Distribution and Genetic Variability Among Campylobacter spp. Isolates from Different Animal Species and Humans in Switzerland
Summary
In Switzerland, a national database with 1028 Campylobacter isolates from poultry, pigs, cats, dogs, cattle, humans, zoo animals and water has been created. The database contains the genetic fingerprint and background information of each Campylobacter isolate. Dominant species could be identified in the different sources with a majority of Campylobacter jejuni in poultry (73%), humans (79%), cattle (95%), zoo animals (40%) and water (100%), of Campylobacter coli in pigs (72%), and of Campylobacter upsaliensis/helveticus in cats and dogs (55%). The comparison of three genotyping methods, amplified fragment length polymorphism (AFLP), pulsed field gel electrophoresis and restriction fragment length polymorphism, revealed that AFLP allows discrimination between the different Campylobacter species and is the most appropriate method to distinguish specific strains within the same species. Genotyping analysis demonstrated that the Campylobacter population is heterogeneous among the different sources and that no dominant clone is spread in the country. Genotyping and the resulting database are useful tools to trace back future Campylobacter infections.
Introduction
Campylobacter is an important cause of acute bacterial enteritis worldwide (Aarestrup et al., 1997; Engberg et al., 2000) and since 1995 it has been the most important reported cause of human gastro-enteritis in Switzerland. Over the last 3 years (2003–2005), the annual incidence of Campylobacter infections in humans in Switzerland reached up to 90 cases per 100 000 inhabitants (Bundesamt für Gesundheit, 2005). Livestock animals and pets represent the main reservoirs for Campylobacter species (Blaser, 1997). However, most Campylobacter infections in humans are associated with handling and consumption of poultry meat (Smith et al., 1999; Duim et al., 2000; McDermott et al., 2002; Siemer et al., 2005). Observed cases are mainly sporadic and outbreaks are reported rather rarely.
As antibiotic resistance have emerged in Campylobacter, better control and understanding has become a major public health concern (Engberg et al., 2001; Mølbak, 2005; Perreten, 2005). In Switzerland, the epidemiology of Campylobacter is not well known. Thus, during the last few years various projects has been launched in this country in collaboration with the Swiss Federal Veterinary Office and the Swiss National Science Foundation. The projects allowed the collection of Campylobacter strains isolated from different hosts, the creation of a database with background information on the strains, and the investigation of other characteristics such as the antibiotic resistance profiles (Ledergerber et al., 2003b; Regula et al., 2003; Schuppers et al., 2005; Wittwer et al., 2005), antibiotic resistance mechanisms (Keller and Perreten, 2006), and exposure assessment (Wieland et al., 2006).
In the present study, the identification of the contamination sources and of Campylobacter species thereof indicates which species are the most prevalent in the different reservoirs. The molecular characterization of different Campylobacter strains and the creation of a database containing Campylobacter fingerprints, will allow the comparison of strains and give a basis for tracing back the route of transmission and the origin of the contaminants (Alter et al., 2005). Additionally, genotyping helps to determine whether dominant clones are spreading within Switzerland.
A better knowledge on the distribution of Campylobacter in Switzerland will increase the understanding of its epidemiology. This should contribute to limit the dissemination of these bacteria, and hence, reduce the number of sporadic infections and prevent major outbreaks with strains resistant to multiple antibiotics.
Materials and Methods
Isolates
Since 2001, Campylobacter strains from different sources have been obtained from cloacal, meat and faecal samples using isolation methods previously described (Ledergerber et al., 2003a; Wieland et al., 2005; Wittwer et al., 2005). To date, strains from poultry, pigs, cats and dogs, cattle, human, zoo animals and water have been recorded in the database. Campylobacter upsaliensis and Campylobacter helveticus were not differentiated and Campylobacter belonging to either one of these species were considered as C. upsaliensis/helveticus.
Genotyping and database
Three different genotyping methods were used. Fluorescent amplified fragment length polymorphism (AFLP) was performed according to a protocol previously described (Duim et al., 2001) using the DNA restriction enzymes HindIII and HhaI and the internal standard ROX500 for normalization. The size of the amplified fragments was determined through capillary gel electrophoresis with the ABI Prism 310 sequencer (Applied Biosystems, Foster City, CA, USA).
