Survey on the Listeria Contamination of Ready-to-Eat Food Products and Household Environments in Vienna, Austria
Summary
Qualitative and quantitative contamination of ready-to-eat food-stuffs with the pathogen Listeria monocytogenes was studied in 1586 samples collected from 103 supermarkets (n = 946) and 61 households (n = 640) in Vienna, Austria. Seventeen groups of ready-to-eat foods were classified into three risk categories for contamination (CP1–CP3). Three to four samples were randomly collected at the retail level from each CP. Regarding the households, the sampling procedure was started with food items of CP1, and if not available, was continued with sampling of food items of CP2 and finally of CP3. Additionally, 184 environmental samples (swabs from the kitchen area, dust samples from the vacuum cleaner) and faecal samples (household members and pet animals) were included. One-hundred and twenty-four (13.1%) and 45 (4.8%) samples out of 946 food samples collected from food retailers tested positive for Listeria spp. and L. monocytogenes, respectively, with five smoked fish samples exceeding the tolerated limit of 100 CFU/g food. Food-stuffs associated with the highest risk of contamination were twice as frequently contaminated with L. monocytogenes as food-stuffs associated with a medium risk of contamination. Products showing the highest contamination rate were fish and seafood (19.4%), followed by raw meat sausages (6.3%), soft cheese (5.5%) and cooked meat products/patés (4.5%). The overall contamination rate of foods collected at the household level was more than two times lower. Only 5.6% and 1.7% of 640 food-stuffs analysed tested positive for Listeria spp. and L. monocytogenes, respectively. However, CP1 foods were rarely collected. Pulsed-field gel electrophoresis (PFGE) typing of the collected L. monocytogenes isolates revealed a high degree of diversity between the isolates, with some exceptions. PFGE typing of isolates harvested from green-veined cheese revealed a match among strains, although the manufacturer seemed to be distinguishable. Typing of household strains revealed an epidemiological link within one family. In this case, food-stuffs and the kitchen environment were contaminated by an indistinguishable isolate. In addition, the same isolate was collected from a pooled faecal sample of the household members suggesting that consumption of even low contaminated food items (<100 CFU/g) results in Listeria shedding after the passage through the gut.
Introduction
Mainly as a result of food plant contamination, L. monocytogenes predominantly enters the food chain in the post-harvest area (Norton et al., 2001). Therefore, surveys on food contamination have to consider the fate of Listeria during processing and at post-processing stages. The consumers’ risk because of L. monocytogenes contamination is increased by the adaptation of the bacteria to the environmental conditions otherwise protective in food technology, such as low pH and high salt contents (Lou and Yousef, 1999). As plant environment contamination is the initial point of the contamination chain, L. monocytogenes can be found in various types of food chains including dairy, meat and fish processing chains. Therefore, a survey on L. monocytogenes contamination of food products should not be limited to a particular food group of concern, even ready-to-eat (RTE) food-stuffs are generally considered as vehicles for transmission of L. monocytogenes to the consumer. According to the Austrian Codex Alimentarius, RTE-foods comprise 17 groups of foods. Sampling of such a large number of food groups is challenging, especially when considering large retail premises where a large variability of food types is offered. This holds true also for sampling actions to be taken at the household level. Moreover, data on the qualitative and quantitative occurrence of L. monocytogenes in food-stuffs stored in households are, although unequivocally needed, unfathomably rare. We, therefore, were interested in determining the presence of L. monocytogenes in two pivotal elements of the food chain, the retail level and the household level. To provide a comprehensive view on a contamination scenario, food at lower and higher risk for contamination should be represented in the sample collection. Thus, a sampling scheme based on predictions of possible contamination was used. To explore possible contamination chains the recovered isolates were finally subjected to molecular typing, by pulsed-field gel electrophoresis (PFGE). The data achieved are the first describing Listeria contamination of RTE-foods in Austria.
Materials and Methods
Materials
Ranking of food-stuffs according to probability of contamination
From April 2003 to February 2004, we determined the occurrence of L. monocytogenes in RTE-food-stuffs collected at the retail and household level, and investigated environmental samples which were exclusively collected from the household environments.
