 1 day prior to bacterial challenge had a minor effect (60% survival versus 40% survival for the controls); however, simultaneous inoculation of phage induced significant protection against IP infection with P. aeruginosa.
Four different bacteriophages obtained from commercial broiler houses (CB4) and 45 bacteriophages from a municipal wastewater treatment plant (WT45) were evaluated for effectiveness against Salmonella enterica Serovar Enteritidis in broiler chickens by Filho et al.[37] A primary poultry isolate of Salmonella enteritidis was obtained from the USDA National Veterinary Services Laboratory. In one experiment, day-of-hatch chicks were challenged orally with 9 × 103 CFU/chick S. enteritidis and treated via oral gavage with 1 × 108 CB4 PFU/chick, 1.2 × 108 WT45 PFU/chick or a combination of both, 1 h post challenge. The commercially available probiotic Floramax-B11 (41069, IVS-Wynco LLC, Springdale, AR) was used for this experiment. The product consisted of a defined bacterial probiotic containing Lactobacillus fermentum, Lactobacillus helveticus, Lactobacillus paracasei, Lactobacillus salivarius and Pediococcus parvulus. The culture was diluted in reconstituted powdered skim milk to treat chicks cloacally. All treatments significantly reduced the numbers of S. enteritidis recovered from cecal tonsils at 24 h, as compared with untreated controls. No significant differences were observed at 48 h following treatment. These data suggest that some bacteriophages can be efficacious in reducing S. enteritidis colonisation in poultry, however the effect is temporarily limited and thus dosing interval is a critical factor for the success of phage therapy.
The influence of the mode of administration of a phage cocktail on the dissemination of three coliphages (phage cocktail consisted of ΦF78E, ΦF258E and ΦF61E) in chickens was examined.[32] In-vivo trials were conducted by infecting chickens orally, by spray (applied directly to the beak) and intramuscularly with 106, 107 and 108 PFU/ml suspensions of this phage cocktail. The formulation consisted of the three coliphages in LB broth. Groups of three animals were challenged with 1 ml of the phage suspensions at each concentration. The suspensions were administered orally with a syringe, by spray directly to the beak or by IM injection to the chest muscle. A control group was not treated with phage. The birds were administered isofluorane by inhalation 3, 10 and 24 h after challenge. Lungs and air sac membranes, liver, duodenum and spleen were carefully excised, weighed and emulsified individually in LB broth and assayed for phage concentration. Results were presented simply as either presence or absence of phage. In each case, results were concentration-, administration-route- and phage-dependent. When administered by spray, all three phages reached the respiratory organs. When orally administered, all three phages were recovered in the lungs, but only phi F78E was recovered from the duodenum, the liver and the spleen. The author suggests that these differences could be explained by the possible replication of phi F78E in commensal E. coli strains present in the chicken gut. This led to a higher concentration of this phage in the intestines and the systemic circulation. As predicted, the IM route of administration resulted in all phages being detected in all organs. This study illustrated that some phages can successfully be absorbed into the systemic circulation following oral administration. However, this is dependent on the characteristics of each individual phage.
Local delivery of phages
Local delivery of phages has proven very successful, and numerous studies are reported in the literature, especially from former Soviet/Eastern Bloc countries.[3,5] One of the therapeutic areas that has received much attention has been wound healing. The development of hydrogel and impregnated wound-healing formulations has increased the success rates of phage therapy in topical applications. An example of a successful commercially available product is the Phagebioderm® system developed by the Eliava Institute in Georgia, which targets P. aeruginosa, S. aureus and Streptococcus spp. This formulation consists of a cocktail of bacteriophages and antibiotics impregnated in a stabilised hydrogel system for topical application.[38] Recently, local phage therapy for areas other than the skin has also received attention (i.e. otic and oral applications)
Topical administration of phages
The use of lytic bacteriophages to reduce the contamination of chicken skin by Salmonella and Campylobacter spp. has recently been reported by Goode et al.[39] The effect of the MOI – the ratio of phage particles to bacterial cells – on Salmonella and C. jejuni counts on chicken skin was assessed. Phage preparations were cultured on the target organisms, harvested in LB broth, centrifuged and filtered (0.22 µm filter) with a filtration unit. Large sections of skin (60 cm2 in area) in triplicate were inoculated with S. enterica Serovar Enteritidis phage type 4 strain P125589, and the inoculum was distributed over the surface using a glass spreader to produce a density of approximately 103 CFU/cm2.
