Stem cell factor induces ERM proteins phosphorylation through PI3K activation to mediate melanocyte proliferation and migration
Summary
Stem cell factor (SCF) activates a variety of signals associated with stimulation of proliferation, differentiation, migration, and survival in melanocytes. However, the molecular mechanisms by which SCF and its receptor Kit activates these signaling pathways simultaneously and independently are still poorly defined. Here, we examined whether SCF induces ezrin/radixin/moesin (ERM) proteins phosphorylation as a downstream target of PI3K in melanocytes. ERM proteins are cross-linkers between the plasma membrane and the actin cytoskeleton and are activated by phosphorylation of a C-terminal threonine residue. Our results demonstrated that SCF-induced ERM proteins phosphorylation on threonine residue and Rac1 activation in cultured normal human melanocytes through the activation of PI3K. The functional role of phosphorylated-ERM proteins was examined using melanocytes infected with adenovirus carrying a dominant negative mutant (Ala-558, TA) or wild type of moesin. In the TA moesin-overexpressing melanocytes, SCF-induced cell proliferation and migration were inhibited. Thus, our results indicate that phosphorylation of ERM proteins plays an important role in the regulation of SCF-induced melanocyte proliferation and migration.
Introduction
Melanocytes are specialized cells of the epidermis that synthesize melanin, the molecule that is responsible for skin pigmentation. It is well-accepted that paracrine growth factors produced by keratinocytes such as endothelin-1, stem cell factor (SCF), and basic fibroblast growth factor (bFGF), regulate melanocyte proliferation along with other diverse melanocyte functions such as melanin synthesis and dendricity (Hirobe et al., 2004; Imokawa, 2004). Among them, the Kit receptor ligand, SCF, is sufficient to sustain proliferation on its own under serum-free conditions in human melanocytes (Imokawa et al., 1996). SCF binding to the Kit receptor mediates dimerization, activation of its intrinsic tyrosine kinase activity, and autophosphorylation (Blume-Jensen et al., 1991). The activated receptor then phosphorylates various substrates and associates with various signaling molecules, including phosphatidylinositol 3-kinase (PI3K), the Shc and Grb2 adaptor proteins, the guanine nucleotide exchange factor, SOS, and components of the Ras-MAPK pathway (Cutler et al., 1993; Liu et al., 1994; Lennartsson et al., 1999). However, the molecular mechanisms by which SCF and its receptor, Kit, mediate migration, survival, proliferation, and differentiation in melanocytes are still poorly defined. Recently, PI3K and its downstream effecter protein kinase Akt (or PKB) were implicated as important mediators of survival in neuronal cells and human melanocytes (Dudek et al., 1997; Kennedy et al., 1997; Kulik et al., 1997; Larribere et al., 2004). PI3K regulates actin dynamics induced by growth factor stimulation through the regulation of Rac (Nobes et al., 1995). In melanocytes, SCF-induced lamellipodium formation is Rac1-dependent (Ballestrem et al., 2000), suggesting that Rac may be a downstream mediator of PI3K stimulated by SCF in melanocytes. Recently, we reported that the KCl-induced phosphorylation of moesin was Rac-dependent in PC 12 cells (Jeon et al., 2005) and that nerve growth factor-induced phosphorylation of moesin was mediated through PI3K/Rac/Akt activation in PC12 cells (S. Jeon, B. S. Koo, Y. S. Kim, C. D. Bae and J. Park, unpublished data), which suggest the possibility that the ERM (ezrin/radixin/moesin) proteins family could be a downstream target of Rac in SCF-treated melanocytes.
