Retracted: Natural course of nonalcoholic fatty liver disease in southern China: A prospective cohort study
Abstract
OBJECTIVE: Nonalcoholic fatty liver disease (NAFLD) is a common chronic liver disease, the natural course of which has not been well documented. This study aimed to perform a prospective cohort study to investigate NAFLD in a Chinese population.
METHODS: Using our previous epidemiological survey, 3543 patients were followed-up for a median of 4 years (range 3.6–4.8 years). Of these patients, 624 participated in a new survey. Interviews, physical examinations, biochemical tests and abdominal ultrasonography were repeated for these patient.
RESULTS: The annual incidence of NAFLD was 9.1% (male 7.3% vs female 9.7%, P = 0.047). Among 117 NAFLD patients at baseline, 51 (43.6%) remained unchanged, 26 (22.2%) became worse, and 40 (34.2%) improved. Patients with simultaneous metabolic syndrome (MS) showed accelerated progression (P = 0.026). For the NAFLD patients, both general annual mortality rates and cardiovascular disease deaths (both 0.54%) were significantly higher than those of patients without NAFLD (0.19% and 0.17%, P = 0.005). Age and several variables related to MS were risk factors for NAFLD progression.
CONCLUSIONS: The incidence of NAFLD in southern China is relatively lower in comparison with that of the developed countries. Patients with NAFLD have a benign prognosis. Variables related to MS are risk factors for NAFLD occurrence and progression.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is often associated with metabolic syndrome (MS), which consists of obesity, type 2 diabetes, dyslipidemia and high blood pressure. NAFLD is one of the most common forms of chronic liver disease and a worldwide cause of elevated serum aminotransferases. The prevalence of NAFLD in the general population of Western countries ranges from 20% to 30%.1,2 With the improvement of standard of living and changes in dietary habits, the prevalence of NAFLD in developing countries, including China, has been increasing rapidly.3,4
NAFLD encompasses a morphological spectrum of simple fatty liver (SFL), nonalcoholic steatohepatitis (NASH) and hepatic cirrhosis. SFL generally has a benign prognosis but it may progress to NASH and cirrhosis, even hepatocellular carcinoma.5,6
Little has been published on the incidence and natural course of NAFLD in the general population. Longitudinal data from clinical series seldom reflect the condition of the disease. Our group reported a prevalence of 11.7% in Guangdong Province in southern China.7 The rate was comparable to the 15.3% noted in Shanghai (Eastern China).8 In this study, the patients from the previous survey7 from 2005 were followed-up for a median time of 4 years to investigate the incidence, natural history and characteristics of NAFLD in the general population of southern China.
PATIENTS AND METHODS
Study design and data source
Our previous epidemiological study was performed between April and November 2005. It was a cross-sectional survey with multiple-stage stratified clustering and random sampling of 3543 inhabitants from six urban and rural areas of Guangdong Province in southern China.7 All 3543 residents were enrolled in mortality analysis to investigate survival and causes of death. Of these, 219 patients (6.2%) dropped out due to loss of contact. The remaining 3324 including 467 patients with NAFLD and 2857 without NAFLD at baseline were recruited (Fig. 1). Median duration of follow-up was 4.0 years (range 3.6–4.8 years), from November 2005 (baseline) to November 2009 (end-point). From April to November 2009, we conducted a follow-up of 814 (23.0%) participants who were willing to participate in a new study (Fig. 1). Among these, 190 were excluded because of alcohol intake, viral hepatitis, chronic medication or concomitant diseases resulting in liver problems during the 4-year interval. The remaining 624 participants (117 with and 507 without NAFLD at baseline) were enrolled in the new survey group.
