Prevalence and Genetic Diversity of Anaplasma marginale Strains in Cattle in South Africa
Summary
Bovine anaplasmosis, caused by the tick-borne rickettsia Anaplasma marginale, is endemic in South Africa and results in considerable economic loss to the cattle industry. This study was designed to characterize strains of A. marginale at the molecular level from cattle raised in communal and commercial farms in the north-eastern and south-western regions of the Free State Province, South Africa, that varied in rainfall and vegetation. Seroprevalence to A. marginale was determined in 755 cattle by an Anaplasma spp. competitive enzyme-linked immunosorbent assay and ranged from 44% to 98% and was similar in both regions. While Anaplasma centrale was not targeted in this study, A. marginale infections were identified by species-specific msp1α polymerase chain reaction in 129 of 215 of the samples studied. Similar genetic diversity of A. marginale strains was found in both the north-eastern and south-western regions. The sequences of 29 A. marginalemsp1α amplicons from South African strains revealed considerable genetic diversity providing 14 new repeat sequences. However, 42% of MSP1a repeat sequences were not unique to this region. These results indicated the presence of common genotypes between South African, American and European strains of A. marginale. Cattle movement between different parts of South Africa was suggested by the presence of identical A. marginale MSP1a genotypes in north-eastern and south-western regions of the Free State Province. Control strategies for anaplasmosis in South Africa should therefore be designed to be protective against genetically heterogeneous strains of A. marginale.
Introduction
Anaplasmosis or gallsickness is a tick-transmitted disease of cattle caused by the intraerythrocytic rickettsia, Anaplasma marginale (Rickettsiales: Anaplasmataceae). Anaplasma centrale, also infective for cattle, causes a milder form of anaplasmosis and is used as a live vaccine in many areas of the world including Israel, South Africa, South America and Australia (Callow and Dalgliesh, 1980; Pipano et al., 1986; Kocan et al., 2003).
Ticks Boophilus decoloratus, Boophilus microplus, Hyalomma marginatum rufipes, Rhipicephalus evertsi evertsi and Rhipicephalus simus have been implicated as vectors of A. marginale in South Africa (Dreyer et al., 1998). Anaplasmosis is endemic throughout most of South Africa and Namibia, except in the low-rainfall areas where tick populations are minimal (De Waal, 2000). Seasonal incidence of anaplasmosis outbreaks occur more frequently during summer and fall because of the increased abundance of ticks and blood-sucking flies and is also affected by rainfall and the implementation of tick-control measures (Kocan et al., 2000). Losses because of anaplasmosis result from reduced weight gains, reduction in milk production, abortion, increased veterinary costs and mortality (Kocan et al., 2003).
Cattle farming in the Free State Province, South Africa, varies in scale and practices from commercial to small scale communal resource-poor farming systems. During the day, cattle graze on communal pastures for approximately 7–9 h, which may vary according to season. At night cattle are herded into kraals with a manure floor (Mtshali et al., 2004).
Geographic isolates of A. marginale have been identified which differ in biology, morphology, protein sequence, antigenic characteristics and transmissibility by ticks and have been characterized by the major surface proteins (MSPs) 1a and 4. MSP1a varies in sequence and molecular weight because of different numbers of tandem 28–31 amino acid repeats (reviewed by de la Fuente et al., 2005a). The MSP1a tandem repeats are located after a conserved decapeptide in the amino terminal region of the protein and are exposed extracellularly for interaction with host cell receptors (de la Fuente et al., 2003a). The frequency of variable amino acid positions within geographic isolates is higher in this region than in the rest of the protein (de la Fuente et al., 2001a). While msp1α has not been identified in A. centrale, msp4 sequence allows for differentiation of A. marginale from A. centrale (de la Fuente et al., 2005a). Despite the importance of anaplasmosis in South Africa and other African countries, A. marginale strains have not been genetically characterized in Africa. The study reported herein was designed to characterize the genetic diversity of A. marginale strains from cattle from the north-eastern and south-western regions of the Free State Province, South Africa, where cattle production varies from communal to commercial farming systems.