Ezrin/radixin/moesin proteins consist of three domains: a globular domain in the N-terminal region, which is conserved among the members of the band 4.1 superfamily and is referred to as the FERM (4.1 and ERM) domain, a membrane-binding domain, and an extended alpha-helical, charged C-terminal domain, which contains a consensus sequence motif for actin binding. Thus, ERM proteins have been suggested to function as cross-linkers between actin filaments and plasma membranes, and to be involved in microvilli formation, cell adhesion, cell motility, cytokinesis, phagocytosis, and the integration of membrane transport with signaling pathways (Bretscher et al., 2002). Phosphorylation at a C-terminal threonine residue (Thr-567 of ezrin, Thr-564 of radixin, Thr-558 of moesin) is required for the function of ERM proteins (Bretscher, 1989). This threonine residue was shown to be effectively phosphorylated in vitro by Rho-kinase, phosphatidyl-inositol 4-phosphate 5-kinase, PKC-θ, myotonic dystropy kinase-related Cdc42-binding kinase, and Akt (Matsui et al., 1998; Nakamura et al., 2000; Oshiro et al., 1998; Pietromonaco et al., 1998; Wu et al., 2004).
In this study, we found that SCF induces phosphorylation of the critical C-terminal threonine of ERM proteins in human primary melanocytes through the activation of PI3K, and phosphorylated-ERM proteins are required for SCF-induced melanocyte proliferation and migration.
Results
SCF induces ERM proteins phosphorylation at the C-terminal threonine in human melanocytes
Ezrin/radixin/moesin protein expression is tissue type-dependent (Amieva and Furthmayr, 1995). However, in human melanocytes, the expression of ERM proteins had not yet been determined. Therefore, we examined the levels of each ERM family protein in melanocytes. Anti-ERM antibody detected three bands; upper two bands are ezrin and radixin and lower is moesin; molecular weight of ERM proteins, ezrin is 82 kDa, radixin is 80 kDa and moesin is 75 kDa. Moesin was predominant ERM proteins compared with ezrin and radixin in melanocytes (Figure 1A, left panel). Moreover, p-ThrERM antibody detected one main band in the control melanocytes, and this band corresponded to moesin as compared with molecular weight. ERM proteins were phosphorylated at threonine residue in melanocytes even without stimulation, but 50 ng/ml SCF treatment rapidly increased ERM proteins phosphorylation, which was transient; it increased at 5 min, peaked at 30 min (triple the basal level), and returned to basal levels after 60 min of SCF treatment (Figure 1B).
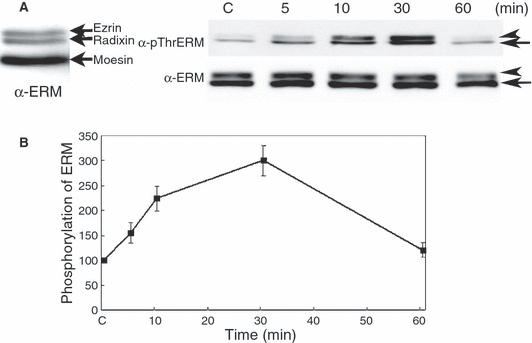
Stem cell factor induces ezrin/radixin/moesin proteins phosphorylation at the C-terminal threonine residue in human melanocytes. (A) Supplement-starved melanocytes were stimulated with 50 ng/ml of SCF for 5, 10, 30, or 60 min. Whole cell lysates were subjected to SDS–PAGE and analyzed by immunoblotting with anti-p-ThrERM or ERM antibody. Before measuring the phosphorylation levels of ERM proteins, the distribution of ERM proteins in melanocytes was examined with unstimulated melanocytes in 6% SDS–PAGE. The upper two bands are ezrin and radixin and lower band is moesin (arrow indicated, left panel). The faint upper bands in the top lanes are phospho-ezrin and radixin (arrow head) and lower band is moesin (arrow) (left panel). (B) The intensity of phosphorylated and total moesin and ezrin bands in (A) were quantified by densitometric analysis and the amounts of phosphorylated-ERM proteins were normalized to total ERM proteins. The data represent the means ± SE of four independent experiments. C: unstimulated melanocytes.
SCF-induced ERM proteins phosphorylation is dependent on PI3K activation in human melanocytes
As a next step to determine the involvement of PI3K in SCF-induced ERM proteins phosphorylation, we used an inhibitor of PI3K, LY294002. The inhibitor (30 nM) was added to the medium for 30 min and the ERM proteins phosphorylation was examined after 5 or 30 min of SCF treatment. In the presence of LY294002, SCF-induced ERM proteins and Akt phosphorylation were completely suppressed in melanocytes (Figure 2A, B). Moreover, the phosphorylation of Akt was maximally increased at 5 min and remained high for 30 min following SCF treatment, which preceded moesin phosphorylation. Thus, these results indicate that PI3K/Akt activation is required for SCF-induced ERM proteins phosphorylation in melanocytes.
