Topical levofloxacin 1.5% overcomes in vitro resistance in rabbit keratitis models
Abstract.
Purpose: To determine whether topical levofloxacin 1.5% will successfully treat both levofloxacin-resistant and susceptible Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) in rabbit keratitis models.
Methods: For levofloxacin-resistant and susceptible SA, respectively, 32 New Zealand White (NZW) rabbits were intrastromally injected with 1000 colony-forming units (CFU). After 4 hr, the corneas of eight rabbits were homogenized to determine onset CFU/ml. Twenty-four rabbits were divided into three treatments: levofloxacin, vancomycin (cefazolin for levofloxacin-susceptible SA) and saline. Twenty-one drops were administered over 5 hr. One hour post-treatment, the corneas were homogenized for CFU/ml. For levofloxacin-resistant and susceptible PA, respectively, 32 NZW rabbits were intrastromally injected with 1000 CFU. After 16 hr, the corneas of eight rabbits were homogenized for CFU/ml. Twenty-four rabbits were divided into three treatments: levofloxacin, tobramycin (ciprofloxacin for levofloxacin-susceptible PA) and saline. Nineteen drops were administered over 8 hr. One hour post-treatment, the corneas were homogenized for CFU/ml. The CFU/ml data were analysed for sterilization and non-parametrically for reduction.
Results: Levofloxacin 1.5% significantly reduced more (p < 0.05) levofloxacin-resistant SA than vancomycin; was equivalent to cefazolin (p > 0.05) for levofloxacin-susceptible SA; was equivalent to tobramycin for levofloxacin-resistant PA; was equivalent to ciprofloxacin for levofloxacin-susceptible PA; and significantly reduced more SA and PA than saline and onset. Levofloxacin 1.5% sterilized the corneas in the levofloxacin-resistant and susceptible PA groups (32/32) and levofloxacin-susceptible SA group (16/16), but not the levofloxacin-resistant SA group (0/16).
Conclusion: Levofloxacin 1.5% was effective for reducing SA and PA in the rabbit keratitis models regardless of in vitro resistance.
Introduction
The fluoroquinolones (FQs) have proven to be successful for treating ocular infections because the introduction of ciprofloxacin and ofloxacin; the improvement in ofloxacin to levofloxacin, the active l-isomer of ofloxacin; and the targeting of Gram-positive bacteria by moxifloxacin and gatifloxacin with a dual mechanism of action. The in vitro testing of the FQs has determined that moxifloxacin and gatifloxacin are more potent anti-infectives with lower minimum inhibitory concentrations (MICs) to Gram-positive bacteria than the earlier generations of FQs, but no real advantage for in vitro susceptibility has been noted among the FQs for Gram-negative bacteria (Mather et al. 2002; Kowalski et al. 2003; Kowalski et al. 2005).
Although the in vitro susceptibility differences are evident, the accuracy of predicting in vivo ocular clinical success based on in vitro data has not been established. There are no standards for interpreting the susceptibility of ocular bacterial isolates for topically applied FQs. At present for ocular bacterial isolates, in vitro FQ susceptibility is based on a MIC value interpreted with a standard based on blood serum concentrations (CLSI 2006) and not the concentration of a FQ level reached in the ocular tissues after topical administration. The blood serum standard can give an indication of the susceptibility of an ocular isolate to a FQ, if it is assumed that the concentration of the topically applied FQ in the ocular tissues is greater or equal to the concentration of FQ reached in the blood serum after systemic administration. As the FQs were developed for systemic therapy to penetrate the body tissue, it is believed intuitively that the concentrations of FQs in the ocular tissue are elevated with topical therapy in comparison with lower concentrations attained in the blood serum. Subsequently, this would indicate that the blood serum standards would interpret more ocular bacterial isolates to be resistant.
The hypothesis of the present study is levofloxacin 1.5% overcomes in vitro resistance by effectively reducing the colony counts of both levofloxacin-susceptible and levofloxacin-resistant bacteria in rabbit keratitis models. The hypothesis will be tested by (1) selecting two bacterial keratitis pathogens that are represented by isolates that were deemed either resistant or susceptible to levofloxacin (Staphylococcus aureus and a Pseudomonas aeruginosa); (2) infecting rabbit corneas with the bacterial isolates in separate experiments; (3) treating topically the infected corneas with levofloxacin, standard therapies and saline in respective groups; and (4) analysing statistically the reduction in bacterial loads with comparison of levofloxacin to other treatment groups.