The same technique was then used to inoculate half of the chicken skin portions with Salmonella typing phage 12 at an approximate density of 103 PFU/cm2. Similar experiments were carried out using identical chicken skin pieces inoculated with approximately 104 CFU of C. jejuni strain C222. C. jejuni phage 12673v was used to treat half of the chicken skins at an approximate density of 106 PFU/cm2. Phages were also applied at higher MOIs in a similar way. The efficacy of phage treatment was dependent primarily on phage concentration. Phages applied at an MOI of between 100 and 1000 rapidly reduced recoverable bacterial numbers by up to 2 log10 units over 48 h. When the level of Salmonella contamination was low (<log10 2 per unit area of skin) and the MOI was 105, no organisms were recovered. By increasing the number of phage particles administered, other strains of Salmonella were eliminated. This study demonstrates that increasing the MOI increases the success of phage therapy by reducing bacterial numbers.
The treatment of K. pneumoniae B5055-induced burn wound infection in mice using natural products, such as aloe vera and honey, was assessed by Kumari et al.[40]K. pneumoniae B5055 was obtained from the Department of Medical Microbiology and Hygiene, Ulm University Hospital, Ulm, Germany. Briefly, the skin was denuded with a commercially available hair-removing cream. Mice were anesthetised with ether fumes and thermal injury was induced by applying a heated brass bar (10 × 10 × 100 mm) for 45 s. Immediately after the burn, all the mice were injected intraperitoneally with 0.5 ml of sterile physiological saline for fluid replacement to prevent overt shock, and acetaminophen (0.25 mg/ml) was given in drinking water as a post-burn analgesic. These treatments were compared to phage therapy using the Klebsiella-specific phage Kpn5. A full thickness burn wound was induced in mice and infected with K. pneumoniae B5055 via the topical route. The efficacy of natural antimicrobial agents (honey and aloe vera gel) applied daily to a murine burn wound was compared with the efficacy of phage Kpn5 suspended in 3% hydroxypropylmethylcellulose hydrogel, also applied daily to a similar murine burn wound. The efficacy of these antimicrobial agents was assessed on the basis of the percentage of infected mice that survived following treatment. In group I, all the burned mice challenged with bacterial inoculum acted as controls. In groups II and III mice were burned, infected and treated with a single application of 0.5 ml of Kpn5 phage at 108 PFU/ml and 1010 PFU/ml hydrogel. All other groups were treated with 0.5 ml aloe vera and honey. When applied topically, the phage Kpn5 formulation provided protection on the first day, with survival rates of 86.66% for low titre and 100% for high titre being observed, compared to 86.66% survival in the phage untreated (control) group. A survival rate of 80% at low titre and 96.66% at high titre was observed in comparison to mortality in the control group of 83.34% on the second day post phage treatment. Over time, the high titre phage-treated group showed a high level of protection compared to the untreated (control) group (0% survival). All the animals in the low titre phage-treated group (0% survival) succumbed to infection eventually. The results of this study are in general agreement with a number of similar studies that also indicate that low-titre phage administration is unlikely to be successful.
Otic phage administration
Comprehensive data from a clinical trial of a therapeutic bacteriophage preparation in chronic otitis caused by antibiotic-resistant P. aeruginosa have been reported recently.[41] Each patient had, at the time of entry to the trial, an ear infection because of an antibiotic-resistant P. aeruginosa strain that is sensitive to one or more of the six phages present in Biophage-PA® (Biocontrol Inc., UK). Formulation details of Biophage-PA® were not made available. Participants were randomised in two groups of 12 treated with either a single dose of Biophage-PA® or placebo and followed up at 7, 21 and 42 days after treatment. Ears were thoroughly cleaned on each occasion, and clinical and microbiological indicators measured. Relative to day 0, pooled patient- and physician-reported clinical indicators improved for the phage-treated group relative to the placebo group. P. aeruginosa counts were significantly lower in the phage-treated group only. The mean recovery of bacteriophage from swabs taken from the ears of the phage group over all three post-treatment clinic visits was 1.27 × 108. This compares with an input dose of 6 × 105, suggesting an average amplification in the treated ear in excess of 200 times the input dose, allowing only for bacteriophage collected on the swab. No treatment-related adverse events were reported. The results show that administration of this topical bacteriophage mixture leads to lysis of P. aeruginosa in the ear and improvement of clinical manifestation of the infection.