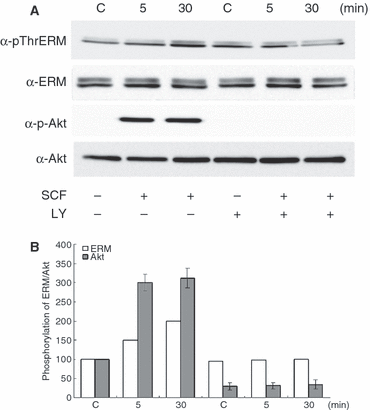
Stem cell factor-induced ezrin/radixin/moesin proteins phosphorylation is dependent on PI3K activation in human melanocytes. (A) Supplement-starved melanocytes were preincubated with 30 nM LY294002 for 30 min and then stimulated or not stimulated with 50 ng/ml of SCF for 5 and 30 min. Whole cell lysates were subjected to SDS–PAGE and analyzed by immunoblotting with anti-p-ThrERM, ERM, p-Akt or Akt antibodies. (B) The intensities of phosphorylated and total bands in A were quantified by densitometry, and the amounts of phosphorylated proteins were normalized to total protein levels. The data shown represent means ± SE of three independent experiments. C: untreated melanocytes.
SCF-induced Rac1 activation is dependent on PI3K activation in human melanocytes
The Rho family proteins, RhoA, Rac1, and Cdc42 have been implicated in the phosphorylation of moesin in various cell lines (Nakamura et al., 2000). SCF was shown to activate Rac1 in mast cells (Samayawardhena et al., 2007). Here, Rac1 activity following 50 ng/ml SCF treatment was examined in melanocytes by measuring the amounts of Rac1/Cdc42 bound to a glutathione S-transferase (GST)-fused Rac/Cdc42-binding domain of PAK3 (GST-PBD). Increased Rac1 activity was observed after 5 min of SCF treatment and it continued to increase gradually for 30 min. This time course coincided with that of ERM proteins phosphorylation, suggesting that Rac1 might be involved in the phosphorylation of ERM proteins that is induced by SCF treatment. Moreover, SCF-induced Rac1 activation was completely inhibited by pretreatment with LY294002, a PI3K inhibitor (Figure 3A, B), suggesting that SCF-induced Rac1 activation is mediated through PI3K in melanocytes.
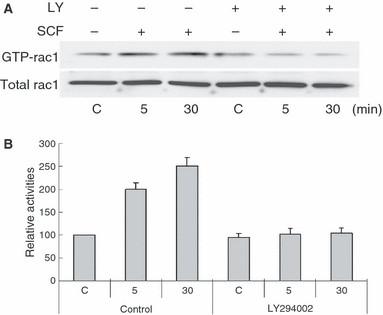
Stem cell factor activates Rac1 through PI3K activation in melanocytes. Supplement-starved melanocytes were preincubated with 30 nM LY294002 (LY) and then stimulated or not stimulated with 50 ng/ml SCF for 5 and 30 min. Whole cell lysates were incubated with GST-PBD, and the amounts of GTP-bound Rac1 were determined by immunoblotting with anti-Rac1 antibody (A) and densitometry (B). The data shown represent the means ± SE of three independent experiments. C: untreated melanocytes.