Pulsed field gel electrophoresis (PFGE) was performed using the evaluated ‘Campynet’ prototype standardized protocol (http://campynet.vetinst.dk/PFGE.html). DNA was digested as recommended with restriction enzymes KpnI or SmaI.
The flaA restriction fragment length polymorphism (flaA-RFLP) was performed according to the protocol described in ‘Campynet’ (http://campynet.vetinst.dk/Fla.htm), but using primers described previously (Wassenaar et al., 1995). The resulting PCR fragments were digested with the restriction endonuclease DdeI and subsequently separated by gel electrophoresis.
To assess similarity between strains, the Pearson correlation coefficient was used to calculate the similarity matrix for the AFLP and flaA-RFLP profiles, and the band-based Dice coefficient was used to calculate the similarity matrix for the PFGE profiles. The dendrogram construction was performed using the unweighted paired group method with arithmetic mean values.
The genotypic profiles were normalized and analysed using BioNumerics 3.0 (Applied Maths, Kortrijk, Belgium). BioNumerics is a software platform programmed to integrate and analyse a broad range of data such as electrophoresis gels, protein gels, phenotype characters, microarray images and sequences. Entries of the database are managed with Access software (Microsoft Office) which is directly connected to BioNumerics through a Open Database Connectivity (ODBC) link. This approach allows the straight forward inclusion of various information on the Campylobacter isolates such as animal source, sampling date, farm details, geographical location, flock or herd size, and housing system.
Results
Isolates
A total of 1028 Campylobacter isolates were characterized and included into the database. Four hundred and thirty-three isolates originated from poultry, 166 from pigs, 222 from cats and dogs, 65 from cattle, 135 from humans and seven from other sources. In poultry, the dominant Campylobacter species was Campylobacter jejuni with 315 isolates, followed by 23 Campylobacter coli. Ninety-five additional isolates were only identified as Campylobacter spp. based on Gram-staining and microscopic assessment and were not further differentiated to species level. In pigs, 120 C. coli, four C. jejuni and 42 Campylobacter spp. were identified. In cats and dogs, 123 C. upsaliensis/helveticus, 81 C. jejuni, four C. coli, two Campylobacter lari, two Campylobacter concisus, and 10 Campylobacter spp. were identified. In cattle, 62 C. jejuni, two C. lari and one Campylobacter hyointestinalis could be identified. The collection of human isolates consisted of C. jejuni (107), C. coli (18), C. lari (five), and C. upsaliensis/helveticus (five). Four C. jejuni, two C. coli and one C. upsaliensis/helveticus were found in other sources (Fig. 1).
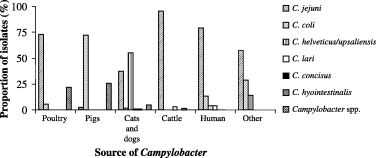
Distribution of Campylobacter species in different sources.
Genotyping
Amplified fragment length polymorphism typing was performed on 651 Campylobacter isolates: 266 profiles were obtained from poultry isolates (177 C. jejuni and 89 Campylobacter spp.), 46 from pigs (42 C. coli and four C. jejuni), 179 from cats and dogs (94 C. upsaliensis/helveticus, 79 C. jejuni, four C. coli, one C. lari and one C. concisus), 51 from cattle (48 C. jejuni, two C. lari and one C. hyointestinalis), 102 from humans (92 C. jejuni and 10 C. coli), and seven from other sources (four C. jejuni, two C. coli and one C. upsaliensis/helveticus).
Pulsed field gel electrophoresis was performed on 119 isolates analysed with the restriction enzyme KpnI and on 152 isolates analysed with SmaI. Sixty-six KpnI profiles (38 C. jejuni, 18 C. coli, five C. upsaliensis/helveticus and five C. lari) and 70 SmaI profiles (47 C. jejuni, 18 C. coli and five C. lari) were obtained from human isolates, 30 \KpnI profiles (28 C. jejuni and two C. lari) and 58 SmaI profiles (55 C. jejuni, two C. lari and one C. hyointestinalis) from cattle isolates, 19 KpnI profiles (18 C. upsaliensis/helveticus and one C. lari) and 19 SmaI profiles (11 C. upsaliensis/helveticus and eight C. jejuni) from cats and dogs isolates, and four KpnI and five SmaI profiles from poultry isolates (all C. jejuni). Sixty-one isolates from humans were analysed with both KpnI and SmaI (38 C. jejuni, 18 C. coli, five C. lari), as well as 27 isolates from cattle (25 C. jejuni, two C. lari), four C. jejuni isolates from poultry, and two C. upsaliensis/helveticus isolates from cats and dogs. As the profiles of the PFGE band patterns generated with the restriction enzyme SmaI were superior to the ones obtained with KpnI, the former were used for the comparison of different genotyping methods (see below).