Methods
Rabbit keratitis models
Rabbit keratitis models were previously established to test topical anti-infectives to treat keratitis (Callegan et al. 1994; Dajcs et al. 2001; Dajcs et al. 2004; Kowalski et al. 2001; Rhee et al. 2004; Romanowski et al. 2005; Romanowski et al. 2008). The rabbit keratitis models were developed to determine the efficacy of an anti-infective to penetrate and eradicate bacteria in the cornea. The treatment regimens were not a primary focus of the models, nor were cure and resolution. O’Callaghan (Callegan et al. 1994; Dajcs et al. 2001; Dajcs et al. 2004) and associates reported that an early phase S. aureus keratitis model, in which the infection was allowed to progress for 4 hr prior to treatment, was the better model to eradicate bacteria, whereas bacterial eradication was problematic in the late-phase (stationary) model (progression over 10 hr). Our group (Kowalski et al. 2001; Rhee et al. 2004) determined that both early and late-phase P. aeruginosa keratitis models were appropriate to demonstrate anti-infective efficacy. We chose the early phase keratitis model for S. aureus and the late-phase keratitis model for Pseudomonas aeruginosa to test for anti-infective efficacy.
The present study (IACUC Protocol #0708035 ‘The Evaluation of IQUIX® in NZW Rabbit Keratitis Models’) conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research and was approved prior to initiation of the study by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC).
The S. aureus rabbit keratitis studies consisted of two experiments. For experiment 1, 32 New Zealand White rabbits (NZW) were anesthetized with intramuscular injections of ketamine (40 mg/kg) (Ketajet; Phoenix Pharmaceuticals Inc., St Joseph, MO, USA) and xylazine (4 mg/kg) (TranquiVed; Vedco, Inc., St. Joseph, MO, USA) into the rear flank muscle. The rabbit eyes were administered topical 0.5% proparacaine and proptosed to stabilize and expose the corneas for injection of bacteria. Using a 100-μl Hamilton syringe and a 30-gauge ½ inch needle, 25 μl containing 1000 colony-forming units (CFU) of levofloxacin-resistant S. aureus was injected intrastromally into both corneas of each rabbit. Four hours after bacterial challenge (onset), eight rabbits were killed with an overdose of Euthasol Solution (390 mg/ml pentobarbitol, 50 mg/ml phenytoin sodium) (Virbac AH, Inc., Fort Worth, TX, USA) following systemic anaesthesia. A 9.5-mm corneal button that encompassed the inoculation site was excised from the cornea and added to a tube containing 1 ml of PBS. The corneal buttons were then homogenized using a Pro Scientific motorized homogenizer (Pro Scientific, Oxford, CT, USA). These corneas established the bacterial load at the onset of topical treatment and were necessary to determine the bactericidal effect between the final and onset colony counts. In establishing our rabbit keratitis models in earlier reports, we determined growth curves that demonstrated an increase in growth from the initial inoculum (1000 CFU) to different times points over a 24 hr period (data not include). We did not reproduce the growth curve for this experiment but experience from our previous studies indicated that similar growth patterns would be present (Kowalski et al. 2001; Rhee et al. 2004; Romanowski et al. 2005; Romanowski et al. 2008).
The remaining 24 rabbits were divided into three topical treatment groups: (1) levofloxacin 1.5%, (2) vancomycin 5% and (3) saline. Treatment consisted of drops every 15 min for 5 hr (21 drops). One hour after treatment, the rabbits were killed, and the corneas were homogenized for colony counts. The data were non-parametrically analysed for bacterial load reduction among the treatment groups. The authors believe that appropriate scientific method includes precise colony counts at the onset of treatment, at the time of completion of anti-bacterial treatment and at the time of completion of treatment with saline as a control.
For experiment 2, the protocol was similar except levofloxacin-susceptible S. aureus was injected and cefazolin 5% was substituted for vancomycin 5%.