SCF-induced ERM proteins phosphorylation is involved in melanocyte proliferation
To study the role of phosphorylated ERM proteins in melanocytes, we made a wild type moesin (WT moesin) and phosphorylation-defective mutant moesin adenoviruses (TA moesin; Thr-558 is substituted by Ala). Melanocytes were infected with adenovirus carrying WT moesin (WT), TA moesin (TA) or a control virus carrying blue florescent protein (BFP). We examined whether moesin phosphorylation is involved in melanocyte proliferation with or without SCF treatment. Three days after melanocytes were infected with the indicated viruses, the expression levels of ERM proteins and the phosphorylation of endogenous moesin, as well as ezrin and radixin, was examined. In WT and TA moesin-expressing cells, moesin expression was increased by twofold compared with that of BFP-infected cells and there was no effect on the expression other ERM proteins, ezrin and radixin (Figure 4A). The phosphorylation of ERM proteins was decreased in TA moesin-overexpressing cells compared with that of WT or BFP-overexpressing cells (Figure 4A), which indicated that TA moesin inhibits the function of endogenous ERM proteins by suppressing phosphorylation and confirmed previous findings (Oshiro et al., 1998). To examine the functional role of phosphorylated-ERM proteins after SCF treatment in melanocytes, we counted the numbers of BFP, WT or TA moesin-expressing cells at 4 days after SCF treatment. In BFP virus-infected cells, melanocyte proliferation was increased by SCF treatment (147 ± 14%). On the contrary, in WT and TA moesin-expressing cells, the number of cells was significantly increased compared to that in BFP-infected cells at 3 days after each virus infection (125 ± 11% and 119 ± 6%, respectively) without stimulation of SCF (Figure 4B). In that time, each cell was treated with SCF. SCF further induced melanocyte proliferation in WT moesin-expressing cells, but not in TA moesin-expressing cells (Figure 4B). To confirm that the threonine residue phosphorylation of ERM proteins is required for the SCF-induced melanocyte proliferation, the activity of cyclin-dependent kinase 2 (CDK2) was measured by using anti-phospho-CDK2 antibody. As shown in Figure 4C, the phosphorylation level of CDK2 was decreased in TA moesin-expressing cells compared with that of BFP or WT moesin-expressing cells after SCF treatment, indicating that the cell cycle progression from G1 to S-phase was reduced in the TA moesin-expressing cells.
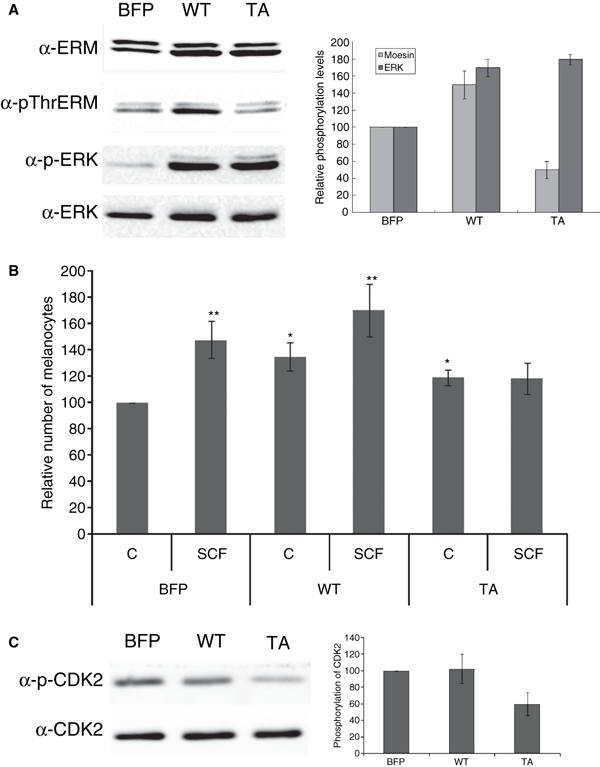
Stem cell factor-induced ezrin/radixin/moesin proteins phosphorylation is involved in melanocyte proliferation. (A) Melanocytes were infected with adenovirus carrying wild type (WT), mutant moesin (TA) and a control virus carrying BFP (10 MOI) for 3 days. Melanocytes were then stimulated with 50 ng/ml of SCF for 4 days. Whole cell lysates of 4 days after viral transfection were subjected to SDS–PAGE and analyzed by immunoblotting with anti-p-ThrERM, ERM, p-ERK or ERK antibody. The intensities of phosphorylated and total bands in A were quantified by densitometry, and the amounts of phosphorylated proteins were normalized to total protein levels. The data shown represent means ± S.E. of four independent experiments. (B) The number of melanocytes was examined by using the MTT assay at 3 days after viral transfection (C) and then the cells were treated with SCF for 4 days (SCF). Each cell lysates were subjected to SDS–PAGE and analyzed by immunoblotting with anti-phospho-CDK2 or CDK2 antibody. The error bars indicate SE (n = 3); *P < 0.01, **P < 0.001.