Seventeen groups of RTE-foods were assigned to risk-associated groups considering: (i) previous associated outbreaks, (ii) evidence for elevated contamination rates from private Austrian food investigation laboratories and (iii) the potential of the food-stuff to foster growth of Listeria. After a careful consideration of each of the three aspects, a score of zero (no relevance) to three points (high relevance) was allocated to the groups of RTE-foods. Groups of food-stuffs allocated seven to nine points in total ranked high with respect to the probability for L. monocytogenes contamination (CP1), whereas groups of foods of a lower impact, (5–6 and ≤4 points) were assigned to categories of medium contamination probability (CP2) and low contamination probability (CP3), respectively. The scheme was helpful in achieving a representation of high-risk foods and low risk foods in the sample collections at the supermarket and the household level.
Collection of samples at retail and household levels
Eight to nine RTE samples were randomly collected from 103 supermarkets and retail outlets (in total 946 food samples, approximately three to four food samples for each of CP1, CP2, and CP3). According to the register of the Vienna Chamber of Trade, the sampled food shops represented 6.3% of all food shops located in the Viennese urban area. At the household level, six to seven food samples were taken from the refrigerators of 61 households, beginning with the sampling of CP1 food-stuffs, and if not stored there, proceeding to CP2 and CP3 food-stuffs (n = 640). Four out of the 61 households were sampled every fortnight during 5 months, and 57 households were sampled only once. This resulted in a total of 93 sampling events (nSE = 93). The household types sampled were: (i) households of singles (nSE = 29), (ii) households of families (nSE = 33), (iii) households of vegetarians (nSE = 12), (iv) households of elderly people (>65 years, nSE = 10) and (v) a household of a citizen with a foreign cultural background (nSE = 9).
To predict the occurrence of listeriae in the food preparation area and household environment, a swab sample from the kitchen surface (the area around the sink was swabbed with a moisturized sterile cotton pad of a size of 5 × 6 cm) and approximately 3 g of floor dust from the vacuum cleaner were collected. A pooled faecal sample including 1–2 g faecal material of all individuals living in the household was included. If the apartment was shared with pets, faecal material of the animal was collected. In total, 184 environmental samples (92 swab and dust samples each), 90 faecal samples from household members and 27 faecal samples from pets were collected for analysis.
Methods
Microbiological and molecularmicrobiological methods
Twenty-five grams of a food sample and 1 g of a faecal or dust sample were added to pre-warmed (30°C) Half-Frazer broth (BioMerieux, Marcy D‘Etoile, France) stored in stomacher bags (1 : 10 dilution, w/v). Cotton pads were put into a sterile plastic bag and 100 ml of Half-Frazer broth was added. The stomacher bags were stomached twice for 3 min at medium speed. The two-step enrichment was performed using the standard protocols (ISO 11290-1 and ISO 11290-2; Anonymus, 1996 and Anonymus, 1998, respectively). Instead of using the recommended Palcam and Oxford media (all agars except ALOA agar: Oxoid, Basingstoke, UK) for plating, Palcam and the chromogenic medium ALOA (Biolife, Milan, Italy) were tested. Part of presumptively positive colonies were picked from the Palcam and ALOA media with a loop and confirmed using the polymerase chain reaction (PCR) (Bubert et al., 1999). Additionally, part of the same colony was streaked on to TSA agar and used for subtyping by PFGE, in cases where L. monocytogenes was identified by PCR (Graves and Swaminathan, 2001).
Food samples shown to be positive for L. monocytogenes after qualitative analysis were stored at 4°C until the end of the products shelf-life. The number of L. monocytogenes was then re-quantified according to ISO 11290-2.
Statistical analysis
Data were compared using either the χ2 test or the Fisher-Exact test (http://www.physics.csbsju.edu/stats/contingency_NROW_NCOLUMN_form.html).
