Evidence-Based Medicine and Knowledge Dissemination, Translation, and Utilization: Challenges of Getting Evidence-Based Treatments to Patient Care and Service Delivery
Abstract
The aim of this article is to explore challenges associated with effective utilization of best available scientific evidence on what works and what is potentially harmful in health, mental health, and social care. A gap exists between the production of high quality evidence on what has proven to be successful and the utilization of this evidence in human services. Patients and service users do not always receive interventions supported by the best available scientific evidence. Methods of evidence-based medicine and evidence-based practice have emerged as promising approaches to remedy this deficit. Furthermore, implementation and translational research, supported by multi-disciplinary efforts, is emerging as another approach to bring best available evidence to service delivery settings. Although these approaches are promoted by diverse stakeholders in many countries, we are still waiting for rigorous evaluations of these approaches to understand their effectiveness in comparison to traditional ways of delivering interventions and other types of services in human services organizations.

Introduction
The aim of this article is to explore problems associated with effective utilization of best available scientific evidence on what works and what is potentially harmful in health, mental health, and social care.
As much as progress pertaining to increasingly sophisticated means of searching, retrieving, appraising, and synthesizing rigorous evidence on what is effective and what is potentially detrimental in professional human services has taken place over the last 10 to 15 years, stakeholders are concerned about whether those evidence-based interventions are effectively implemented in patient and client care. The itinerary from “bench to bedside” is long. This itinerary includes evidence production through basic and applied research, dissemination of research results, receiving and acceptance of research results by professionals, willingness and ability of professionals to use research results, translation of research results to local and patient/client circumstances, and implementation of research results in real-life treatment situations.
In the United States of America, several reports have pointed out the gap between evidence production and evidence utilization. In the field of health and mental health, three pivotal reports have indicated this problem and warned that the time gap between production and utilization might be up to 20 years (1–3). Another report prepared by McGlynn and her colleagues has demonstrated the deficit pertaining to preventive, acute, and long term health care provided to Americans (4). The results are discouraging. McGlynn and her colleagues employed 439 indicators of quality of care applied to 30 acute and chronic conditions, and preventive care.
Table 1, adopted from Table 5 in McGlynn et al (4), shows the scope of the problem of not being able to get recommended care interventions. Although breast cancer patients on average are treated much better compared with other groups of patients, approximately 25% were not treated in alignment with recommended guidelines. Another “better-off” condition was coronary artery disease; only 68% of these patients received recommended care treatment, meaning, one third of the patients did not receive recommended care. Only 36.7% of the sexually transmitted diseases or vaginitis patients were treated using the recommended guidelines. Lowest on the scale was alcohol dependency; approximately 90% of these patients were not treated with the recommended care processes. Altogether, Americans in this study received only 50% of recommended preventive, acute, and long-term health care. McGlynn and her colleagues concluded that the deficits they identified in “adherence to recommended processes for basic care pose serious threats to the health of the American people” (4).
| Condition | Number of indicators | Patients | Percent of care received recommended |
|---|---|---|---|
| Breast cancer | 9 | 192 | 75.7 |
| Coronary artery disease | 37 | 410 | 68.0 |
| Depression | 14 | 770 | 57.7 |
| STD or vaginitis | 26 | 410 | 36.7 |
| Hip fracture | 9 | 110 | 22.8 |
| Alcohol dependency | 5 | 280 | 10.5 |
We may then assume: if the deficit in adherence to recommended health care is severe in the United States, it must even be worse in countries where the training of health professionals, opportunities for continuing education, health care infrastructure, and other pertinent factors are less developed. Likewise, if adherence to recommended care guidelines is poor in the fields of health and mental health, social care, within the same country, must be even worse. Nevertheless, we have reason to be cautious about the relationship between national wealth and health. The United States, one of the richest countries in the world, spends more than any country on medical care (USD 6,350 in 2005). In the world ranking, life expectancy from birth to age 65 in this country is 36th for men and 42nd for women (5).
Against this backdrop, we can understand that the production of high quality efficacy and effectiveness research and high quality systematic reviews that summarize the state of the science with regard to what works and what is potentially harmful in service professions is necessary but not sufficient for the delivery of high quality care to the end-users. Many networks such as the Cochrane (6) and Campbell (7) Collaborations perform exemplary work not only in terms of producing high quality systematic reviews, but also in terms of disseminating those reviews. Many networks that focus on dissemination of evidence-based interventions also perform excellent work; certainly, the California Evidence-based Clearinghouse for Child Welfare (8) and the National Registry of Evidence-based Programs and Practices (9) are among such networks. These networks’ dissemination efforts, while essential, have not proven to be sufficient in making sure that evidence-based interventions are in fact used by professionals.