A recent canine clinical trial on the treatment of P. aeruginosa otitis using a bacteriophage cocktail has shown similar promise.[42] Ten dogs with chronic P. aeruginosa otitis received, directly into the auditory canal of one ear, a single dose of a topical preparation containing approximately 1 × 105 PFU of each of six bacteriophage strains (formulation details/phage strain identity not provided). The bacteriophage preparation was stored frozen at 70°C and consisted of 0.2 ml doses, each containing 1 × 105 PFU of each of the six therapeutic bacteriophages (designated BC-BP-01 to BC-BP-06) in 10% (v/v) glycerol/phosphate buffered saline (PBS). At the time of bacteriophage administration and 48 h later, each dog's core temperature was taken, its ear was assigned a clinical score (higher = worse condition) and aural swabs were taken for bacteriophage and P. aeruginosa counts. Forty-eight hours after treatment, the clinical score and P. aeruginosa count of all ears had fallen (mean score fall 30.1%, range 7.7–56.3%, mean count fall 67%, range 29.4–96.8%). The bacteriophage counts had risen from the administered dose (mean rise 99.1-fold, range 2.8–433.3-fold). All results were statistically significant. No treatment-related inflammation or other adverse events were detected. This topical bacteriophage formulation has the potential to be a convenient and effective treatment for P. aeruginosa otitis in dogs.
Dental phage administration
The use of bacteriophages in the treatment or prevention of dental infections has been the subject of a number of papers and patent filings. The effect of bacteriophages on the viability of Enterococcus faecalis (ATTC 29212) in human dental roots was assessed by Paisano et al.[43] Human teeth with a single root and with complete root formation were used in this study. The crowns of the teeth were removed and canals were rinsed with sterile PBS and fully sterilised before inoculation with E. faecalis. Teeth were split into groups of five. Group 1 was inoculated with bacteria and phages at an MOI of 1. Details of the phage formulation were not given in the paper. Phage titre was 2 × 108 PFU/ml in each case, but phage names were not disclosed in the paper. Groups 2 and 3 contained bacteria and phages at MOIs of 10 and 0.1, respectively. The samples were homogenised and aliquots of 20 µl were inoculated into each canal. The inoculated teeth were kept at 37°C for 3 h, after which viable counts of bacteria were carried out. Group 4 underwent a similar experiment but was not treated with bacteriophage until 6 days after bacterial inoculation. This was to allow bacterial penetration into the teeth tubules.
No bacterial growth was detected for groups 1 and 2 after 3 h of incubation, with negligible growth in group 3. In group 4, no bacteria were observed after 24, 48 or 72 h of the treatment with the phage lysate. There was a substantial reduction in bacterial growth, which indicates that the phages were able to suppress bacteria growth in the tubules. The paper concluded that phage therapy may be an important alternative for the treatment of root canal infections refractory to conventional endodontic therapy.
Inhalation of bacteriophages
The application of inhalation technologies to phage therapy has been one of the most recent advances within the field. Taking into account the previous successes of bacteriophage therapy in local and systemic applications, the use of bacteriophages to combat bacterial lung infections seems to be a logical step. The development of modern inhalation and process technologies has allowed great advances in this field. Recently, nebulisers have been used to deliver bacteriophage solutions. The Golshahi et al.[44] study focused on the efficiency of nebuliser delivery and the particle-size distribution of droplets. The results suggested that phages can be nebulised and delivered successfully. No in-vivo studies were carried out in this work. A recent paper by the same group further examined the in-vitro delivery of bacteriophages using a dry-powder inhalation formulation as a potential therapeutic approach for cystic fibrosis pulmonary infections.[45] Although it was an in-vitro study, it provided detailed information on the formulation of bacteriophages within an aerosolised powder for lung delivery. Endotoxin-removed bacteriophages KS4-M and ΦKZ were lyophilised in lactose/lactoferrin 60 : 40 w/w matrix and deagglomerated in a mixer mill to formulate respirable powders. The powders were then aerosolised using an Aerolizer® capsule inhaler. Along with aerodynamic diameter measurements, the viability of the bacteriophages delivered distal to an idealised mouth–throat replica was determined from bioassays. Pulmonary delivery was determined by measuring the amount of powder delivered as a percentage of inhaler load. The results were 33.7 ± 0.3% for KS4-M and 32.7 ± 0.9% for ΦKZ. Phage titres collected following delivery were high. A titre of 3.4 × 106 PFU of phage KS4-M was recovered after an initial loading of 9.8 × 106 PFU. Similarly, a titre of 1.9 × 107 PFU of phage ΦKZ was recovered after an initial loading of 6.5 × 107 PFU. Phage stability studies within the formulation were carried out at 4°C and 22°C over a 3-month period. Both phages maintained stability at both 4°C and 22°C over the 3-month period. The promising data in this paper warrant further investigation into the development of dry-powder formulations containing bacteriophages that are active against antibiotic-resistant bacteria.