Materials and Methods
Study area
Cattle farms in the north-eastern and south-western regions of the Free State Province of South Africa were included in the study. The north-eastern Free State (Fig. 1) is predominantly a hilly and mountainous area situated between latitudes 28° and 30°S, 28° and 30°E. This region is bounded to the south by Lesotho and to the east by KwaZulu-Natal, and is situated at a height of about 1500 to more than 3000 m above sea level (Kritzinger and Pieterse, 1987). The summer rainfall in the north-eastern region during the period of September–March is more than 85% of the annual precipitation. The north-eastern region is in the grassland biome, with five vegetation types, namely moist cool highveld, wet cold highveld, afro-montane and alti-montane grassvelds (Moffett, 1997). The south-western Free State region (Fig. 1) is the hotter and more arid area of the province. Average summer temperature is 23°C and the average winter temperature is 8°C. January is the hottest month with a temperature range of 15–32°C, while June is the coldest, it ranges from a cold 1°C to a mild 17°C, and the average annual rainfall is 500–600 mm (Mostert et al., 1971). Both the north-eastern and south-western regions have similar farming systems which include communal and commercial farming systems.
A map of the Free State Province, South Africa, with the two study regions encircled.
Blood sample collection
Twenty-nine cattle farms in the north-eastern and south-western regions of Free State Province, South Africa, were included in the study (Table 1). Farms were selected for the study in A. marginale endemic areas that covered the main characteristics of cattle husbandry and production in South Africa, including commercial and communal farming systems. All cattle ≥1 year old were included in the study. Blood was collected from 755 cattle into sterile tubes with and without anti-coagulant (lithium heparin) and maintained at 4°C until arrival at the laboratory. Plasma and serum were then separated after centrifugation and stored at −20°C.
| Location (farm) | Farming system | Seroprevalence Anaplasma spp., positive cELISA/total (%) | Prevalence Anaplasma marginale, positive PCR/total (%) |
|---|---|---|---|
| North-eastern Free State | |||
| Bethlehem (Africaskop) | Commercial | 18/25 (72) | 17/22 (77) |
| Anndeil | Commercial | 17/20 (85) | 9/11 (82) |
| Bethlehem (Versien) | Commercial | 38/40 (95) | 7/8 (88) |
| Bethlehem (Retreat) | Commercial | 19/20 (95) | 0/5 (0) |
| Endor | Commercial | 19/20 (95) | 5/9 (56) |
| Harrismith (Uithoek) | Commercial | 19/25 (76) | 17/22 (77) |
| Kestell | Commercial | 41/45 (91) | ND |
| Plelegraaf | Commercial | 15/20 (75) | 4/8 (50) |
| Reitz | Commercial | 26/30 (87) | ND |
| Harrismith (Saaihoek) | Commercial | 17/20 (85) | 2/4 (50) |
| Ficksburg | Communal | 61/70 (87) | ND |
| Fouriesburg | Communal | 46/60 (77) | 22/26 (85) |
| Harrismith (Khalanyoni) | Communal | 49/50 (98) | ND |
| QwaQwa | Communal | 48/50 (96) | ND |
| South-western Free State | |||
| Baphurst | Commercial | 13/15 (87) | 7/8 (88) |
| Bloemspruit | Commercial | 18/20 (90) | 5/9 (57) |
| Kaalspruit | Commercial | 11/25 (44) | 0/11 (0) |
| Lappiesland | Commercial | 12/15 (80) | 5/5 (100) |
| MacDaline | Commercial | 23/30 (77) | 14/20 (70) |
| Martindale | Commercial | 16/20 (80) | 6/9 (67) |
| Botshabelo | Communal | 21/25 (84) | ND |
| Phillipolis | Communal | 49/50 (98) | 14/38 (38) |
| Thaba Nchu | Communal | 35/40 (88) | ND |
| Trompsburg | Communal | 17/20 (85) | ND |
- cELISA, competitive enzyme-linked immunosorbent assay; ND, not determined; PCR, polymerase chain reaction.
Serology
The anaplasmosis competitive enzyme-linked immunosorbent assay (cELISA) was performed using the Anaplasma specific cELISA (VMRD, Inc., Pullman, WA, USA) following the manufacturer's instructions. This cELISA specifically detects the presence of serum antibodies that targets the MSP5 protein of Anaplasma spp. (Knowles et al., 1996). Per cent inhibition values >30% were considered positive for this assay (de la Fuente et al., 2003b).