The overexpression of WT and TA moesin increased melanocyte proliferation compared to that of BFP (Figure 4B). Thus, to determine which signaling molecules are involved in this process, we examined ERK activation in melanocytes. Activation of ERK1/2 is critical for the mitogenic response of melanocytes. Failure to activate this kinase is evident in terminally differentiated melanocytes (Medrano et al., 1994). In WT and TA moesin-expressing cells, ERK1/2 phosphorylation was significantly increased compared to that in BFP-infected cells at 3 days post-infection (Figure 4A).
SCF-induced ERM proteins phosphorylation is involved in melanocyte migration
As migration of melanocytes was shown to be associated with changes in expression of adhesion proteins such as E-cadherin and N-cadherin (Haass et al., 2005), we examined the expression of these proteins in BFP- WT- and TA-infected cells and determined the cell migration abilities of both cell types using a Boyden chamber assay. In the absence of SCF, a greater number of cell migrations occurred in WT and TA moesin-expressing cells than in BFP-infected cells (Figure 5B). Moreover, levels of N-cadherin expression were elevated in WT and TA moesin-infected cells compared to BFP-infected cells, whereas those of E-cadherin were similar between each cell (Figure 5A). Boyden chamber assays showed that SCF additionally stimulated melanocyte migration in BFP and WT-infected cells and not in TA-infected cells (Figure 5B), which is consistent with western blot data and N-cadherin protein expression.
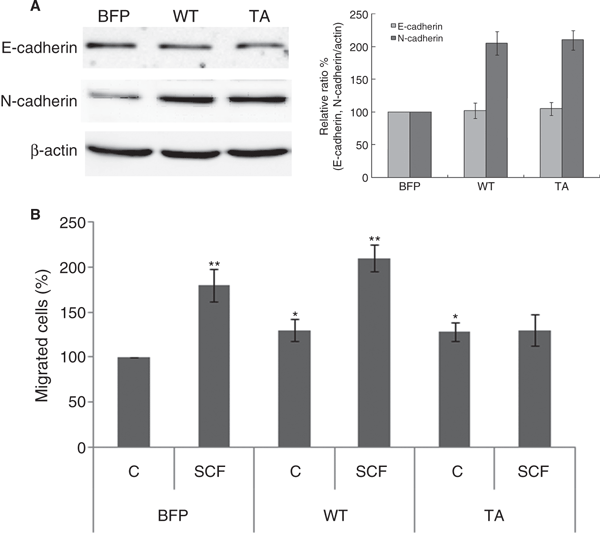
Stem cell factor-induced ezrin/radixin/moesin proteins phosphorylation is involved in melanocyte migration. (A) Whole cell lysates of 3 days after viral transfection were subjected to SDS–PAGE and analyzed by immunoblotting with anti-N-cadherin, E-cadherin and β-actin antibodies. The intensities of bands were quantified by densitometry. The data shown represent means ± SE of three independent experiments. (B) Both BFP, WT or TA moesin-infected melanocytes were plated onto Boyden chambers and treated with/without SCF for 72 h. The migrated cells were photographed and the quantitation was carried out by determining the percentage of migrated cells compared with the control. The error bars indicate SE (n = 3); *P < 0.01, **P < 0.001.
Discussion
Kit is a receptor tyrosine kinase that binds to the ligand SCF, resulting in the stimulation of various signaling pathways in melanocytes. However, at present it is unclear whether activated Kit transmits signals to different target molecules simultaneously and independently, or whether there is crosstalk between different downstream signaling pathways of kit that enhance or oppose one another and regulate/coordinate the receptor’s numerous roles. In this study, we demonstrated for the first time that phosphorylated ERM proteins, specifically moesin, may be downstream effectors of PI3K for the regulation of SCF-induced melanocyte proliferation and migration.