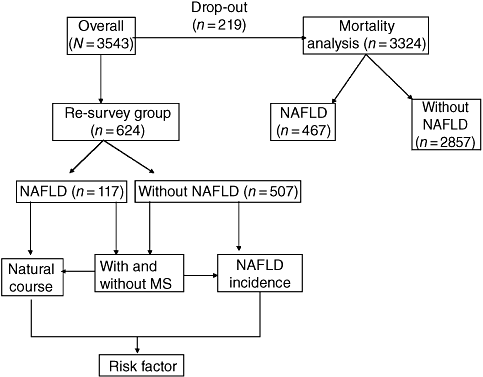
Study design. A total of 3543 residents surveyed in 2005 were enrolled in mortality analysis to investigate survival and cause of death. The new survey group included 624 patients (117 with NAFLD and 507 without NAFLD at baseline). NAFLD, nonalcoholic fatty liver disease.
Each participant in the new survey group underwent the same investigation as that in 2005, including questionnaire, physical examinations, serum biochemical tests and abdominal ultrasound examination.7 Before starting the study, all investigators were trained intensively to ensure standardized methods were used in the new survey. Information for survival and cause of death were determined by home visits, telephone inquiries and searches of patients' medical charts for mortality data.
This research was approved by the Ethics Committee of Guangzhou First Municipal People's Hospital. Written informed consent was obtained from each participant in the new survey group.
Interview and physical examinations
A face-to-face interview was carried out by specially trained postgraduates of Guangzhou Medical College, with the supervision of the experienced investigators. Standard questionnaires designed in the collaboration of epidemiologists and hepatologists consisted of items on the participants' baseline characteristics, their current medication, medical history and health-relevant behavior, that is, alcohol consumption, smoking and dietary habits, and physical activity. Physical examinations included the participants' height, body weight, blood pressure, waist circumference (WC), waist-to-hip ratio (WHR) and routine anthropometric parameters, and were conducted through health checkups.
Biochemical tests
Laboratory assessments included fasting blood glucose (FBG), fasting plasma insulin, plasma lipid profiles, that is, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) low-density lipoprotein cholesterol (LDL-C); serum liver function, that is, alanine transaminase (ALT), aspartate transaminase (AST), bilirubin and albumin levels, and viral markers of hepatitis A, B and C.
Diagnostic criteria
NAFLD was diagnosed using the diagnosis and treatment guidelines of the Chinese Liver Disease Association.9 Which is based on abdominal ultrasonic findings combined with patients' medical history, clinical symptoms and laboratory results. Viral hepatitis and other liver diseases were ruled out. Patients with heavy alcohol intake (more than 140 g weekly in male, 70 g weekly in female) were excluded. Liver biopsy is considered as the only diagnostic method for NASH, therefore, we avoided using the term NASH in this epidemiological study.
MS was diagnosed by the Asian definition10 modified from the criteria issued by the American College of Endocrinology,11 if the following three or more items were met (i) central obesity measured as WC >90 cm (male), >80 cm (female), and/or body mass index (BMI) > 25 kg/m2 in both genders; (ii) hypertriglyceridemia as TG ≥1.7 mmol/L; (iii) HDL-C <1.03 mmol/L (male), <1.29 mmol/L (female); (iv) elevated blood pressure of ≥130/85 mmHg; and (v) elevated FBG of ≥5.6 mmol/L or previously diagnosed type 2 diabetes. In addition, patients receiving treatment for MS were considered to have met the criteria.