Results
Results from samples collected at the retail level
One-hundred and twenty-four (13.1%) and 45 (4.8%) samples out of the 946 food samples collected from food retailers tested positive for Listeria spp. and L. monocytogenes, respectively. Groups of food-stuffs assigned to the category with the highest CP were about two times more frequently contaminated with L. monocytogenes than food-stuffs assigned to the CP 2 (Fig. 1). These differences were statistically significant (CP1 versus CP2: P =0.003; CP1 versus CP3: P < 0.0001). Product groups showing the highest contamination rate with L. monocytogenes were fish and seafood (19.4%), followed by soft cheese (5.5%), raw meat sausages (4.9%), and cooked meat products/pates (4.5%) (Table 1). Within the categories fresh cheese, meat/cheese spread and fermented and cured meat products, only one sample each was positive for L. monocytogenes. Food samples drawn from the remaining nine groups, all of them of non-meat origin, tested negative for the presence of L. monocytogenes. With respect to the prevalence of L. monocytogenes in re-packaged or unpackaged foods, the type of outlet, either supermarket or small-size food shop, did not result in a statistically significant difference in prevalence (P = 0.43). Overall, packaged foods were at an average twice as frequently contaminated as unpackaged food items (Table 2). However, when ignoring the group of fish products which were invariably sold packaged, the contamination rate of the unpackaged and the repackaged food-stuffs (3.2% and 5%, respectively) were more frequently contaminated than the packaged food-stuffs. However, this difference was not statistically significant (P = 0.328).
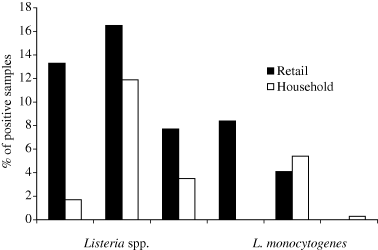
Prevalence of Listeria spp. in groups of food of a high contamination probability (CP1), medium contamination probability (CP2) and low contamination probability 3 (CP3).
| Food group | CP* | Samples analysed | Samples positive for | ||||
|---|---|---|---|---|---|---|---|
| R* | H* | Listeria spp. | L. monocytogenes | ||||
| R (%) | H (%) | R (%) | H (%) | ||||
| Fish/seafood | 1 | 93 | 3 | 29 (31.2) | 0 (0) | 18 (19.4) | 0 (0) |
| Raw meat sausage | 2 | 144 | 37 | 36 (25.0) | 7 (21.9) | 7 (4.9) | 4 (10.8) |
| Soft cheeses | 1 | 200 | 33 | 17 (8.5) | 1 (3) | 11 (5.5) | 0 (0) |
| Cooked sausage-Patè | 1 | 112 | 23 | 8 (7.1) | 0 (0) | 5 (4.5) | 0 (0) |
| Fresh cheese | 3 | 25 | 27 | 1 (4.0) | 1 (3.7) | 1 (4.0) | 0 (0) |
| Meat/cheese spread | 2 | 34 | 53 | 7 (20.6) | 3 (5.66) | 1 (2.9) | 1 (1.9) |
| Fermented sausages | 2 | 65 | 50 | 4 (6.2) | 1 (2) | 1 (1.5) | 1 (2) |
| Cured meat products | 2 | 77 | 45 | 6 (7.8) | 11 (24.4) | 1 (1.3) | 4 (8.9) |
| Sandwiches | 3 | 10 | 0 | 4 (40.0) | 0(0) | 0 (0) | 0 (0) |
| Salad | 3 | 60 | 34 | 6 (10.0) | 6 (17.6) | 0 (0) | 0 (0) |
| Spices and sprouts | 3 | 28 | 0 | 3 (13.6) | 0 (0) | 0 (0) | 0 (0) |
| Sheep milk cheese | 3 | 27 | 9 | 1 (8.3) | 1 (3.7) | 0 (0) | 0 (0) |
| Produce | 3 | 33 | 110 | 1 (3.0) | 1 (0.9) | 0 (0) | 0 (0) |
| Other | 3 | 12 | 193 | 1 (8.3) | 4 (2.2) | 0 (0) | 1 (1.6) |
| Fruits | 3 | 11 | 7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Seeds | 3 | 7 | 7 | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) |
| Dried fruits | 3 | 8 | 9 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 946 | 640 | 124 (13.1) | 37 (5.8) | 45 (4.8) | 11 (1.7) | |
- Table 1 lists the total numbers collected from the R- and H-stage.