The use of an aerosolised bacteriophage spray containing phages SPRO2 and DAF6 for the prevention of E. coli infection in broiler chickens has also been described previously.[46]E. coli was isolated from municipal sewer treatment facilities or poultry processing plants. Three separate studies were conducted. In the first study, chickens at 7 days old were treated with an aerosol spray containing 3.6 × 107 and 4.6 × 107 PFU/ml of the bacteriophages DAF6 and SPR02, and challenged by injecting 0.1 ml of a 2.5 h culture of E. coli (5.6 × 105 CFU/ml) into the thoracic air sac at 7, 8 or 10 days old. In studies 2 and 3, the titres of the two bacteriophages were increased, providing approximately 108 PFU/ml of SPRO2 and 109 PFU/ ml of DAF6, whereas the E. coli challenge was approximately the same with a 0.1 ml injection of 6.12 × 105 CFU/ml. No details of the aerosol formulations were given in this paper. The results were analysed by examining the protection provided by the administered bacteriophage at day 7. The protection afforded by bacteriophage administration was not complete, but there was a significant decrease in mortality compared to the control. Bacteriophage titre dictated the degree of protection afforded by the aerosol formulation, with the best overall protection observed in study 2, with phage titres of 2.6 × 108 and 2.35 × 109 PFU/ml for SPR02 and DAF6, respectively. The least protection was observed in study 1, with phage titres of 4.6 × 107 and 3.6 × 107 PFU/ml for SPR02 and DAF6, respectively. Yet again this study illustrates the importance of phage titre for the successful application of phage therapy.
A recent study demonstrating the in-vivo efficacy of phage therapy for Burkholderia cepacia respiratory tract infections[47] is discussed below. Burkholderia cenocepacia strains AU0728 and K56-2 were isolated from the sputum of patients with cystic fibrosis. Using a mouse model of acute lung infection, the effect of treatment with a single phage strain on bacterial load and lung inflammation was examined. Before phage administration into mice, the phage liquid lysate was purified using methods modified from Sambrook et al. The 9- to 12-week-old C57BL/6 mice were infected via tracheotomy with 1 × 107 or 1 × 108 CFU B. cenocepacia, suspended in 50 ml sterile PBS. Twenty-four hours post infection, mice were administered, either by intranasal inhalation or by IP injection, phage suspended in 50 or 100 ml of SM buffer at an MOI of 100. Bacterial load, macrophage inflammatory protein 2 (MIP-2) and tumour necrosis factor α (TNF-α) levels were significantly reduced in lungs of mice treated with IP-administered phages. No significant differences in lung bacterial density or MIP-2 levels were found between untreated mice and mice treated with intranasal phages, IP ultraviolet-inactivated phages, or IP phage control mice. Systemic phage administration was more effective than inhalational administration, suggesting that circulating phages enjoy improved access to bacteria in the lungs than topically administered phages.
The ability of bacteriophages to treat bacterial lung infections caused by antibiotic-resistant P. aeruginosa strains was recently examined.[48] Bioluminescent P. aeruginosa strain PAK was used to record a real-time view of the lung infection. Photon emission of the luminescent bacteria in the lungs of infected mice was quantified using an IVIS 100 imaging system. For the animal experiments, bacteriophages prepared by caesium chloride ultracentrifugation were diluted in PBS. The infectious dose was 1 × 107 luminescent bacteria resuspended in 50 ml of PBS, and was injected intraperitoneally. After 2 h, the bioluminescence was recorded and 30 ml of bacteriophages were applied intranasally while the mice were anaesthetised (isofluorane inhalation). In preventive experiments, 24 h before infection, the animals received intranasally 30 ml of bacteriophages or PBS while under light anaesthesia by means of isofluorane inhalation. Mice treated with bacteriophages in a phage-to-bacterium ratio of 1 : 10 died within 5 days of inoculation with PAK. Mice treated with higher bacteriophage-to-bacterium ratios (1 : 1 and 10 : 1) survived until the end of the experiment (12 days). Bioluminescence imaging showed that early inoculation (2 h) is pivotal in resolving PAK infection, as during this early inoculation stage the growth of bacteria is at its fastest. Under such conditions, susceptibility of bacteria to bacteriophage infection is also at its highest. Thus, infection is rapidly reduced, with a reduction of the inflammatory response in the host, as shown by the levels of IL-6 and TNF-a.