Ultrasonography
Ultrasound examination of the upper abdomen was performed for each patient by two experienced physicians who were blinded to the research program at baseline and end-point using a scanner equipped with a convex-array probe (3.5 MHz; Siemens Adama, Siemens AG, Medical Solutions Henkestr, Erlangen, Germany). Diagnostic patterns and score systems were based on the Chinese guidelines for NAFLD diagnosis and treatment,9 supported by data from the literature on Western populations.12–14 The ultrasound patterns of NAFLD included: (i) a bright liver or hepatorenal echo contrast as an essential item (bright liver was determined as high-level intensive echoes arose from the hepatic parenchyma; hepatorenal echo contrast was based on a clear ultrasonic contrast between the hepatic and right renal parenchyma of the right intercostal sonogram in the mid-axillary line; either bright liver or hepatorenal echo contrast was considered to be indicative of NAFLD); (ii) structural blurring of the intrahepatic bile ducts; (iii) increase in liver volume and blunt liver edge; (iv) hepatic vessel blurring and (v) deep attenuation and blurring echoes in the right lobe of the liver envelope and the diaphragm. The severity of NAFLD was scored according to ultrasound patterns: 0, absence of all the patterns listed; 1, mild hepatosteatosis (echo increase in one-third of the liver area) plus any item in (ii)–(iv); 2, moderate hepatosteatosis (echo increase in two-thirds of the liver area) plus any two items in (ii)–(iv); 3, severe hepatosteatosis, or item (i) and item (v) plus any two items in (ii)–(iv), and a marked increase in the echogenicity with poor penetration of the posterior segment of the right liver lobe and non-visualization of the hepatic vessels and diaphragm. NAFLD development was determined by comparing the ultrasound scores at baseline and at end-point. A score increasing by one point or more indicated worsen of the disease; the same score indicated no change and a score decreasing by one point or more indicated improvement.
Statistical analysis
Data were analyzed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± standard error (SE) and examined by Student's t-test (two-tailed). Categorical variables were expressed as percentages and analyzed by χ2 test. A paired t-test was used to compare the data at baseline and end-point. anova analysis was applied for multiple comparison. Multivariate analyses with binary logistic regression were applied to risk factor analysis. P < 0.05 was considered to be statistically significant.
RESULTS
NAFLD incidence
In the new survey group, 507 were without NAFLD at baseline (Table 1). Among them, 185 developed NAFLD at the end-point with a cumulative NAFLD incidence of 36.5% (185/507) and an annual incidence of 9.1%. The cumulative incidence (36/124, 29.0%) and annual incidence (7.3%) of males differed significantly from those of females (cumulative incidence 149/383, 38.9% and annual incidence 9.7%, P = 0.047). After adjusting for gender and age using the Chinese census of Guangdong Province,15 the overall annual incidence of NAFLD was 4.9%, and the incidence of male (5.1%) was significantly higher than that of female (4.7%, P < 0.001). The incidence of NAFLD increased with age in most of the age stages but decreased in the elderly. The peak age of NAFLD in male was 40–59 years, while that in female was 40–69 years. And the incidence of NAFLD under 40 years was higher in male than that in female, while an opposite trend was noted in those over 40 years (P < 0.001). A cumulative incidence (127/295, 43.1%) in urban residents differed significantly from that observed in rural residents (58/212, 27.4%, P < 0.001). At baseline, the MS prevalence of 9.1% (301/3324) in the general population did not differ significantly from that of 10.3% (64/624) in the new survey (P = 0.342). In the new survey group of 507 patients without NAFLD at baseline, 21 had MS and 486 did not. The cumulative incidence of NAFLD was 66.7% (14/21) and the annual incidence was 16.7% in the MS subgroup, which differed significantly from those of the non-MS subgroup [35.2% (171/486) and 8.8%, respectively; P = 0.003].