- *CP, contamination probability; R, retail level; H, household level.
| Packaged samples | Re-packaged samples | Unpackaged samples | ||||
|---|---|---|---|---|---|---|
| Retail n (%) | Household n (%) | Retail n (%) | Household n (%) | Retail n (%) | Household*n (%) | |
| Listeria spp. | 279 (19.7) | 84 (3,57) | 137 (11.6) | 31 (9.68) | 289 (12.8) | 437 (8.92) |
| Listeria monocytogenes | (8.6) | (0.0) | (6.6) | 0.0 | (4.2) | (2.51) |
- *Includes both unpackaged and opened food-stuffs.
The numbers of L. monocytogenes detected in the food-stuffs were <100 CFU/g for all food samples tested, with the exception of five smoked fish products which contained L. monocytogenes at contamination levels between 200 and 30 000 CFU/g. PFGE analysis of 45 L. monocytogenes isolates recovered from retail food-stuffs revealed 24 distinguishable pulsetypes (Table 3). The detailed analysis of the genetic profiles revealed that frequently contaminated products such as gorgonzola-type green veined cheese or salmon samples, although retailed at different premises, showed a contamination with genetically indistinguishable isolates of pulsetypes ‘e’ and ‘r’, respectively. Evidence for cross-contamination at the retail level (cases where genetically indistinguishable L. monocytogenes were recovered from more than one unpackaged or re-packaged sample per retail outlet) was not observed.
| Origin | Isolate | Sample | Food item | Package type | PT* |
|---|---|---|---|---|---|
| SM-A | 8 | 14 | Roquefort | Re-packaged | a |
| FS-1 | 9 | 92 | Duck in Aspique | Open | b |
| 10 | 93 | Veal liver patè | Open | b | |
| SM-B | 11 | 140 | Brie | Re-packaged | c |
| SM-C | 12 | 165 | Veal liver patè | Open | d |
| SM-D | 13 | 172 | Gorgonzola | Re-packaged | e |
| 14 | 180 | Fermented sausage | Open | f | |
| FS-2 | 15 | 210 | Gorgonzola | Open | e |
| 16 | 211 | Brie | Open | g | |
| SM-E | 17 | 277 | Ham | Original | f |
| 18 | 280 | Trout fillet | Original | h | |
| FS-3 | 19 | 318 | Fermented sausage | Open | i |
| FS-4 | 20 | 346 | Turkey breast | Open | j |
| SM-F | 21 | 413 | Herring filet | Original | k |
| 22 | 415 | Salmon | Original | l | |
| SM-G | 23 | 421 | Salmon | Original | m |
| SM-H | 24 | 448 | Herring filet | Original | n |
| SM-I | 25 | 483 | Raw meat sausage | Original | f |
| SM-J | 26 | 494 | Gorgonzola | Re-packaged | e |
| SM-K | 27 | 500 | Salmon | Original | l |
| FS-5 | 28 | 517 | Salami | Open | f |
| FS-6 | 29 | 530 | Swine liver patè | Open | o |
| SM-L | 30 | 559 | Trout fillet | Original | p |
| SM-M | 31 | 607 | Mozarella | Original | q |
| SM-N | 32 | 620 | Salmon | Original | r |
| SM-O | 33 | 624 | Salmon | Original | l |
| SM-P | 34 | 658 | Salami | Open | m |
| SM-Q | 35 | 663 | Gorgonzola | Re-packaged | e |
| 36 | 667 | Patè | Re-packaged | s | |
| 42 | 686 | Gorgonzola | Re-packaged | e | |
| SM-R | 43 | 721 | Brie | Original | t |
| 44 | 727 | Makerel filet | Original | q | |
| SM-S | 45 | 764 | Salmon | Original | l |
| SM-T | 46 | 786 | Gorgonzola | Re-packaged | e |
| 47 | 793 | Gorgonzola | Re-packaged | e | |
| SM-U | 48 | 805 | Salmon with citron | Original | r |
| 49 | 811 | Salmon with basil | Original | r | |
| SM-V | 50 | 887 | Salami | Original | u |
| 51 | 888 | Rillettes | Original | v | |
| SM-W | 52 | 913 | Herring fillet | Original | w |
| 53 | 916 | Salami | Open | f | |
| SM-X | 54 | 928 | Salmon with basil | Original | r |
| 55 | 929 | Salmon | Original | x | |
| 56 | 930 | Salmon with citron | Original | r | |
| 57 | 932 | Salmon | Original | r |
- *PT, Pulsetype.