The P. aeruginosa rabbit keratitis studies consisted of two experiments. For experiment 1, 32 NZW rabbits were anaesthetized and administered topical anaesthesia to proptose the corneas for injection of bacteria. Using a 100-μl Hamilton syringe and a 32-gauge ½ inch needle, 25 μl containing 1000 CFU of levofloxacin-resistant P. aeruginosa was injected intrastromally into both corneas of each rabbit. Sixteen hours after bacterial challenge (onset), eight rabbits were killed, and the corneas were homogenized for standard colony counts. The remaining 24 rabbits were divided into three topical treatment groups: (1) levofloxacin 1.5%, (2) tobramycin 1.4% and (3) saline. Treatment consisted of drops every 15 min for 1 hr and every 30 min for 7 hr (19 drops). One hour after treatment, the 24 rabbits were killed, and the corneas were homogenized for standard colony counts. The data were non-parametrically analysed for bacterial load reduction among the treatment groups.
For experiment 2, the protocol was similar except levofloxacin-susceptible P. aeruginosa was injected and ciprofloxacin 0.3% was substituted for tobramycin 1.4%.
Bacterial pathogens and inoculum preparation
The S. aureus isolates used in these studies were the following: (1) levofloxacin-resistant, methicillin-resistant S. aureus (K950 – levofloxacin MIC = 32 μg/ml; vancomycin MIC = 2 μg/ml; oxacillin MIC = >256 μg/ml), and (2) levofloxacin-susceptible, methicillin-susceptible S. aureus (K1181 – levofloxacin MIC = 0.19 μg/ml, cephalothin MIC = .75 μg/ml, oxacillin = 0.75 μg/ml).
The P. aeruginosa isolates used in these studies were the following: (1) levofloxacin-resistant P. aeruginosa (PA-E, levofloxacin = MIC 32 g/ml; tobramycin MIC = 0.5 μg/ml), and (2) levofloxacin-susceptible P. aeruginosa (K900, levofloxacin MIC = 0.75 μg/ml; ciprofloxacin MIC = 0.19 μg/ml).
The bacterial isolates were collected from clinical cases of keratitis and are part of our retrospective clinical collection of bacterial isolates that were de-identified and stored for antibiotic validations and resistance monitoring.
The bacterial inoculum to be injected into the rabbit corneas was prepared by growing the isolates overnight at 36°C in a CO2 atmosphere on trypticase soy agar supplemented with 5% sheep blood plates (Becton, Dickinson, and Company, Sparks, MD, USA). A few colonies were suspended in 10 ml of trypticase soy broth (Becton, Dickinson, and Company) to the turbidity of a 0.5 McFarland Standard. The turbidity was measured spectrophotometrically at wave length 650 nm. The optical density was recorded and compared to a predetermined optical density that was equated to a bacterial concentration. Concurrently, a standard colony count was determined on the turbid bacterial suspension. The bacterial suspension was further diluted in trypticase soy broth to achieve an inoculum to be injected of 1000 CFU per 25 μl.
In brief, standard colony counts were determined by serially diluting the corneal homogenates 10-fold for four dilutions (10−1, 10−2, 10−3 and 10−4) and pipetting 0.1 ml of each dilution and the undiluted sample onto duplicate plates containing trypticase soy agar supplemented with 5% sheep blood. After incubation at 37°C for 24 hr, the colonies on the plates containing approximately 20–200 colonies were counted. Using the dilution factor, volume plated and the average colony count of two plates, the bacterial concentration as CFU/ml was calculated (CFU per ml = average colony counts/0.1× dilution factor).