Recent analyses have shown that ERM proteins are not only involved in cytoskeletal organization but are also involved in signaling pathways (Batchelor et al., 2004). However, the functional role of ERM proteins as well as their phosphorylation state in melanocytes has not been elucidated. In this study, we showed for the first time that SCF activation leads to the phosphorylation of ERM proteins through activation of PI3K/Akt and Rac1 in human melanocytes. Moreover, we have studied the functional role of phosphorylated-ERM proteins by using the phosphorylation defective (TA) mutant moesin. Previously it has been reported that the formation of ezrin dimmers in response to EGF stimulation in A431 cells, which partially corresponded with tyrosine-phosphorylation of ezrin (Berryman et al., 1995). Thus, dominant-negative effect of the TA mutant may be explained by the formation of heterodimers between endogeneous ERM proteins and transfected TA-mutant moesin.
Stem cell factor activates ERK and PI3K, two signaling pathways largely involved in proliferation and survival of several cell types including melanocytes (Chang et al., 2003; Larribere et al., 2004; Neri et al., 2002), and Kit-dependent migration of various cell types was previously shown to be associated with activation of PI3K (Dudek et al., 1997). However, the effectors and downstream signaling pathways of PI3K remain unknown in melanocytes. In this study, we first demonstrated that SCF activates Rac1 and ERM proteins through PI3K activation in melanocytes. This is consistent with previous reports that SCF-induced Rac1 activation is mediated by PI3K in mast cells, a process critical for SCF-induced proliferation (Timokhina et al., 1998), and that SCF-induced migration is mediated by Rac1 activation in melanocytes (Ballestrem et al., 2000). Indeed, SCF-induced ERM proteins phosphorylation on threonine residue was also important for SCF-induced proliferation and migration in melanocytes. Overall, these results suggest that SCF-mediated induction of melanocyte proliferation and migration through PI3K is a common signaling pathway and that ERM proteins are downstream targets of this pathway.
Interestingly, in TA and WT moesin-overexpressing cells, ERK phopshorylation, which is involved in melanocyte proliferation and melanogenesis, was increased in the normal melanocytes growth media (4, 5). Moreover, the expression of N-cadherin, a protein associated with cell migration was enhanced in WT and TA moesin overexpressing cells compared with that of control. These results suggest that the other site besides C-terminal threonine residue of ERM proteins may be involved to induce melanocyte proliferation and migration by other growth factors or activating reagents supplemented in the melanocyte growth media such as bFGF and phorbol 12-myristate 13 (PMA). In fact, Tyr145 of ezrin, lies in the N-terminal region is phosphorylated by epidermal growth factor (EGF) in A431 cells and this tyrosine residue and its vicinal amino acids are conserved throughout the family members, including radixin and moesin (Krieg and Hunter, 1992). Furthermore, cells expressing ezrin Y145F shows defect in the proliferation and adhesion-mediated events in epithelial cells (Srivastava et al., 2005) and ezrin expression correlates with tumor thickness and level of invasion in primary cutaneous melanoma (Ilmonen et al., 2005). Moreover, it has been reported that ezrin and moesin localise to the nucleus in a cell density-dependent manner and phosphorylation in the actin-binding domain is not a prerequisite for nuclear localization (Batchelor et al., 2004). These suggest that tyrosine residue or other site rather than threonine residue phosphorylation of ERM proteins may play a functional role in the melanocyte proliferation and migration. However, it remains to be examined how ERM proteins up-regulates ERK phosphorylation and N-cadherin expression and what is the downstream target of ERM proteins phosphorylation.
In this study, we also present evidence that SCF-induced ERM proteins phosphorylation is primarily responsible for the stimulation of proliferation and migration rather than differentiation of melanocytes. Previously, it has been reported that inhibition of RhoA and its effector, Rho-kinase, is required for cAMP-induced melanoma cell differentiation (Buscà et al., 1998). Although RhoA and Rho-kinase are well-known to be part of an upstream signaling pathway of ERM proteins in various cells (Fukata et al., 1998; Jeon et al., 2002; Oshiro et al., 1998), other effecters of Rho-kinase such as myosin light chain kinase have been reported in cAMP-induced melanogenesis (Buscà et al., 1998), suggesting that the effect may be different according to the signaling pathway involved.