| Age (years) | Male | Female | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| n | New patients (n) | Annual incidence (%) | n | New patients (n) | Annual incidence (%) | n | Annual incidence (%) | |
| 11–29 | 23 | 2 | 2.2 | 23 | 1 | 1.1 | 46 | 1.6 |
| 30–39 | 15 | 5 | 8.3 | 53 | 16 | 7.5 | 68 | 7.7 |
| 40–49 | 10 | 4 | 10.0 | 83 | 37 | 11.1 | 93 | 11.0 |
| 50–59 | 20 | 8 | 10.0 | 119 | 52 | 10.9 | 139 | 10.8 |
| 60–69 | 36 | 11 | 7.6 | 64 | 28 | 10.9 | 100 | 9.8 |
| ≥70 | 20 | 6 | 7.5 | 41 | 15 | 9.1 | 61 | 8.6 |
| Total | 124 | 36 | 7.3 | 383 | 149 | 9.7 | 507 | 9.1 |
Natural course of NAFLD patients at baseline
Of the 624 participants in the new survey group, 117 had NAFLD at baseline. Among these patients, 51 (43.6%) remained unchanged, 26 (22.2%) became worse (including one patient with cirrhosis) and 40 (34.2%) improved at the end-point. Stratified analysis was applied for further evaluation of the two subgroups, that is, patients with and without MS. Eighteen patients (41.9%) remained unchanged, 15 (34.9%) became worse and 10 (23.2%) improved at the end-point in the MS group, while 33 (44.6%) remained unchanged, 11 (14.9%) became worse and 30 (40.5%) improved at the end-point in the non-MS group (P = 0.021). MS significantly worsened the natural course of NAFLD.
Mortality analysis included 467 patients with NAFLD and 2857 without NAFLD at baseline. In the NAFLD subgroup, 68.7% (321/467) had cardiovascular disease (CVD) compared to 38.6% (1102/2857) in the subgroup without NAFLD (P < 0.001). In the NAFLD subgroup, 10 (2.14%) died of CVD during the 4-year follow-up period, with an annual mortality rate of 0.54%. In the subgroup without NAFLD, 22 patients (0.77%) died, in which 19 died of CVD, two of malignancy (gastric and nasopharyngeal carcinoma) and one of traffic accident, with an annual mortality rate of 0.19% and a CVD mortality rate of 0.17%. The overall mortality rate by CVD in the NAFLD subgroup was significantly higher than that in the subgroup without NAFLD (P = 0.005).
Risk factors
Clinical dynamic changes at baseline and end-point were evaluated under two conditions. The progressive group (n = 211) included patients without NAFLD at baseline who had NAFLD at the end-point (n = 185), plus patients with NAFLD at baseline who became worse at the end-point (n = 26). The regressive group (n = 40) included patients who had NAFLD at baseline but improved at the end-point.
Using binary multivariate regression logistic analysis with a probability for entry of 0.05 and removal of 0.1, the risk factors in the progressive group were found to be age (odds ratio [OR] = 1.025, 95% confidence interval [CI] 1.003–1.047) and WC (OR = 1.122, 95% CI 1.048–1.202) (Table 2). In the regressive group, a decrease of LDL-C (OR = 4.976, 95% CI 1.492–16.593), TG (OR = 0.401, 95% CI 0.201–0.800) and diastolic blood pressure (DBP) (OR = 1.100, 95% CI 1.005–1.204) were all protective factors against NAFLD development (Table 3). In general, several variables related to MS (WC, LDL-C, TG and DBP) were also risk factors for the development and acceleration of NAFLD.
| β | SE | χ2 | P | OR | 95% CI for EXP(B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gender | 0.051 | 0.341 | 0.022 | 0.882 | 1.052 | 0.539 | 2.052 |
| Age | 0.024 | 0.011 | 4.818 | 0.028 | 1.025 | 1.003 | 1.047 |
| WC | 0.115 | 0.035 | 10.895 | 0.001 | 1.122 | 1.048 | 1.202 |
| Hips | −0.021 | 0.031 | 0.429 | 0.513 | 0.980 | 0.921 | 1.042 |
| TC | −0.179 | 0.156 | 1.318 | 0.251 | 0.836 | 0.617 | 1.135 |
| HDL-C | 0.352 | 0.303 | 1.349 | 0.245 | 1.423 | 0.785 | 2.578 |
| LDL-C | 0.042 | 0.204 | 0.042 | 0.839 | 1.042 | 0.699 | 1.555 |
| TG | 0.162 | 0.111 | 2.111 | 0.146 | 1.176 | 0.945 | 1.463 |
| FBG | −0.004 | 0.077 | 0.003 | 0.957 | 0.996 | 0.856 | 1.158 |
| BMI | 0.112 | 0.102 | 1.210 | 0.271 | 1.119 | 0.916 | 1.367 |
| SBP | −0.003 | 0.009 | 0.087 | 0.769 | 0.997 | 0.980 | 1.015 |
| DBP | −0.014 | 0.016 | 0.743 | 0.389 | 0.986 | 0.955 | 1.018 |
- EXP(B) = OR.