Results from samples collected at the household level
The overall contamination rate of RTE-foods collected at the household level was about three times lower, and 5.8% and 1.7% of 640 food-stuffs tested positive for Listeria spp. and L. monocytogenes, respectively. All positive samples were contaminated by low numbers (<100 CFU/g or ml). Noticeably, the frequency of L. monocytogenes contamination of re-packaged and unpackaged samples was two times lower at the household level than at the retail level (P = 0.038). Listeria monocytogenes was isolated at least in one food sample from 14.3% of the single households, 16% of the family households and 20% of the households of elderly. Each 2%, 1.3%, and 5.6% of the samples of these household types were positive. The samples collected from households of vegetarians and citizens with a foreign cultural background were negative for L. monocytogenes. The purchase behaviour could have an influence on this result, as the highest percentage of CP3 food-stuffs was consumed in the households of vegetarians (Fig. 2). The difference in the distribution of samples assigned to the different CPs between households of vegetarians and all other households types was statistically highly significant in all cases (P < 0.0001). When comparing this distribution between the other households types, each type had a unique sample distribution as well. However, the significance levels were lower (single versus family: P = 0.27; single versus elderly: P = 0.045; family versus elderly: P = 0.176). The single household from the person of the different cultural background was not included in the statistical analysis because of the limited household number. When comparing the number of positive households for the different houshold types, no difference could be detected between single, family and elderly persons households (single versus family: P = 0.601; single versus elderly: P = 0.528; family versus elderly: P = 0.564). This was different, when comparing the number of positive samples assigned to the different household types. In this case, a statistically significant difference could be detected between single or family households and housholds of the elderly (single versus elderly: P = 0.111; family versus elderly: P = 0.048). An over-representation of positive samples (n = 3) in one household of the elderly was the obvious cause for that finding.

Presence of CP1, CP2 and CP3 foods in five types of households (family household; single household; household of a vegetarian; household of elderly persons age >65 years; household of a person with a non-Austrian cultural background).
Pulsed-field gel electrophoresis typing of the 11 L. monocytogenes isolates recovered at the household level resulted in six genetically distinguishable pulsetypes. From one household of elderly individuals, three food items, the kitchen swab and a faecal sample were positive for a genetically indistinguishable L. monocytogenes pulsetype, thus confirming a massive cross-contamination scenario.
With respect to the environmental and faecal samples, Listeria spp. was isolated from 3.3% (n = 90) of the human faecal samples, 7.4% (n = 27) of the animal faecal samples and 8.7% (n = 92) of the dust samples. The kitchen swabs tested negative for the presence of Listeria spp. Listeria monocytogenes was present in 1.1% and 2.2% of the dust samples and the human faecal samples, respectively. Numbers of L. monocytogenes in faecal samples were <100 CFUg in all cases.
Discussion
This study reports the contamination of ready-to-eat food-stuffs with the pathogen L. monocytogenes at the retail and the household level in Vienna, Austria. The study design is innovative as no survey has yet followed the prevalence of L. monocytogenes beyond the retail level. The use of the CP scheme allowed us to sample the premises and households so that foods with a higher risk for contamination were equally represented in the sample collection. The average prevalence determined in the three CP categories supported the initial categorization, as groups of foods assigned to CP1 were more frequently contaminated than food-stuffs in CP2 and CP3. However, the high contamination rates of raw meat sausages of 21.9% at the retail level and 10.8% at the household level suggested that this type of food should be rather included in the CP1 group in further studies.