The Rho family of GTP-binding proteins plays a critical role in cytoskeletal organization in all cell types tested, as well as determination of cell polarity, cell–cell adhesion, cell cycle regulation, apoptosis, exocytosis, and endocytosis (Doussau et al., 2000; Lamaze et al., 1996). In most cell types, RhoA mediates stress fibre formation (Ridley and Hall, 1992), Rac1 mediates membrane ruffling and lamellipodia formation (Ridley et al., 1992) and Cdc42 mediates filopodia formation (Kozma et al., 1995). In Melb-a, a melanoblast cell line, SCF induces large lamellipodia formation within 10 min (Ballestrem et al., 2000). These Rho family proteins also have been implicated in the C-terminal threonine phosphorylation of ERM proteins in various cell lines (Nakamura et al., 2000). In this study, we examined the SCF-induced Rac1 activation and ERM proteins phosphorylation in melanocytes. However, SCF-induced changes in cell shape after 30 min of treatment such as lamellipodia formation in BFP, WT and TA moesin-transfected cells was not observed (data not shown). This discrepancy may be explained by morphological difference as the differentiation state of melanocytes. Ballestrem et al. (2000) used melanoblast cell line showing the morphology like fibroblasts, but we used differentiated malanocytes showing polydendritic or bipolar morphology. But, when we stained these cells with anti-pERM antibody, we could observed the increased filopodia and microspike formation among WT moesin-overexpressing cells showing strongly stained with the antibody while shortened and widen cell body in TA moesin-overexpressing cells showing weekly stained with the antibody even without SCF stimulation (data not shown). Filopodia and microspike formation are dynamically active and frequently served as attachment sites from the dendrite to the keratinocyte membrane. Thus, we are studying whether ERM proteins phosphorylation is involved in the vesicular traffic such as melanosome transfer.
In conclusion, we demonstrated that SCF induces the phosphorylation of ERM proteins at the C-terminal threonine residue and Rac1 activation in human melanocytes and that these effects are mediated by PI3K activation. In addition, we found that ERM proteins phosphorylation plays important roles in regulating melanocyte proliferation and migration.
Methods
Normal human epidermal melanocyte culture
Skin specimens obtained from repeated cesarean sections and circumcisions were used for cell cultures. The cells were suspended in Medium 254 (#M-254–500; Cascade Biologics, Portland, OR, USA) supplemented with bovine pituitary extract, fetal bovine serum, bovine insulin, hydrocortisone, bFGF, bovine transferrin (#S-001-5; Cascade Biologics), heparin, and phorbol 12-myristate 13-acetate (#S-002-5; Cascade Biologics).
Treatment of melanocytes with SCF with/without Akt inhibitors
Melanocytes at passage numbers between 7 and 15 were used for the experiments. Medium 254 with supplements was changed to supplement-starved medium for 18 h. The cells were then treated with or without 50 ng/ml SCF (Cell Signaling Technology, Beverly, MA, USA) with or without 30 nM LY294002 (Calbiochem, Darmstadt, Germany). Four cell lines were examined.
MTT assay
Melanocyte proliferation was assessed with an MTT assay kit (R&D Systems, Minneapolis, MN, USA). Each absorbance was measured at a wavelength of 570 nm. The MTT assay was conducted with melanocytes treated with or without SCF after adenovirus infection.