The lack of adherence to recommended practices pertains to the issue of not implementing interventions that are proven to be effective. One way of approaching this problem has been the use of evidence-based medicine (EBM) in health related practices and evidence-based practice (EBP) in social care services.
Implementation of EBP as a process of delivering high quality care to the end-users and implementing research and translational research, stand today as promising efforts that may be able to provide guidance for how to bring best available scientific evidence from bench to bedside and to other service providing units, such as social services agencies, school classrooms, probation services, etcetera.
Evidence-based medicine, practice, and policy
The concept of EBM was originally designed for the health related sciences and practices, and aimed at introducing a new and generic method of interaction between patient and health professionals. Sackett and his colleagues defined EBM as “the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients” (10). This perspective emphasizes three equally important elements of successful and ethical care delivery, which include clinical circumstances, patient's values and preferences, and the best available research evidence. This perspective goes beyond the presence of scientific evidence on what works and what is potentially harmful, indicating that scientific evidence is necessary, but not sufficient, for conscientious and judicious use of that evidence.
More specifically, Sackett and colleagues wrote: “The practice of evidence-based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research. By individual clinical expertise, we mean the proficiency and judgment that individual clinicians acquire through clinical experience and clinical practice. Increased expertise is reflected in many ways, but especially in more effective and efficient diagnosis and in the more thoughtful identification and compassionate use of individual patients’ predicaments, rights and preferences in making clinical decisions about their care. By best available external clinical evidence, we mean clinically relevant research, often from the basic sciences of medicine, but especially from patient-centered clinical research into the accuracy and precision of diagnostic tests (including the clinical examination), the power of prognostic markers and the efficacy and safety of therapeutic, rehabilitative and preventive regimens. External clinical evidence both invalidates previously accepted diagnostic tests and treatments and replaces them with ones that are more powerful, more accurate, more efficacious and safer” (10).
A few years later, a graphic representation of EBM was made available by Haynes and colleagues (11) (see Figure 1).
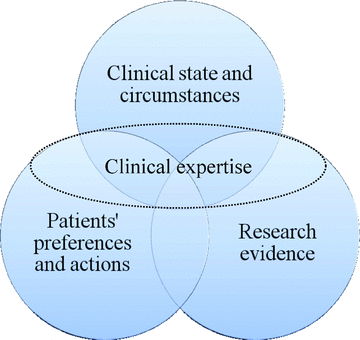
Early model of the key elements for evidence-based clinical decisions
The concept of EBM was adopted and adapted to serve human service practices under the name Evidence-based Practice (EBP) or Evidence-based Policymaking (EBPM) (12–13). For social work practice, an American social worker, Leonard Gibbs (14), prescribed the following steps as the process stages of evidence-based practice:
- •
Step 1 – Become motivated to apply evidence-based practice.
- •
Step 2 – Convert information need into an answerable question.
- •
Step 3 – Track down best available evidence to answer the question.
- •
Step 4 – Appraise the evidence critically.
- •
Step 5 – Integrate evidence with practice experience and characteristics of the client or situation.
- •
Step 6 – Evaluate effectiveness and efficiency in exercising the steps.
- •
Step 7 – Teach others to do the same.
Although evidence-based medicine and evidence-based practice remain a promising approach to patient and client work, there is an urgent need for empirical and high standard studies to explore questions such as:
What is the extent to which EBM and EBP are understood and used in different countries and in different geographical parts within the same country, and what groups of professionals use or do not use EBM and EBP? What factors specifically impede or advance implementation of EBM and EBP as a work process? And does the implementation of EBM and EBP matter in terms of benefiting patients and clients, and if so, how?
Implementation and translation
Implementation and translational research in health care and other human services is emerging as yet another response to the disadvantages generated by the time gap and to concerns such as:
Is implementation of evidence-based interventions feasible? What are the costs if implemented successfully? For what patient/client groups, in what settings, and under what conditions, do evidence-based interventions work? To what extent can results of effectiveness studies conducted in one context be applied to other contexts? What is the optimum balance between adaptability vs. fidelity of evidence-based interventions when they are translated into diverse contexts? And, very importantly, what factors support or impede effective and successful implementation of evidence-based interventions?
The concept of “translational research” was launched by the National Institutes of Health (NIH), the major American federal research foundation that funds research in health, mental health, and related human services areas. Before describing the intended nature of translational research, it would be useful to briefly touch upon three related terms: diffusion, dissemination, and implementation. All of these terms have been around for a long time, and aim to capture various aspects of making innovations reach the end-users for utilization. They make a backdrop to translational research. In the following, reference is made to J. Lomas’ descriptions of these terms. (15) Lomas characterizes “diffusion” as a passive and unplanned process that lacks targeted receivers. Diffused information reaches or rather is captured only by active information seekers who are highly motivated to get specific information. Typically, diffused products are scientific journal articles, and it is less likely that busy physicians in general find time to search, retrieve, appraise, and read primary sources such as journal articles.