DNA extraction, PCR and sequence analysis
DNA was extracted from 0.5 to 1 ml erythrocytes using the QIAamp blood kit (Qiagen, Hilden, Germany). The DNA was resuspended in sterile distilled water and stored at −20°C until used in PCRs. The msp1α and msp4 genes were amplified from 2 to 5 μl (1–10 ng) A. marginale DNA by PCR using 10 pmol of each primer (MSP1a, MSP1aP: 5′GCATTACAACGCAACGCTTGAG3′ and MSP1a3: 5′GCTTTACGCCGCCGCCTGCGCC3′ and MSP4, MSP45: 5′GGGAGCTCCTATGAATTACAGAGAATTGTTTAC3′ and MSP43: 5′CCGGATCCTTAGCTGAACAGGAATCTTGC3′) in a 50-μl volume (1.5 mm MgSO4, 0.2 mm dNTP, 1X AMV/Tfl 5X reaction buffer, 5u Tfl DNA polymerase) employing the Access RT-PCR system (Promega, Madison, WI, USA) (de la Fuente et al., 2001b, 2003b). Reactions were performed in an automated DNA thermal cycler (Eppendorf Mastercycler® personal, Westbury, NY, USA) for 35 cycles. After an initial denaturation step of 30 s at 94°C, each cycle consisted of a denaturing step of 30 s at 94°C and an annealing-extension step of 2.5 min at 68°C (msp1α) or a denaturing step of 30 s at 94°C, annealing for 30 s at 60°C and an extension step of 1 min at 68°C (msp4). The programme ended by storing the reactions at 10°C.
Amplified fragments were resin purified (PureLink, Invitrogen, Carlsbad, CA, USA) and cloned into pGEM-T vector (Promega) or used directly for sequencing both strands by double-stranded dye-termination cycle sequencing (Core Sequencing Facility, Department of Biochemistry and Molecular Biology, Noble Research Center, Oklahoma State University). Only the fragment containing the upstream and variable regions of the msp1α gene was sequenced. When cloned, at least three clones were sequenced from each PCR. Multiple sequence alignment was performed with the programme AlignX (Vector NTI Suite, version 5.5; InforMax, North Bethesda, MD, USA) with an engine based on the Clustal W algorithm (Thompson et al., 1994).
Statistical analysis
The observed prevalence of A. marginale infections in cattle herds was analyzed by cELISA and PCR and sequence analysis of msp1α amplicons. Non-parametric Mann–Witney tests were employed to test statistical differences in A. marginale prevalence. Comparisons were conducted (i) between north-eastern and south-western regions, (ii) between production systems between regions and (iii) between farming systems within each region. Anaplasma marginale prevalence was log10-transformed and included as dependent variable in analyses where geographic location or farming systems were considered the factor. Paired comparisons of the genetic diversity of A. marginale strains were conducted between north-eastern and south-western regions using the genetic diversity index described in Table 2 by two-by-two chi-squared tests. The statistical analyses were performed using the SPSS 11.0 statistical programme (SPSS Inc., Chicago, IL, USA). The differences were considered statistically significant at P ≤ 0.05. The confidence intervals (CI) and standard errors (SE) at 95% confidence level of the genetic diversity of A. marginale strains were calculated for G1 and G3 (both index are true proportions of binomial presence in each strain) based on Martin et al. (1987).
| Regiona | No. of strainsb | No. of MSP1a repeatsc | No. of MSP1a repeat sequencesd | No. of MSP1a genotypese | Genetic diversity index (GD) | |||
|---|---|---|---|---|---|---|---|---|
| GD1f | GD2g | GD3h | combined GDi | |||||
| North-east | 16 | 6 | 17 | 13 | 38 ± 24 | 106 | 81 ± 19 | 3263 |
| South-west | 13 | 4 | 14 | 10 | 31 ± 25 | 108 | 77 ± 23 | 2578 |
| South Africa | 29 | 7 | 24 | 20 | 24 ± 8 | 83 | 69 ± 9 | 1374 |
- CI, confidence interval; MSP1a, major surface protein (1a); SE, standard error.