Use of bacteriophages for preventing biofilms on medical devices
Indwelling medical devices provide efficacious, cost-effective and often simple solutions in a diverse range of clinical scenarios and, as a result, have become a cornerstone of modern clinical and surgical practice. However, their use in practice is significantly compromised by their propensity to become colonised by microorganisms, leading to medical-device-associated infections in a large proportion of patients. Indeed, the use of indwelling medical devices, such as catheters or endotracheal tubes, is associated with at least half of all incidences of healthcare-associated infections.[49] Recently, the use of lytic bacteriophages for the eradication of bacterial biofilms has received increasing scrutiny, with a number of studies reporting promising results.[23]
Donlan and co-workers[50] investigated the ability of pre-treatment with bacteriophage 456 to reduce Staphylococcus epidermidis biofilm formation on hydrogel-coated catheters. For phage pre-treatment experiments, a crude Mueller Hinton broth (MNB) culture of phage 456 with a titre between 1 × 1010 and 2.2 × 1010 PFU/ml was used. Each catheter segment was filled with the phage culture, which was incubated at 37°C for 1 h within the catheter lumens before removal. The silicone catheters were installed in a modified drip flow reactor (mDFR). Biofilms were grown by circulating the S. epidermidis culture (mid-exponential phase) through the mDFR for 2 h (1 ml/min), which irrigated the catheter segments attached inside. The mean CFU per milliltre of the batch culture ranged from 108 to109 during this 2-h period. This was followed by irrigation for 22 h with sterile half-strength MHB (0.5 ml/min) to establish a biofilm. The untreated mean biofilm cell count was approximately 107 CFU/cm2 of catheter. Bacteriophage treatment, with and without supplemental divalent cations, resulted in log-CFU/cm2 reductions of 4.47 and 2.34, respectively.
An investigation into the use of a bacteriophage cocktail for the prevention of biofilm formation by P. aeruginosa on catheters was carried out by Fu et al.[51] Pre-treatment, post-treatment and recharge treatment were carried out on Foley catheters in an mDFR. Bacteriophages at concentrations of 1.0 × 1010 to 2.2 × 1010 PFU/ml were used for all experiments. Phage M4 with a concentration of 1.0 × 1010 to 2.2 × 1010 PFU/ml was used and phage M4 plus four environmental phages –ΦE2005-24-39, ΦE2005-40-16, ΦW2005-24-39 and ΦW2005-37-18–03 – with a final cocktail titre of 7.0 × 109 PFU/ml was used for the second round of treatment. For pre-treatment, the phage lysate was pumped through the catheter segments for 2 h at 1 ml/min prior to exposure to bacterial medium for 2 h. This was followed by sterile medium for 22 or 46 h. For post-treatment, catheters were exposed first to bacterial inoculum for 2 h, then bacteriophage for 2 h, followed by sterile medium as above. Recharge experiments followed the same method as pre-treated, except that after 22 or 46 h of sterile medium exposure catheters were treated with phage again for 2 h, followed by sterile medium for 22 h. The mean viable biofilm count on untreated catheters was 6.87 log 10 CFU/cm2 after 24 h. Pre-treatment of catheters with phage reduced this value to 4.03 log 10 CFU/cm2. Phage treatment immediately following bacterial inoculation also reduced biofilm viable counts (4.37 log10 CFU/cm2 reduction). Regrowth of biofilms on phage-treated catheters occurred between 24 and 48 h, but supplemental treatment with phage at 24 h significantly reduced biofilm regrowth. Pre-treatment of catheters with the phage cocktail reduced the 48-h mean biofilm cell density by 99.9% (from 7.13 log 10 CFU/cm2 to 4.13 log 10 CFU/cm2), but fewer biofilm isolates were resistant to these phages. These results suggest the potential to apply phages, especially phage cocktails, to the surfaces of indwelling medical devices for mitigation of biofilm formation by clinically relevant bacteria.