“Dissemination” is an intentional process in which the information is tailored and adapted to the needs of the targeted group and then actively communicated to them. Typically, systematic reviews, practice guidelines, and consensus statements are disseminated rather than diffused to well-defined and targeted groups. Lomas (15) suggests that dissemination is an effective form of communication if the goal is to generate awareness, the information receivers are well-defined, and the message is tailored to the needs of the target group.
“Implementation” goes beyond dissemination and aims at making an intervention work by identifying and facilitating mechanisms that promote or impede utilization of an intervention.
“The public policy decisions of governments,” writes Lomas “are diffused through technical, legislative and regulatory statutes available to the knowledgeable and motivated interests. Dissemination of public policy relies, however, on the media and targeted information campaigns originating with government to communicate the intent and practical implications of a legislative statute. Implementation of these same decisions is dependent on a complex framework of sanctions and incentives, reinforced by monitoring and adjustment, and often adapted to fit differing environments at more local levels” (16) The process of getting the information on medical innovations follows a similar path.
To bridge the gap between research and utilization of medical evidence, the NIH launched a major program in 2004, which came to be known as the NIH Roadmap for Medical Research. The Roadmap was originally designed to address specific deficits or gaps in biomedical research. The major themes to be addressed included: fostering high-risk/high-reward research; enabling the development of transformative tools and methodologies; filling fundamental knowledge gaps; and changing academic culture to foster collaboration (17)
One of the sub-programs included in the Roadmap is the Translational Research Program, which was designed to re-engineer the clinical research enterprise. The main purpose of bringing evidence-based interventions to the clinical practice as quickly and efficiently as possible was to bridge basic research and human clinical research. The then-director of NIH, Dr. Zerhouni wrote: “…exciting basic science discoveries demand that clinical research continue and even expand, while striving to improve efficiency and better inform basic science. […] Clinical research needs to develop new partnerships among organized patient communities, community-based physicians, and academic researchers. In the past, all research for a clinical trial could be conducted in one academic center; that is unlikely to be true in the future. In these initiatives, NIH will promote creation of better integrated networks of academic centers that work jointly on clinical trials and include community-based physicians who care for large groups of well-characterized patients. Implementing this vision will require new ways to organize how clinical research information is recorded, new standards for clinical research protocols, modern information technology, new models of cooperation between NIH and patient advocacy alliances, and new strategies to reenergize the clinical research workforce” (18).
Today, a master part of NIH funded translational research is conducted within the frame of the Clinical and Translational Science Awards (CTSA) Consortium that was launched in October, 2006. The consortium started with 12 academic health centers across America, and established another 12 centers by September, 2007. It is prospected that when the consortium reaches full scale, about 60 CTSA centers will be in place. The goal of CTSA is designed to: “1) captivate, advance, and nurture a cadre of well-trained multi- and inter-disciplinary investigators and research teams; 2) create an incubator for innovative research tools and information technologies; and 3) synergize multi-disciplinary and inter-disciplinary clinical and translational research and researchers to catalyze the application of new knowledge and techniques to clinical practice at the front lines of patient care” (19).
These centers bring together researchers from diverse fields such as social marketing, behavioral change, organizational change, social anthropology, social work, finance and economics, and medicine. The idea of translational research has now also transferred to social work, which is seen as a leading practice and discipline in the delivery of human services.
Westfall and his colleagues (20) provide an illustration of the current NIH Roadmap for medical research, and suggest further development of the Roadmap by introducing “practice-based research” as a potential vehicle to improve practice.
The original NIH Roadmap for biomedical research involves three stages: basic science research (bench), human clinical research (bedside), and utilization of results in clinical practice. Basic science research includes preclinical and animal-based studies that may or may not lead to any useful results for human clinical application. Human clinical research is studies of basic research outcomes, implemented in human clinical conditions. These studies are conducted in controlled settings and thus do not integrate everyday (uncontrolled or uncontrollable) variations of treatment settings. Useful results of clinical research are usually refined in systematic reviews and meta-analyses such as those generated by the Cochrane Collaboration (6) in practice guidelines and recommendations processed by national agencies. In the Roadmap, the translation between bench and bedside is known as “T1” and the translation between bedside and clinical practice as “T2.”