Experimental drugs
IQUIX® (levofloxacin ophthalmic solution 1.5%; Vistakon Pharmaceuticals LLC, Jacksonville, FL, USA) (Lot 116154; Exp. 10/2010; Lot 116876; Exp. 01/2011) was provided by Vistakon Pharmaceuticals LLC. The generic equivalent of the commercial preparation Ciloxan® [Ciprofloxacin Hydrochloride Ophthalmic Solution, 0.3% (Falcon Pharmaceuticals, Ltd., Fort Worth, TX, USA) Lot #147438F Exp. May 2010] and the fortified preparations of cefazolin (50 mg/ml, 5%), vancomycin (50 mg/ml, 5%) and tobramycin (14 mg/ml, 1.4%) were prepared as for patient use and purchased from the pharmacy at the University of Pittsburgh Medical Center, Pittsburgh, PA. Cefazolin is a standard treatment of Gram-positive bacterial keratitis, and vancomycin is the standard treatment for MRSA keratitis. Ciloxan® and fortified tobramycin are standard treatments for P. aeruginosa keratitis. Fortified antibiotics were prepared by the pharmacist as the standard preparations used in patients with keratitis. Sodium Chloride Injection 0.9% USP (Baxter Healthcare Corp. Deerfield, IL, USA) (Lot # C725663, Exp. May 2009) was also purchased from the pharmacy at the University of Pittsburgh Medical Center, Pittsburgh, PA and served as control drops. The saline control was instilled using a reusable glass medicine dropper.
Animals
Three to four pound New Zealand white rabbits were purchased from Myrtle’s Rabbitry, Thompson Station, Tennessee, USA.
Data analysis
The corneal colony counts values from each rabbit cornea were converted to log values (i.e. 105 CFU was converted to 5) using a log10 (CFU + 1) transformation. The data (treatment groups) were non-parametrically analysed using Kruskal–Wallis anova using Duncan’s Multiple Comparisons (True Epistat, Mesquite, TX, USA) with significance set at p = 0.05. A 3 log10 (99.9%) decrease in median colony counts between treated groups and the onset of therapy control was deemed a bactericidal effect. The ratio of sterile corneas of the topical treatment groups was statistically compared with the Fisher’s exact test (True Epistat) for small group randomization with significance set at p = 0.05. A sterile cornea demonstrated no colony counts from the homogenized corneas.
Results
Figure 1 compares the anti-bacterial activity of levofloxacin 1.5% to vancomycin 5% and saline against levofloxacin-resistant, vancomycin-susceptible (methicillin-resistant) S. aureus. Vancomycin is the first-line treatment for methicillin-resistant S. aureus keratitis. Levofloxacin 1.5% was more effective than fortified vancomycin 5% in reducing the number of corneal colony counts (p < 0.05, Kruskal–Wallis). Based on the decrease in colony counts determined at the onset of treatment, only levofloxacin 1.5% was bactericidal with a 3 log decrease after treatment. In comparison, vancomycin 5% demonstrated a 2 log decrease and reduced significantly the number of colony counts in comparison with saline (p < 0.05, Kruskal–Wallis). Table 1 presents the number of eyes that were sterilized (no bacterial growth after treatment) by each topical antibiotic treatment. All homogenized corneas in the levofloxacin-resistant, vancomycin-susceptible S. aureus group were positive for bacterial growth except for one cornea in the vancomycin 5% group. In contrast, topical levofloxacin 1.5% sterilized the corneas infected with levofloxacin-susceptible S. aureus and levofloxacin-susceptible and resistant P. aeruginosa. This data indicated that long-term therapy with levofloxacin 1.5% or vancomycin 5% may be necessary to effectively eradicate levofloxacin-resistant S. aureus.
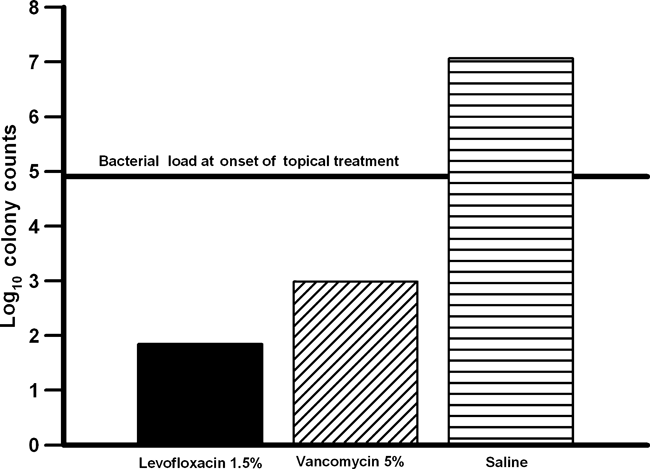
Bar graph compares the anti-bacterial activity of levofloxacin 1.5% (IQUIX®) to vancomycin 5% and saline for levofloxacin-resistant, vancomycin-susceptible Staphylococcus aureus.