Western blot analysis
The cultured melanocytes treated with SCF with or without inhibitor were homogenized in ice-cold homogenization buffer containing 50 mM Tris-base (pH 7.4), 150 mM NaCl, 10 mM EDTA, 0.1% Tween-20, and protease inhibitors. Equal amounts of extracted proteins (30 μg) were resolved via 10% SDS–PAGE and transferred to nitrocellulose membranes. After incubation in a blocking solution of 5% non-fat dry milk in Tris-buffered saline (TTBS) containing 10 mM Tris (pH 7.6), 150 mM NaCl, and 0.1% Tween-20, the membranes were incubated overnight at 4°C with anti-phospho-ERM (pThrERM), anti-ERM, anti-Rac, anti-phospho-ERK (p-ERK), anti-ERK, anti-phospho-Akt (p-Akt), anti-Akt, anti-E-cadherin, anti-N-cadherin, ani-phospho-CDK2 (Thr160) (Cell Signaling Technology), anti-CDK2 (Epitomics, Burlingame, CA, USA) and anti-β-actin (Sigma, St Louis, MO, USA) antibodies diluted 1:1000 in blocking solution. The membranes were then further incubated with anti-rabbit or anti-mouse horseradish peroxidase-conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and treated with an enhanced chemiluminescence solution (Pierce Biotechnology, Rockford, IL, USA). The signals were then captured on an Image Reader (LAS-3000; Fuji Photo Film, Tokyo, Japan). The protein bands were analyzed via densitometry.
Rac1 activity assays
Rac1 activities were measured as described by Jeon et al. (2002). Melanocytes (5 × 106 cells) were seeded in 100-mm culture dishes and supplement-starved for 18 h. The cells were then treated with or without SCF in the presence or absence of 30 nM LY294002.
For Rac1 activity assays, cells were resuspended in 200 μl of NS buffer (25 mM Tris, pH 7.5, 1.5 mM MgCl2, 1 mM sodium orthovanadate, 1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin) for 10 min on ice, and then passed 10 times through a 23-gauge needle. After adding 200 μl of 2× no-salt-lysis buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, 2% NonidetP-40, 1 mM sodium orthovanadate, 1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin), cell lysates were passed through a 23-gauge needle 10 times, and then placed on ice for 5 min. Four hundred microliters of high salt-binding buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, 100 mM NaCl, 0.5% NonidetP-40, 1 mM sodium orthovanadate, 1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin) were then added, and the cell lysates were centrifuged at 10 000 × g for 10 min at 4°C. Supernatants were incubated with 30 μg of the GST-Rac/Cdc42-binding domain of rat PAK3 (GST-PBD)-bound glutathione-Sepharose beads for 60 min at 4°C. Beads were washed three times with wash buffer (25 mM Tris, pH 7.5, 40 mM NaCl, 30 mM MgCl2, 1% Nonidet P-40, and 1 mM dithiothreitol), and bound proteins were eluted with Laemmli sample buffer, separated by 15% SDS–PAGE, and analyzed by immunoblotting with anti-Rac1 antibody.
Adenovirus production
The AdEasy system (Q-bio gene, Carlsbad, CA, USA) was used to construct adenoviruses carrying WT and TA moesin. The cDNA of the TA moesin mutant, which has an Ala substituted for Thr at residue 558, was generated with a site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) using pCMV-moesin as template DNA. The entire sequence of the subcloned cDNA was verified by DNA sequencing. BFP virus was constructed as a control. Viruses were propagated in 293A cells lines. Viral titers were measured in a limiting-dilution bioassay using 293A cells. Recombinant adenovirus infections of the cell lines were carried out by dilution of the viral stocks to appropriate concentrations, addition of viral solutions to cell monolayers, and incubation at 37°C incubators for the indicated time.
Boyden chamber cell migration assay
Nucleopore filters with an 8-μm pore size (Corning Inc., Corning, NY, USA) were coated with type I collagen. The human melanocytes (1 × 104) were added to the upper chamber and lower chambers were filled with or without 50 ng/ml SCF. After 72 h of incubation, the filters were stained with Hoechst and cells on the upper side of the insert were removed with a cotton swab. Three randomly selected fields were photographed, and the cells that had migrated were counted. The migration was expressed as the percentage of migrated cells compared to the positive control melanocytes.
Statistical analysis
Statistical significance was tested with Student t-test. All results are presented as the mean ± SE of the combined data from replicate experiments.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Project (A080980), Ministry of Health Welfare and Family Affairs, Republic of Korea.