Smoked fish products encompassed the vast majority of samples (88 out of 93) tested in the fish/seafood group. Every fifth smoked fish sample was contaminated with L. monocytogenes, and every 20th sample exceeded the limit of 100 CFU/g. Two vacuum-packaged samples were contaminated with >104 CFU L. monocytogenes/g. Both the prevalence data and infrequent high contamination of smoked fish products with L. monocytogenes is in line with other survey data (Hartemink and Georgsson, 1991; Dominguez et al., 2001; Gombas et al., 2003). Higher prevalence data were reported by Jorgensen and Huss (1998) who found 43% of the cold smoked salmon products and 60% of the halibutt samples positive for L. monocytogenes. Although the problem of contaminated fish products is known for years, and the literature on the control of L. monocytogenes in fish products is numerous (for a review see Duffes, 1999), there hasn't been a solution found to this problem yet. When contaminated salmon samples were produced in the same food plant, PFGE typing revealed that the recovered isolates were genetically indistinguishable. In the group of soft cheeses which encompassed 10 different cheese types, every fourth Gorgonzola-type cheese was contaminated with L. monocytogenes, but smear-ripened soft cheeses (n = 24) were negative. Although the number of smear-ripened cheese samples tested was low, our data do not support the high contamination rate of smear-ripened soft cheeses as published by Rudolf and Scherer (2001). PFGE typing demonstrated that genetically indistinguishable isolates of pulsetype ‘e’ were the cause for the Gorgonzola contamination in almost all cases. From the product information it could be derived that the cheeses were produced at a timely distance of 3–5 weeks in two different factories. The genetically indistinguishable isolates were recovered from the products of, to our knowledge unrelated producers, could suggest an adaptation of particular types of L. monocytogenes to green-veined cheese products or maybe the processing companies used the same sources of raw product supply. Low genetic diversity of strains isolated from a single Gorgonzola production plant was reported by Manfreda et al. (2005), obviously because of the re-isolation of persistent L. monocytogenes types from the product and plant environment.
The frequency of L. monocytogenes contamination found in various groups of meat and meat products (1.3% for cured meat products and up to 4.9% in raw meat sausages) was rather low (Uyttendaele et al., 1999; Vitas and Garcia-Jalon, 2004) or comparable to other studies (Breer and Schopfer, 1989; Levine et al., 2001).
Quantitative determinations at the end of product shelf-life revealed numbers of L. monocytogenes in the dairy and meat products of <100 CFU L. monocytogenes/g, thus being lower than the numbers thought to pose a risk to the consumers (Chen et al., 2003).
Data from the contamination of food-stuffs with L. monocytogenes stored at the household level are generally few. The lack of data is unsatisfactory as Listeria contamination usually becomes evident at the late stages of food processing (Norton et al., 2001; Wagner et al., 2006), meaning that the survival and growth capabilities of L. monocytogenes at the retail and household levels are fundamental to understanding the risk of transmission through the food chain. The high rate of contamination of household environments as described by Beumer et al. (1996) was not substantiated in our study. Sergelidis et al. (1997) reported a very low rate of L. monocytogenes contamination at refrigerator surfaces. Interestingly, the contamination level of re-packaged or unpackaged food was lower at the household level than at the retail level. This could be either due to the limited number of CP1 samples (n = 59) present in the households or to a reduction in the contamination level of samples upon storage at home. The absence of L. monocytogenes in the groups of RTE-products assigned to CP1 at the household level may also result from the fact that few CP1 samples were present, whereas the vast majority of food samples consumed in the households tested in our study belonged to groups of foods assigned to CP3 (n = 327). Further studies including more households of each type should be made to clarify these topics. The majority of the CP1 samples, however, were bought by households of elderly people resulting in a relatively high percentage of L. monocytogenes positive samples in this household type in comparison to households of other types. This observation could stimulate the consideration that surveys on the prevalence of L. monocytogenes should be designed with respect to existing risk consumer groups. Such a procedure could foster decision making whether particular recommendations on diet composition should be provided to individuals in an increased risk category. An illustrative description of a contamination cycle was achieved in one household of elderly where three refrigerated food samples (two CP2 samples, one CP3 sample), a dust sample and the faecal sample of the household members were contaminated with a genetically indistinguishable type of L. monocytogenes. This finding suggests that even food-stuffs contaminated with low numbers (<100 CFU/g) could contaminate the household environment, exposing individuals to L. monocytogenes so that on being passaged through the gut into the faeces is still detectable.
Acknowledgements
The study was supported by the Research Focus IV ‘Food Safety and Risk Assessment’ initiated at the University of Veterinary Medicine, Vienna.