The prevention of biofilm formation on Foley catheter biomaterials following impregnation of neutral hydrogel (Bard Lubri-Sil®) coated catheter sections with lytic bacteriophages was also investigated by Carson et al.[52]E. coli ATCC 11303 (LGC Standards, Middlesex, UK) and P. mirabilis 13 HER1094 (Felix d'Herelle Reference Centre for Bacterial Viruses, Quebec, Canada) were used as host strains. E. coli-specific phage T4 and P. mirabilis-specific coli-proteus bacteriophage, which were isolated from a commercially available bacteriophage preparation, were coated onto a section of Foley catheter coated in a neutral hydrogel by incubating catheter segments in bacteriophage culture. Catheters were incubated for 1 h at 37°C with a titre of 1 × 106 PFU/ml of either the T4 bacteriophage or coli-proteus bacteriophage in MHB. Catheters were rinsed in saline to remove excess bacteriophage, then suspended in MHB inoculated with P. mirabilis or E. coli for 24 h at 37°C. The results showed a reduction in biofilm formation on the surface of bacteriophage-treated catheters of approximately 90%. The prevention of biofilm formation on catheter coatings was also visually observed using confocal microscopy, with a marked reduction in biofilm formation on phage-treated catheters.
Conclusion
This review has focused on recent research into bacteriophage therapy using lytic bacteriophages, with a particular view to assessing the wide range of delivery routes used. For systemic applications, the most clinically successful route appears to be parenteral and, more specifically, IP delivery. Oral delivery has shown excellent success rates for combating gastrointestinal E. coli infections, while local applications onto the skin, the ear and oral cavity/dental surfaces, the inhalation of phages to combat lung infections and application of phages to medical devices to reduce biofilm formation have all exhibited positive and conclusive results. One major area that has been overlooked in recent phage therapy papers has been delivery formulations. There is generally a paucity of information regarding formulation effects on phage therapeutic outcome; in a majority of the studies simple aqueous formulations have been employed. Phage formulation development would broaden the range of applications suitable for phage therapy. By diversifying formulations, for example by development of controlled-release formulations, the delivery profiles of phages could be expanded to suit specific bacterial infections. Also, long-term stability studies of phages within formulations are essential to ensure no unacceptable loss of activity occurs that may prove detrimental to treatment. These specific areas require significant industry if phage therapy is to enjoy widespread clinical application.
Similarly, minimal information is available regarding phage pharmacokinetics, a subject first broached over a decade ago[53] with the development of a proposed pharmacokinetic profile for these self-replicating organisms. Phages are thought to control bacterial infections in two ways, firstly via ‘active treatment’, where most of the bacteria are killed by secondary infections after the extensive reproduction and transmission of the phage. Secondly ‘passive treatment’, whereby phages do not increase in number but the initial dose is large enough to inundate the bacteria by primary infection alone. Furthermore, numerous variables are at play during phage therapy, and timing is paramount for successful clinical outcomes. Replication and infection processes of both bacteria and phage are density-dependent in ways that give rise to novel phenomena that do not occur in non-replicating pharmaceuticals. A computerised simulated generalised phage–bacteria system was devised to provide a model of phage–bacteria interactions. The author concluded that early inoculation does not ensure successful treatment and the optimal inoculation time will depend on the particular phage–bacteria system. The author also stressed that the determination of proliferation onset and failure threshold times for real phage–bacteria systems will be an important objective of future studies. This concept was further developed by the development of a complex set of mathematical algorithms to predict phage pharmacokinetics.[54] The paper concluded that the development of customised kinetic models as an intrinsic part of exploratory studies are the important next steps in phage pharmacokinetics. Unfortunately, recent phage studies have not taken this approach, with most papers only exploring phage concentrations and timing of administration. No further pharmacokinetic information is available in any of the papers reviewed. A pharmacokinetic study of bacteriophage K in the treatment of subclinical S. aureus mastitis in lactating dairy cattle was also conducted, but results proved inconclusive.[55] This further illustrates the difficulties associated with predicting phage pharmacokinetics.
These studies, and others discussed in this review, indicate that phage concentration and timing of administration are of paramount importance for the success of phage therapy.[53,54] For each bacteriophage and delivery route, a bacterial and bacteriophage concentration threshold must be reached before successful phage therapy can occur. This threshold must be individually characterised for each phage or cocktail of phages and their host systems. Phages will be cleared from the body before they can come into contact with the bacterial cells if administered too early. However, if administration is left too late, the bacteria will already have caused too much physiological damage within the system for phages to have a significant effect, or they may have developed phenotypically resistant biofilm communities. However, as with bacteriophage concentration, this effect of timing of administration must be characterised independently for each phage or phage cocktail, and for each delivery route.