| Topical agent | LevR-SA | LevS-SA | LevR-PA | LevS-PS |
|---|---|---|---|---|
| Levofloxacin 1.5% | 0/16 (0%) | 16/16 (100%) | 16/16 (100%) | 16/16 (100%) |
| Vancomycin 5% | 1/16 (6.3%) | – | – | – |
| Cefazolin 5% | – | 8/16 (50%) | – | – |
| Tobramycin 1.4% | – | – | 13/16 (81.3%) | – |
| Ciprofloxacin 0.3% | – | – | – | 16/16 (100%) |
| Saline | 0/16 (0%) | 0/16 (0%) | 0/14 (0%) | 0/16 (0%) |
- 0/16 indicates no corneas were sterilized with 16 tested.
- 16/16 indicates that 16 corneas were sterilized with 16 tested.
- 0/14 – Two rabbits were killed prematurely because of unrelated health problems.
- (–) denotes not tested.
- LevR-SA = levofloxacin-resistant Staphylococcus aureus; LevS-SA = levofloxacin-susceptible Staphylococcus aureus; LevR-PA = levofloxacin-resistant Pseudomonas aeruginosa; LevS-PA = levofloxacin-susceptible Pseudomonas aeruginosa.
Figure 2 compares the anti-bacterial activity of levofloxacin 1.5% to cefazolin 5% and saline against levofloxacinsusceptible, cefazolin-susceptible (methicillin-susceptible) S. aureus. Cefazolin 5% is a first-line treatment for methicillin-susceptible S. aureus. Levofloxacin 1.5% was as effective (p > 0.05, Kruskal–Wallis) as fortified cefazolin 5% in reducing the number of corneal colony counts and both reduced significantly more colony counts than saline (p < 0.05, Kruskal–Wallis). Based on the decrease in colony counts determined at the onset of treatment, both levofloxacin 1.5% (4 log) and cefazolin 5% (3 log) were determined to be bactericidal after treatment. Levofloxacin 1.5% (16/16) was more effective (p = 0.0024, Fisher’s Exact) than fortified cefazolin 5% (8/16) in sterilizing the infected corneas of the levofloxacin-susceptible, cefazolin-susceptible S. aureus group (Table 1). This data indicated that levofloxacin 1.5% may eradicate levofloxacin-susceptible, cefazolin-susceptible S. aureus at a faster rate than cefazolin 5%.

Bar graph compares the anti-bacterial activity of levofloxacin 1.5% (IQUIX®) to cefazolin 5% and saline for levofloxacin-susceptible, cefazolin-susceptible Staphylococcus aureus.
Figure 3 compares the anti-bacterial activity of levofloxacin 1.5% to fortified tobramycin 1.4% and saline for levofloxacin-resistant, tobramycin-susceptible P. aeruginosa. Fortified tobramycin 1.5% is a first-line treatment of levofloxacin-resistant P. aeruginosa keratitis. Levofloxacin 1.5% was as effective (p > 0.05) as fortified tobramycin 1.4% in reducing the number of corneal colony counts and both reduced significantly more colony counts than saline (p < 0.05, Kruskal–Wallis). Based on the decrease in colony counts determined at the onset of treatment, both levofloxacin 1.5% (≈3 log) and tobramycin (≈3 log) were determined to be near bactericidal after treatment. Levofloxacin 1.5% sterilized 16 of 16 corneas, and tobramycin 1.4% sterilized 13 of 16. Statistically, there was no difference (16/16 versus 13/16) (p = 0.23, Fisher’s Exact) (Table 1). The data indicated that levofloxacin 1.5% eliminated levofloxacin-resistant P. aeruginosa faster than tobramycin 1.4%. Long-term therapy with fortified tobramycin may be necessary to effectively treat levofloxacin-resistant P. aeruginosa keratitis.