- aThe analysis was done for north-eastern and south-western regions of Free State and for all strains from South Africa.
- bNumber of A. marginale strains analyzed.
- cNumber of different counts of MSP1a repeats in A. marginale strains.
- dNumber of different MSP1a repeat sequences in A. marginale strains.
- eNumber of different MSP1a genotypes.
- fGD1 = (no. of MSP1a repeats/no. of strains) × 100, expressed as per cent diversity ±SE at 95% CI.
- gGD2 = (no. of MSP1a repeat sequences/no. of strains) × 100.
- hGD3 = (no. of MSP1a genotypes/no. of strains) × 100, expressed as per cent diversity ±SE at 95% CI.
- iCombined GD = (GD1 × GD2 × GD3)/100.
Sequence accession numbers
The GenBank accession numbers for msp1α sequences of A. marginale strains are DQ813536–DQ813564.
Results and Discussion
Seropositivity for Anaplasma in the combined study areas as determined by cELISA was detected in 648 of the 755 cattle studied and ranged from 44% to 98% (mean ± SD, 84 ± 12) (Table 1). The observed seroprevalence was similar in the north-eastern (87 ± 9) and south-western (81 ± 14) regions (Mann–Witney U = 47.5, P = 0.19).
Anaplasma marginale infections were analyzed by species-specific msp1α PCR in 215 seropositive cattle from the study area. Anaplasma marginale infections were identified in 129 of the 215 cattle analyzed (Table 1). The observed prevalence of A. marginale infections in cattle herds ranged from 0% to 100% (mean ± SD at 95% CI, 62 ± 29) (Table 1). Again, the observed prevalence of A. marginale infections was similar in north-eastern (63 ± 28) and south-western (60 ± 33) regions (Mann–Witney U = 33.5, P = 0.66). Differences in the prevalence of A. marginale were not observed between commercial and communal farming systems (cELISA, Mann–Witney U = 48.8, P = 0.35; PCR, Mann–Witney U = 11.0, P = 0.48) and within (cELISA, north-east, Mann–Witney U = 16.0, P = 0.19; south-west, Mann–Witney U = 5.5, P = 0.16) regions.
The results of A. marginale observed prevalence in South Africa were similar to those obtained in Sicily, Italy and Spain using similar analyses, which confirms the high seropositivity and observed prevalence of A. marginale infections in endemic areas (de la Fuente et al., 2005b,c). The discrepancies between serology and PCR results could be explained by the absence of detectable levels of rickettsemias in individual samples, a finding particularly common among persistently infected carrier cattle (Kocan et al., 2004; de la Fuente et al., 2005b). However, the cELISA does not differentiate between A. marginale and A. centrale (Dreher et al., 2005). Anaplasma centrale is used as a live blood vaccine in South Africa and co-infection of A. marginale and A. centrale can occur in cattle (Molad et al., 2006). However, the only Anaplasma spp. targeted in this study was A. marginale.
The sequence of A. marginalemsp1α amplicons was analyzed in 29 strains (Figs 2a and b). The South African A. marginale strains showed high genetic diversity as evidenced by the sequence analysis of MSP1a (Table 2). The MSP1a sequences varied in the number and sequence of the repeats, as previously reported for other regions where bovine anaplasmosis is endemic (reviewed by de la Fuente et al., 2005a). The MSP1a of South African strains of A. marginale revealed 14 new repeat sequences (designated as 33–46; Fig. 2a) when compared with sequences reported previously (de la Fuente et al., 2005a and de la Fuente et al., submitted data). Statistical analysis of genetic diversity of A. marginale strains did not result in differences between north-eastern and south-western regions (G1 index, Chi2 = 0.14, P = 0.70; G2 index, Chi2 = 0.00, P = 0.97; G3 index, Chi2 = 0.08, P = 0.77).
