Better understanding and new insight of genetic risk loci for gestational diabetes mellitus
Gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy, and women with GDM are at increased risk for type 2 diabetes mellitus1. Uncomplicated pregnancy is characterized by insulin resistance and enhanced insulin secretion as a compensatory mechanism to maintain normal glucose tolerance (NGT). However, previous studies showed that in women with GDM during pregnancy, the decreased sensitivity of insulin is at a similar level to NGT, but with more pronounced insulin resistance than NGT. These phenomena might contribute to hyperglycemia in addition to defective insulin release2. Furthermore, women with a history of GDM have been considered a high-risk group for diabetes mellitus. A study reported from Korea showed that Korean women with a history of GDM have a four- to fivefold greater risk of developing postpartum diabetes than the general population (Figure 1). The incidence of type 2 diabetes mellitus within <2 years postpartum is reported to be approximately 10–15% in Korean women. A prospective follow-up study on Korean women with GDM showed that approximately 40% of women with previous GDM were expected to develop diabetes within 5 years postpartum. However, some studies have reported lower incidence rates. This variability might be related to the ethnic variation, lack of uniformity in diagnostic criteria for GDM, diversity in follow-up care, characteristics of women lost to follow up, and differences in statistical management of data. Despite the high rate or an increased risk of type 2 diabetes mellitus after GDM, the putative risk factors are yet to be identified, but many risk factors have been identified among the various ethnic groups. These risk factors include homocysteine level, older age (>30 years), prepregnant body mass index (>23 kg/m2), family history of type 2 diabetes mellitus, fasting glucose level at the time of GDM diagnosis (>5.8 mmol/L) and early diagnosis of GDM during pregnancy (<26 weeks)3. In contrast, the putative risk factors for type 2 diabetes mellitus in the Korean population include (but are not limited to) old age, urban living, female sex, obesity, smoking, family history of diabetes, impaired liver function, metabolic syndrome, elevated blood pressure and increased triglycerides4. Although all the risk factors have yet to be identified, the aforementioned variables are the key risk factors for diabetes mellitus in Koreans. Most of these risk factors have also been identified as key risk factors in other places including Europe, South America, North America and Africa. As previously mentioned, some of the risk factors, such as family history of diabetes, lipid profile, high blood pressure and obesity, are common risk factors of both type 2 diabetes mellitus and GDM. Thus, one could question whether GDM is another type of diabetes mellitus or a simple identification of existing type 2 diabetes mellitus during pregnancy when physical, metabolic and psychological changes occur dramatically. It was very difficult to answer the question until the advancement of the Genome-Wide Association Study (GWAS), which enabled the investigation of genetic background. The genome-wide association (GWA) approach is an emerging methodology that allows us to identify genetic variants with specific loci that predispose individuals to complex traits and/or diseases.
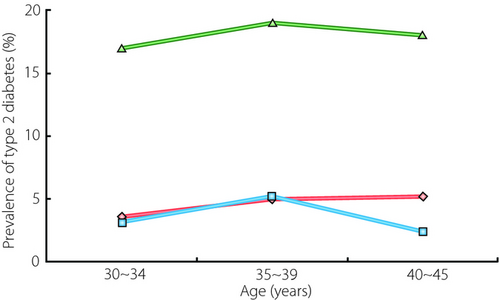
 ,
Prevalence of diabetes in women after gestational diabetes mellitus;
,
Prevalence of diabetes in women after gestational diabetes mellitus;  , prevalence of diabetes in women without a history of gestational diabetes mellitus surveyed in 1998;
, prevalence of diabetes in women without a history of gestational diabetes mellitus surveyed in 1998;  , prevalence of diabetes in women without a history of gestational diabetes mellitus surveyed in 2001.
, prevalence of diabetes in women without a history of gestational diabetes mellitus surveyed in 2001.- small effect size found from the reported GWA studies;
- no insulin resistance gene was found in GDM;
- starting to identify other genes that are specific to GDM.
First, the strength of GWA effect size is not as strong as some of the risk factors, such as family history of diabetes. Therefore, we are inclined to believe that the link between diabetes and genetic susceptibility is genuine; however, it is not a key risk factor for both type 2 diabetes mellitus and GDM. Although the effect sizes are different, the putative risk factors identified from Korean studies are very similar to the risk factors identified from other countries. Furthermore, the genes identified through genome-wide association studies carried out in both Asian and Caucasian populations showed a lower effect size. These genetic studies reported a relative risk of 1.5 or less, indicating that no single gene is associated with the risk of type 2 diabetes mellitus and GDM; however, a strong interaction between genetic factors, and lifestyle, diet and habitual factors were suggested and evident in some studies. In addition, relative risk (RR) and odds ratio (OR) are statistical values that only describe association, not causation. RR and OR also refer to a population and not individual patients. Furthermore, studies of small groups are more likely to find an association that might actually be a result of chance, whereas larger groups are less likely to show an association between a risk factor and an outcome. Finally, when the incidence of an outcome of interest in the study population is low (<10%), OR becomes close to RR; this means that the more frequent the outcome becomes, the more likely OR will overestimate RR when OR is >1 or underestimate RR when OR is <1.
Second, no insulin resistance gene was found in GDM. The reason for these phenomena might be explained by normal pregnancy being characterized by insulin resistance and enhanced insulin secretion as a compensatory mechanism to maintain NGT. Although there were limited numbers, new genes were identified only in the GDM population. Thus, these findings also support that GDM is caused by many different mechanisms and associated with other genes that are yet to be identified.
In conclusion, although genetic factors are independently associated with an onset of type 2 diabetes mellitus even in a high-risk subpopulation, such as women with a previous history of GDM, the strength of an association is less than that of the reported environmental risk factors. Therefore, it is very difficult to be convinced that genes are the sole factors responsible for the disease, but it is rather probable that there might be several channels that lead to the pathogenesis of both type 2 diabetes mellitus and GDM. For example, some cases involved environmental factors only, some genetic factors only, but most cases likely involved gene–environmental interaction as the cause of type 2 diabetes mellitus, as well as GDM. Furthermore, the present study gives new insight into the pathophysiology of GDM in which the genetic component plays a major role.
Acknowledgement
The authors declare no conflict of interest.