The flaA-RFLP analysis was performed on 356 isolates, 241 of them were from poultry (152 C. jejuni and 89 Campylobacter spp.) and 115 were from pigs (C. coli).
Evaluation of the genotyping methods
A certain number of isolates were analysed with more than one genotyping method. This allows to assess the performance of the various genotyping methods in regard to their ability to distinguish between different strains. Two hundred and twenty nine isolates from poultry (140 C. jejuni, 89 Campylobacter spp.) were genotyped with both AFLP and RFLP and comparison of the results was discussed by Wittwer et al. (2005).
One hundred one isolates, namely 48 from human (38 C. jejuni, 10 C. coli), 14 from cats and dogs (C. upsaliensis/helveticus) and 47 from cattle (44 C. jejuni, two C. lari and one C. hyointestinalis), were analysed with both AFLP and SmaI-PFGE. The resulting profiles were then compared and the respective dendrograms were created (Fig. 2). The congruence of experiments comparing the similarity of both dendrograms was 52.3%. The dendrogram of the AFLP profiles showed 74 different AFLP patterns gathering into 12 different clusters at a 66% similarity level. The range of the similarity was between 10.23% and 99%. The PFGE dendrogram showed 57 different patterns distributed into 8 different clusters. The mean similarity within the clusters was 60%. The similarity range between the isolates was 1.63–100% (Fig. 2).
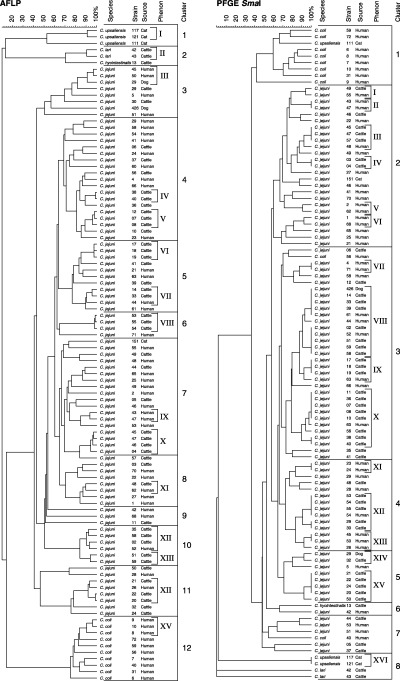
Dendrograms of 101 Campylobacterstrains from different sources based on profiles generated by amplified fragment length polymorphism (AFLP) and pulsed field gel electrophoresis (PFGE) using the restriction enzyme SmaI. The dotted vertical line suggests the >90% similarity threshold. Roman numbering I–XVI represents groups of isolates sharing more than 90% genetic similarity (phena). The clusters represent a formation of different groups containing genetic homogeneous strains with an average similarity of 66% within the clusters of AFLP (1–12) and 60% within the clusters of PFGE (1–8).
Groups of isolates sharing more than 90% similarity were considered as genetically related (clonal lineage) and grouped together to form a phenon (Olive and Bean, 1999). Repeated analysis of isolates showed a repeatability above 90%. Of the 101 isolates included in the comparison AFLP–PFGE (Fig. 2), 15 phena (I–XV) were identified in the AFLP dendrogram. Six of them with two isolates, six with three isolates, and three with four isolates. The PFGE dendrogram displayed 16 different phena (I–XVI), nine of them with two isolates, two with four isolates, and one with three, five, six, nine or 11 isolates (Fig. 2). This indicates that <4% of all isolates belonged to clonal lineages.