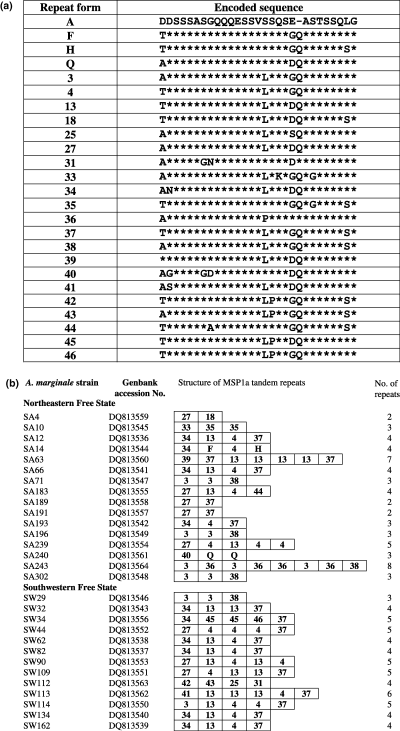
Sequence of MSP1a tandem repeats in South African strains of A. marginale. (a) The one letter amino acid code was used to depict the different sequences found in MSP1a repeats. Asterisks indicate identical amino acids and gaps indicate deletions/insertions with respect to the reference repeat (a). (b) The structure of the MSP1a repeats region was represented using the repeat forms described in (a) for South African strains of A. marginale. Description of MSP1a repeats was updated after de la Fuente et al. (2005a).
In contrast to msp1α sequence results, the sequence of msp4 was identical for all A. marginale strains analyzed from north-eastern and south-western Free State (n = 6) and also identical to the Israel non-tailed strain (GenBank accession No. AY786993), but different in one nucleotide (G × A at position 607 with respect to the adenine in ATG translation initiation codon) from the previously reported sequence of South African strain SWA (AY666005). These results are in agreement with previous reports in which genetic diversity of A. marginalemsp1α was higher than that of msp4 (de la Fuente et al., 2003c, 2005a).
As a result of the high degree of sequence variation within endemic areas, MSP1a sequences failed to provide phylogeographic information (de la Fuente et al., 2002a, 2005a). As discussed previously (de la Fuente et al., 2005a), the genetic heterogeneity observed among strains of A. marginale within endemic regions could be explained by cattle movement and maintenance of different genotypes by independent transmission events, because of infection exclusion of A. marginale in cattle and ticks which commonly results in the establishment of only one genotype per animal (de la Fuente et al., 2002b, 2003d). However, when distantly related genotypes exist in the same region, infections of a single host with multiple A. marginale strains may be possible (Palmer et al., 2004).
In previous studies (de la Fuente et al., 2005a), we have suggested that multiple introductions of A. marginale strains from different geographic locations may have occurred in some regions. In South African A. marginale strains, 42% (10/24) of MSP1a repeat sequences were not unique to this region. These results indicated the presence of common genotypes between South African, American and European strains of A. marginale. Cattle movement between different parts of South Africa was suggested by the presence of identical A. marginale MSP1a genotypes in north-eastern and south-western regions of the Free State Province (Fig. 2b).
Despite the fact that most regions in Africa are endemic for bovine anaplasmosis, this is the first report of the genetic diversity of A. marginale strains on this continent. Characterization of infection prevalence and the diversity of A. marginale strains reported herein are fundamental to the design of epidemiological studies and control strategies for A. marginale. These results suggested that farming systems have little or no influence in the observed prevalence of A. marginale in South Africa and demonstrated the need for anaplasmosis control measures and vaccines in South Africa that are protective against genetically heterogeneous populations of A. marginale.
Acknowledgements
We gratefully acknowledge the cooperation of farmers from the Free State Province in the collection of blood samples from their cattle. We sincerely thank Fumane Nyaile, Mpho Sellane, Puleng Moloi, Nthabiseng Lisene and Bonolo Hlasa for their assistance with data collection. We would like to also express appreciation to Merrs Moletsane and Mokoena for transporting us to the study sites. This study was funded by the National Research Foundation (GUN 2070102), US-AID Cooperative Development Research (CDR) Grant No. TA-MOU-01-C21–027, the University of the Free State, QwaQwa Campus and Tick and Tick-borne Pathogen Vaccine Development Laboratory, Department of Veterinary Pathobiology, Center for Veterinary Health Sciences and Oklahoma Agricultural Experiment Station Project 1669, Oklahoma State University, Stillwater, OK, USA.