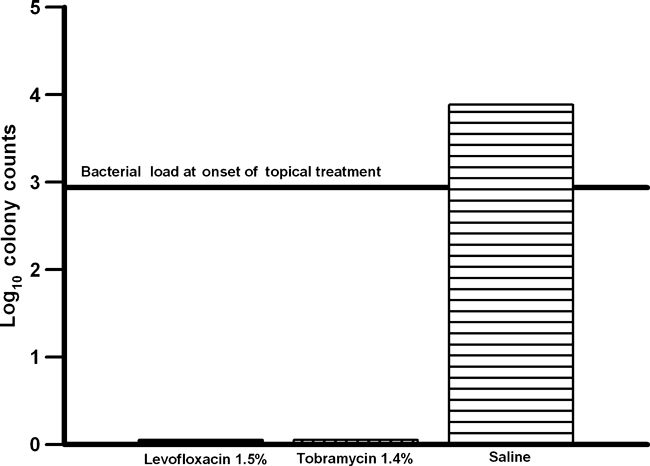
Bar graph compares the anti-bacterial activity of levofloxacin 1.5% (IQUIX®) to tobramycin 1.4% and saline for levofloxacin-resistant, tobramycin-susceptible Pseudomonas aeruginosa.
Figure 4 compares the anti-bacterial activity of levofloxacin 1.5% to ciprofloxacin 0.3% and saline for levofloxacin-susceptible, ciprofloxacin-susceptible P. aeruginosa. Ciprofloxacin 0.3% is a first-line treatment for levofloxacin-susceptible P. aeruginosa keratitis. Levofloxacin 1.5% was as effective (p > 0.05, Kruskal–Wallis) as ciprofloxacin 0.3% in reducing the number of corneal colony counts and both reduced significantly more colony counts than saline (p < 0.05, Kruskal–Wallis). All corneas were sterilized by both levofloxacin 1.5% and ciprofloxacin 0.3% (16 of 16) (p = 1.0) (Table 1). This data indicated that both levofloxacin 1.5% and ciprofloxacin can be considered as first-line therapy for the topical treatment of levofloxacin-susceptible, ciprofloxacin-susceptible P. aeruginosa keratitis.

Bar graph compares the anti-bacterial activity of levofloxacin 1.5% (IQUIX®) to ciprofloxacin 0.3% and saline for levofloxacin-susceptible, ciprofloxacin-susceptible Pseudomonas aeruginosa.
Discussion
Interpretation of ‘susceptible’, as determined by the systemic blood serum standards, can predict in vivo susceptibility with some assurance, but the interpretation of in vitro‘resistance’ may not predict in vivo resistance with the same degree of assurance. The current experimental study and the clinical success of newer and older FQ topical therapy (McLeod 2006; McDonald 2006) suggest that clinical efficacy cannot be based exclusively on MIC data, but that other factors such as tissue penetration and dosing are likely to be important factors in clinical outcome. Levofloxacin 1.5%, IQUIX® (Vistakon, Pharmaceuticals LLC), is formulated as the highest concentration of topical FQ on the market and has FDA (USA Federal Drug Administration) approval for the treatment of keratitis. The high concentration of levofloxacin at 1.5% in the formulated solution has been reported to elevate the concentration of levofloxacin in the ocular tissues (McLeod 2006; McDonald 2006). Our experimental study suggests that an optimized drug concentration and dose regimen presumably explain the efficacious levels of levofloxacin in the ocular tissues that overcame the resistance predicted by the blood serum standard.
There are no other independent studies supporting or refuting the treatment of bacterial keratitis by levofloxacin-resistant bacteria with levofloxacin 1.5%. Dajcs reported that ofloxacin-resistant S. aureus keratitis treated with levofloxacin 0.5% decreased the colony counts in comparison with a non-treated controls but the true bactericidal effect could not be determined because the bacterial load at the onset of treatment was not determined (Dajcs et al. 2004). We do note that in an endophthalmitis prevention model, levofloxacin 0.5% was not able to consistently prevent bacterial infection of a levofloxacin-resistant S. aureus. Whether levofloxacin 1.5% can be more effective, would be conjecture without experimental support (Kowalski et al. 2008).
Levofloxacin 1.5% demonstrated a greater decrease in colony counts in comparison with vancomycin 5%. This difference can be explained by the fact that levofloxacin is a concentration-dependent anti-infective, and the effective concentration was reached within the time frame of experimental testing (hours), whereas vancomycin is a time-dependent antibiotic that requires additional time for effective therapy as occurs in the time frame of clinical dosing (days).