However, as evidenced by the reports referred to in the introduction, not much of these research results reach patients in ambulatory settings. Westfall and colleagues (20) propose that an additional stage, practice-based research, is added into the chain between basic research and ultimate patient treatment. Practice-based research involves observational studies in real-life conditions. In fact, this proposition is not unknown to biomedical and social research. What Westfall and colleagues are proposing is traditionally called effectiveness research, that is, implementation and study of efficient treatments or interventions in real-life conditions. Nevertheless, their proposal is instrumental and helps to clarify a deficit of the NIH Roadmap concept. The third translational stage, T3, refers to moving new medical treatments and innovations into day-to-day medical practice. Translation 3 is supported by dissemination research and implementation and translational research. We agree with Westfall and his colleagues that “[w]ell positioned to conduct translational research, practice-based research is not synonymous with translational research. Practice-based research may be the best setting for studying the process of care and the manner in which diseases are diagnosed, treatments initiated, and chronic conditions managed. It is in practice-based research where effectiveness can be measured, where new clinical questions may arise, where readiness to change and adopt new treatments can be studied and addressed, where patient knowledge and preferences are encountered and managed, and where the interface between patients and their physicians can be explored and medical care improved. Practice-based research is the final common pathway for improving individual patient care and outcomes” (21).
Importance of organizational settings
In an early study, Main and her colleagues (22) showed that perception of depression as an important primary care problem was strongly related to clinician training in depression, beliefs about the burden associated with diagnosis and treating depression, and perceptions of the patient's discomfort. This finding supports the hypothesis that the practitioner's assessment of the day-to-day care is related to the practitioner's individual professional attributes. However, it is equally important to recognize that individual care providers and patients/clients interact within organizational settings. Organizations may promote or impede use of best available evidence in care services.
Introduction, implementation, and use of evidence-based interventions in health, mental health, and social services take place in organizational environments. Thus, health and social care agency organizations constitute a structural arena for treatment and interventions. Introducing evidence-based interventions in an organizational setting is similar to introducing a new way of thinking and working within an agency. This means that the agency will be transferring new technology into its organization and to its staff. Introducing and implementing evidence-based treatment methods will require strategic planning including adoption of the intervention as an innovation, implementation of the innovation, and assurance of sustainability to maintain and improve the quality of intervention within the organizational setting.
Health and social care managers know from experience that organizations can be resistant to attempts to change the organizational culture and to introduce innovations. However, research evidence shows that even rigid organizations can respond strategically by taking corrective actions when they have evidence of their organizational deficits (23).
A promising conceptual framework to guide organizational innovation implementation is being tested in multiple sites to measure an organization's readiness to receive innovations and to test the effectiveness of the framework. This model is called the TCU (Texas Christian University) Program Change Model (24).
The TCU framework lays out a logical and empirically-tested process for efficient transfer of best available evidence and mindset necessary for its implementation into health and social care agencies.
In this model (25) the process of innovation starts out with an examination of the organizational infrastructure of service units in a provider network and of the staff members’ knowledge about the service structure of their program. This examination generates knowledge about the strengths and deficits of the infrastructure that will help to prepare the organization for a successful implementation of best available evidence. At this stage, it is important to acquire information about the staff motivation (e.g., program needs, training needs), institutional resources (e.g., staffing, equipment, internet), staff attributes (e.g., efficacy, influence adaptability), and organizational climate (e.g., cohesion, autonomy, communications, stress).
The implementation process begins after the necessary preparations. The process includes staff training, innovation adoption, and implementation. Finally, after these stages, innovations tend to become standard practice and have to be maintained.
Conclusion
Effective utilization of best available scientific evidence on what works and what is potentially harmful in health, mental health, and social care is a very complex process, and not always easily achieved. During the last two decades, promising advances have been achieved in the human services. These include awareness of the value of using best scientific evidence, development of methods and infrastructure of generating high quality evidence, increased attention to transparency and ethical aspects of interventions, and awareness of difficulties pertaining to effectively bringing best available evidence to the patients and service users. Evidence-based medicine, evidence-based practice, evidence-based policy making, implementation and translational research, and understanding how organizations impact the quality of service delivery have emerged as promising approaches to better patient care and service delivery. None of these approaches alone have yet been empirical proven to be more efficient as compared with other means of patient care and service delivery. Much empirical work remains to be done. Meanwhile, human services professionals need to continue to refine their methods of service delivery and collaborate with the research community to understand effectiveness of these methods. Advancement of the quality of care provided needs to be supported by research, professional experience and skills, training of professionals at universities and colleges, and continued training of human services professionals. Advancement of the quality of care will also greatly benefit from multi-disciplinary collaboration among researchers and developers.




