Visfatin and resistin: Mediators of the pleiotropic effects of incretins?
Adipocytokines play an important role in the pathogenesis of atherosclerosis through inflammation associated with obesity and metabolic syndrome. In many studies, visfatin and resistin are reported as modulators of insulin sensitivity and inflammatory status in humans. Visfatin has been reported to be a beneficial adipocytokine with insulin mimicking/sensitizing effects. In contrast, resistin was originally reported as a molecule mediating between obesity and insulin resistance. Despite the opposite effect on insulin sensitivity, these two adipocytokines include pro-inflammatory properties.
Visfatin represents a new adipocytokine, which is used because of its predominant production in visceral fat that is also named pre-B-cell colony-enhancing factor or nicotinamide phosphoribosyltransferase, reflecting the pleiotropic effect of this adipocytokine. Some previous papers focused on the relationship between insulin resistance and visfatin action. Visfatin was also identified as a pro-inflammatory and predictive marker; for example, indicating the extent of peripancreatic adipose tissue necrosis and clinical severity in acute pancreatitis.
There is increasing evidence that resistin, as well as visfatin itself, might contribute to inflammatory progression by evoking cytokine production and nuclear factor-kappa B (NF-κB) activation. We showed in a previous paper that resistin polymorphism and serum resistin levels might be a risk marker for stroke susceptibility in Japanese type 2 diabetic patients. Resistin levels in blood might be regulated by genetic factors in Asians, but also resistin itself might affect the progression of atherosclerotic diseases1. We and others reported that serum resistin levels are increased in a genotype-dependent manner based on the RETN polymorphism at –420 (C > G). We also showed that the genotyping of this polymorphism might be a good risk marker for stroke susceptibility in Japanese type 2 diabetic patients in a recent paper1. However, there have been some conflicting reports that do not support a relationship between the blood resistin levels and susceptibility to cardiovascular diseases.
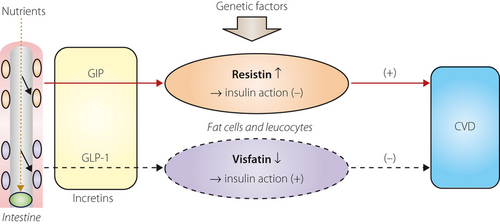
Kim et al.2 previously reported that in 3T3-L1 adipocytes, glucose-dependent insulinotropic polypeptide (GIP), in the presence of insulin, increased resistin secretion through a pathway involving p38 mitogen-activated protein kinase (p38 MAPK) and the stress-activated protein kinase/jun amino-terminal kinase (SAPK/Jun). The other major incretin glucagon-like peptide-1 (GLP-1), however, did not show any significant effects. In in vivo studies of Vancouver Diabetic Fatty Zucker rats, blood GIP and resistin elevation were observed, and the administration of resistin to 3T3-L1 adipocytes mimicked the effects of GIP on the protein kinase B (PKB)/liver kinase B1 (LKB1)/adenosine monophosphate-activated protein kinase (AMPK)/lipoprotein kipase (LPL) pathway. Knockdown of resistin using ribonucleic acid interference attenuated the effect of GIP on the PKB/LKB1/AMPK/LPL pathway in 3T3-L1 adipocytes. From these observations, resistin might be a key mediator of GIP stimulation of lipoprotein kipase (LPL) activity, but not GLP-1. These data might represent a pathophysiological relationship between resistin and GIP.
In a recently published paper in Journal of Clinical Endocrinology and Metabolism, the authors explored the physiological regulation of visfatin release in vivo in healthy, non-diabetic probands and also in an in vitro study using 3T3-L1 adipocytes3. Fasting visfatin levels were not different between sexes or lean/obese individuals, but were negatively correlated with fasting glucose levels. Visfatin levels were rapidly suppressed on an oral glucose load. In vitro experiments showed that hyperglycemia, osmotic stress and sex steroids did not influence visfatin release. In contrast, insulin strongly inhibited visfatin release in vitro by 50%, and this suppression was more pronounced under hyperglycemia. GLP-1 strongly inhibited adipocytic visfatin release by approximately 50%. The authors concluded that insulin and GLP-1 are responsible for the rapid suppression of visfatin levels on an oral glucose intake in healthy probands. Furthermore, they concluded that the inhibitory effect of GLP-1 on adipotic visfatin release in an in vitro study by 3T3L1 cell line together with the absence of direct glucose effects on visfatin release suggests the existence of a novel incretin-like effect represented by a GLP-1–visfatin axis.
This report attracted my interest, because the beneficial effect of GLP-1 on the cardiovascular system has been supposed to work through a non-GLP-1 receptor4. The aforementioned GLP-1–visfatin pathway might contribute to the development of atherosclerotic diseases at least in part.
In a more recently published paper in Diabetologia5, the authors systematically evaluated visfatin expression among several tissues and found that visfatin was predominantly expressed in leukocytes. They also identified higher visfatin protein levels in lysates of granulocytes. They also confirmed the enzymatic activity of visfatin and concluded that leukocytes are a major source of enzymatically active visfatin, which might serve as a biomarker or even a mediator linking obesity, inflammation and insulin resistance. Based on these data, the implications of previous results by Bala et al.3 should be considered more carefully, because in vitro experiments using the 3T3-L1 murine adipocyte cell line cannot be implicated as the only explanation of the suppression of visfatin after rapid oral glucose load. Leukocytes in blood might also respond to GLP-1 stimulated by glucose load, and suppression of visfatin might occur. It is worth mentioning that resistin has also been reported as an inflammatory cytokine expressed dominantly in human macrophages, and has been reported to be elevated in patients with obesity and inflammation. There were some common characteristics observed in these two adipocytokines.
However, the report by Bala et al.3 on the inhibitory effect of GLP-1 on adipocytic visfatin release might lead to new interesting and profound insights into the regulation of inflammatory adipocytokines and pathogenesis of atherosclerosis.
Both adipocytokine visfatin and resistin have similar characteristics as inflammatory mediators of the pleiotropic effect of GLP-1 or GIP on the atherosclerotic process (Figure 1). Experiments using non-active GLP-1 (9–36), which does not activate the GLP-1 receptor, might clarify the role of the possible pathway of the GLP-1–visfatin axis in vitro and in vivo. Elucidating the detail(s) of the GLP-1–visfatin pathway might lead to new therapeutic approaches; that is, for atherosclerotic diseases. As a resistin receptor has not yet been reported, exploration by molecular-biological methods for resistin and GIP is difficult. However, resistin might also be a molecule of therapeutic target, and exploring the relationship between the resistin–GIP pathway might be useful for pathophysiological and clinical settings. Genetic studies on the visfatin and resistin genes, and their mutations, will also contribute to the understanding and explanation of these phenomena.