The future for phage therapy – current phage products and recent clinical trials
Evidence of the recent interest in phage therapy is apparent from the increasing numbers of pharmaceutical companies involved in phage research and carrying out clinical trials (Table 1). An example of a phage product that has already received FDA approval is the LMP-102 bacteriophage preparation, which consists of a cocktail of six phages for use on meat and poultry as an antimicrobial agent against Listeria monocytogenes. A Dutch food safety company, EBI Food Safety, have also received ‘generally recognised as safe’ (GRAS) status for its listeria product LISTEXP100.[57] Companies such as Biocontrol® Ltd are in preparation for phase 2 and 3 clinical trials, concentrating mainly on combating Pseudomonas infections. Sites of administration are mainly topical, with leg ulcers and ear infections being targeted as initial administration sites. A clinical trial on the inhalation of phage is also in preparation.[34,35] Other companies that are active in the development of phage products include Novolytics Limited, Phico Therapeutics and Biophage Pharma Inc., who are all reported to be developing phage products against methicillin-resistant S. aureus (MRSA) and Clostridium difficile.[57]
| Company | Product | Target application |
|---|---|---|
| Omnilytics (USA) | AgriPhage™ | Targets bacterial spot or bacterial speck on crops, with specific formulations for strains of Xanthomonas campestris pv. vesicatoria or Pseudomonas syringae pv. Tomato |
| CheilJedang Corp. (Korea) | BioTector | Animal feed for control of Salmonella in poultry |
| Intralytix (USA) | EcoShield™ | Targets Escherichia coli O157:H7 contamination in foods and food-processing facilities |
| Intralytix (USA) | ListShield™ | Targets Listeria monocytogenes contamination in foods and food-processing facilities |
| Biotech Laboratories (Israel) | FASTPlaque-Response™ | Rapid detection of rifampicin resistance in smear-positive sputum specimens containing Mycobacterium tuberculosis |
| Biotech Laboratories (Israel) | FASTPlaqueTB™ | Rapid detection of Mycobacterium tuberculosis in human sputum samples |
| EBI Food Safety (The Netherlands) | LISTEX™ P100 | A food-processing aid that targets Listeria monocytogenes strains on food products |
| Microphage (USA) | MRSA/MSSA blood culture test | Determining of Staphylococcus aureus methicillin resistance or susceptibility directly from blood cultures |
| Microphage (USA) | MRSA screening test | Identifies methicillin-resistant Staphylococcus aureus (MRSA) for use in infection-control programmes |
| Microphage (USA) | MicroPhage MRSA/MSSA test | Differentiation of methicillin-resistant (MRSA) and methicillin-susceptible (MSSA) Staphylococcus aureus |
| Biocontrol (UK) | Phage products to combat otitis and lung infections | Clinical trials on phage products to combat Pseudomonas aeruginosa infections have been successfully completed |
| Novolytics (UK) | In development – gels for targeting MRSA and Clostridium difficile | Gel containing a cocktail of phages targeted at MRSA to treat nasal carriage of MRSA |
| Also in development are gels for skin infections and indwelling medical devices | ||
| New Horizons Diagnostics Corporation (USA) | In development – phage-associated enzymes | Lysins to be applied directly to the designated area (limited information available) |
| Biophage Pharma Inc (Canada) | Phage-based products for a range of applications | A large bank of phages is being isolated from natural sources for use in phage therapy applications, PDS® Biosensor and Bactrapping® System. |
| Phico Therapeutics (UK) | SASPject™ | Phico modifies a fully characterised bacteriophage for each type of target bacterium |
| SASPject™ vectors target only bacterial cells |
The recent surge in interest in phage therapy, the successful results in animal models, the shift towards clinical trials and the reducing investment in antibiotics are all indications that this therapy is a viable alternative to antibiotic treatment. However, more research into phage pharmacokinetics, the stability of phages and phage cocktails within delivery formulations and the development, optimisation and characterisation of novel formulations, as well as robust clinical trials, must be undertaken to allow phage therapy to make the much-anticipated leap towards widespread clinical application.
Declarations
Conflict of interest
The author(s) declare(s) that they have no conflicts of interest to disclose.