The in vivo results of this experimental study predict that frequent topical therapy with levofloxacin 1.5% may be efficacious in the treatment of S. aureus (predicted by in vitro blood serum standards to be levofloxacin-resistant) and P. aeruginosa (predicted by in vitro blood serum standards to be levofloxacin-resistant). However, an important precaution must be understood. It cannot be assumed automatically that levofloxacin 1.5% will be effective in treating keratitis caused by all bacterial pathogens that are tested to be ‘resistant’ by in vitro susceptibility methods. Rhee demonstrated in a rabbit keratitis study that ciprofloxacin 0.3% was not effective in reducing P. aeruginosa colony counts that was highly resistant to ciprofloxacin by the blood serum standard with an MIC > 128 μg/ml (Rhee et al. 2004). In vitro resistance is generally tiered, whereas low resistance is characterized by MICs near but higher than the serum standard, and high resistance is characterized by high MICs greater than the range of routine testing at about 128 μg/ml and greater. The MICs of both the levofloxacin-resistant S. aureus and levofloxacin-resistant P. aeruginosa used in this experimental study were 32 μg/ml and both were considered to be moderately resistant.
In a clinical setting, proficient bacterial keratitis management should be directed by laboratory investigation with culture and susceptibility testing for all vision-threatening central corneal infections prior to the onset of treatment. The information gained is invaluable for those corneal infections that do not respond to empiric broad spectrum antibiotic therapy and provides a rational basis for the subsequent choice of appropriate therapy.
When laboratory investigations are not available or feasible, the use of empiric broad spectrum therapy then becomes the default approach. In this study, therapy with levofloxacin 1.5% was as or more effective in reducing colony counts of S. aureus and P. aeruginosa, including levofloxacin-resistant, vancomycin-susceptible and levofloxacin-resistant, tobramycin-susceptible strains, compared to other commonly used therapies (fortified antibiotics). However, empiric treatment with levofloxacin 1.5% may not be efficacious with bacteria found to have high resistance (MICs greater than 128 μg/ml) to levofloxacin. It is highly recommended that patients with keratitis who are not responding to empiric therapy then be referred to facilities with laboratory investigation capabilities to determine whether the infecting bacterium has a possible multi-resistant anti-infective profile.
In summary, we accept the hypothesis that in vitro resistance to levofloxacin does not always predict in vivo resistance. Levofloxacin 1.5% was effective in reducing colony counts of S. aureus and P. aeruginosa in the rabbit keratitis model that were not always predicted by in vitro susceptibility testing. Levofloxacin 1.5% was more effective against levofloxacin-resistant S. aureus and as effective against levofloxacin-resistant P. aeruginosa in comparison with standard therapies. Clinically, we recommend that laboratory investigation supports the choice of any treatment in vision-threatening keratitis, including levofloxacin 1.5%, to optimize patient care and to avoid complications because of infecting bacteria with high anti-infective resistance.
Acknowledgements
This study was funded by a grant from Vistakon, Pharmaceuticals LLC Jacksonville, FL, with a research contract through the University of Pittsburgh, Pittsburgh, PA. The Pennsylvania Lions Club has provided support of diagnostic testing development for the Charles T. Campbell Ophthalmic Microbiology Laboratory. The Eye and Ear Foundation of Pittsburgh, Pittsburgh, PA, has provided salary support. Research to Prevent Blindness, Inc. has provided financial support to the Department of Ophthalmology and has provided a Career Development Award to Robert M.Q. Shanks, PhD.
Conflict of interest statement
This study was funded by Vistakon Pharmaceuticals LLC, Jacksonville, FL, with a research contract through the University of Pittsburgh, Pittsburgh, PA. The authors (RPK, EGR, FSM and RMQS) have been paid independent consultant fees by Vistakon Pharmaceuticals LLC, and these fees were deemed not to produce a Conflict of Interest for the authors by the University of Pittsburgh, Pittsburgh, PA. Vistakon Pharmaceuticals LLC did not design, participate, collect data or analyse the data for this study. Vistakon Pharmaceuticals LLC approved the design and was allowed to review the manuscript prior to journal submission. The first author, Regis P. Kowalski, is fully responsible for the content of this manuscript.