Isolates displaying a specific AFLP profile were found to have different profiles when analysed by PFGE. Five specific AFLP profiles corresponded to two different PFGE profiles, and two AFLP profiles corresponded to three different PFGE profiles. On the other hand, isolates displaying a specific PFGE profile were also found to have different profiles when analysed by AFLP. Ten specific PFGE profiles corresponded each to two different AFLP profiles. The other PFGE profiles corresponded to three, four, five or seven different AFLP profiles. This demonstrated that diversity may exist even in the presence of a common pattern and that the methods may play a role in the strains differentiation as shown with AFLP and PFGE. These methods are not 100% accurate to distinguish between Campylobacter strains. Nevertheless, AFLP allowed to differentiate between Campylobacter species. Indeed, clusters three to 11 of the AFLP dendrogram contained only C. jejuni, cluster 12 only C. coli isolates, cluster 1 only C. upsaliensis/helveticus and cluster 2 two C. lari and one C. hyointestinalis isolates. In contrast, in the PFGE dendrogram, isolates were not grouped according to species identification (Fig. 2).
Discussion
The different isolation campaigns of Campylobacter showed that this organism is widespread in various environmental sources in Switzerland. Identification of Campylobacter on species level demonstrated that in some hosts certain Campylobacter species are predominant. Poultry and cattle were mainly carriers for C. jejuni, pigs for C. coli, and cats and dogs for C. upsaliensis/helveticus. The clinical isolates from humans were mostly C. jejuni followed by C. coli, and less frequently C. upsaliensis/helveticus and C. lari suggesting that humans can be infected from different sources, even from pets. This is supported by the fact that Campylobacter strains of the same genotype were found in both humans and animals. A method for assessing the epidemiological significance of possible infection sources for human campylobacteriosis and the transmissions between these sources has recently been developed (Wieland et al., 2006). A similar distribution of Campylobacter species isolated from different sources was also found in other countries (Ishihara et al., 2004; Boes et al., 2005; CDC, 2006). However, different results were obtained in studies in France, Germany, and the USA where the investigators exclusively detected C. coli in pigs (Payot et al., 2004; Alter et al., 2005; Gebreyes et al., 2005; Thakur and Gebreyes, 2005b). Unfortunately, in many countries other Campylobacter species than C. jejuni and C. coli were not identified to species level and therefore precise prevalence data for species such as C. lari, C. hominis, C. hyointestinalis, and C. upsaliensis are not available.
The comparison of the different genotyping methods revealed that actual genotyping methods do not always allow to distinguish exactly between two strains, as some strain displaying the same genetic profile using one method (e.g. AFLP) were found to have different genetic profile when analysed by PFGE and vice versa. AFLP allowed a better differentiation of the strains than PFGE as previously demonstrated for C. jejuni strains (Lindstedt et al., 2000). Using AFLP analysis of different species, it was shown that AFLP grouped strains of the same species together, contrarily to PFGE which generated clusters containing diverse species. Indeed, AFLP was shown to have a potential to distinguish C. upsaliensis from C. helveticus (Wieland et al., 2005). The use of different genotyping methods enabled better discrimination between strains, therefore demonstrating that the Campylobacter population is heterogeneous in Switzerland and that no dominant strains are present in the different sources. Multilocus sequence typing (MLST) should be considered as a further possible method to characterize Campylobacter isolates (Dingle et al., 2001; Thakur and Gebreyes, 2005a), as large databases of MLST sequences are available through Internet (Urwin and Maiden, 2003).
The creation of a national database was essential to provide an accurate reflection of the Campylobacter population from different sources in Switzerland. The database represents a useful tool for the development of better control strategies for Campylobacter and to traceback infection sources. However, it should not be forgotten that prevention of bacterial contamination along the food chain starts by following the basic rules of food safety. Thus, surveillance of Campylobacter occurrence with the implementation of hazard analysis critical control point principles throughout the different stages of the food process (slaughterhouse, food handling, processing and packing, and retail stores), impeccable hygiene and appropriate cooking methods are necessary to reduce foodborne infections and limit the spread of antibiotic-resistant zoonotic bacteria to humans.
Acknowledgements
We thank Denise Howald, Elisabeth Lüthi, and Grethe Sägesser for technical assistance, Gertraud Regula, Andreas Thomann and Isabelle Brodard for helpful discussion. We are grateful to the Institute of Infectiology (K. Mühlemann, University of Bern, Inselspital, Switzerland) and the National Centre for Enteropathogenic Bacteria NENT (H. Hächler, Institute of Medical Microbiology, Cantonal Hospital, Lucerne, Switzerland) who provided human isolates. This work was supported by the research grant no. 1.02.15 of the Swiss Federal Veterinary Office.