- β, unstandardized coefficients beta; BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; Hips, hip circumference; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure; SE, standard error; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
| β | SE | χ2 | P | OR | 95% CI for EXP(B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gender | −0.359 | 0.974 | 0.136 | 0.713 | 0.699 | 0.104 | 4.714 |
| Age | −0.054 | 0.039 | 1.881 | 0.170 | 0.947 | 0.877 | 1.023 |
| WC | −0.102 | 0.079 | 1.641 | 0.200 | 0.903 | 0.773 | 1.055 |
| Hips | −0.097 | 0.085 | 1.316 | 0.251 | 0.907 | 0.768 | 1.071 |
| TC | −0.414 | 0.345 | 1.437 | 0.231 | 0.661 | 0.336 | 1.301 |
| HDL-C | −1.410 | 0.866 | 2.649 | 0.104 | 0.244 | 0.045 | 1.333 |
| LDL-C | 1.605 | 0.614 | 6.819 | 0.009 | 4.976 | 1.492 | 16.593 |
| TG | −0.915 | 0.353 | 6.729 | 0.009 | 0.401 | 0.201 | 0.800 |
| FBG | −0.166 | 0.149 | 1.244 | 0.265 | 0.847 | 0.632 | 1.134 |
| BMI | −0.289 | 0.240 | 1.448 | 0.229 | 0.749 | 0.468 | 1.199 |
| SBP | −0.002 | 0.018 | 0.011 | 0.918 | 0.998 | 0.963 | 1.035 |
| DBP | 0.095 | 0.046 | 4.312 | 0.038 | 1.100 | 1.005 | 1.204 |
- EXP(B) = OR.
- β, unstandardized coefficients beta; BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; Hips, hip circumference; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure; SE, standard error; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
DISCUSSION
There are limited data on the incidence and characteristics of NAFLD in a Chinese population. Discrepancies among the available studies from developed countries (annual incidence ranging from 3.1% to 10%) are probably the result of differences in sample selection methods, diagnostic modalities and diversity of life styles and dietary habits of people from different areas.16–18 We found that Chinese population has a relatively lower incidence of NAFLD (4.9% annually) in comparison with developed countries. The results were consistent with the lower prevalence (11.7%) in our previous findings.7 Incidence increased with age and the peak age was 40–69 years, then decreased in both genders. The incidence was significantly higher in male than in female under 40 years of age and showed an inverse pattern afterwards. This suggested that estrogen might be protective against NAFLD. Incidence by gender and age were consistent with the prevalence data from our previous study (Fig. 2).7

Prevalence in ( ) male and (
) male and ( ) female patients and annual incidence of nonalcoholic fatty liver disease (NAFLD) in (
) female patients and annual incidence of nonalcoholic fatty liver disease (NAFLD) in ( ) male and (
) male and ( ) female patients in 2005.
) female patients in 2005.
The natural history of NAFLD has not been entirely documented.17–19 In the most recent NAFLD guidelines, histology is the only standard method for the diagnosis of NASH.20,21 Therefore, we did not analyze NASH in the present study. Some published data using biopsy database provided histological evidence for the evaluation of the natural course of NAFLD (Table 4).22–26 However, these studies had referral, selection and ascertainment biases, since a patient who was willing to undergo at least two biopsies might not be representative of the general population. A Japanese study followed-up 704 NAFLD patients with ultrasound health checkup for a mean follow-up period of 414 days, and found that 113 (16%) improved.17 In our population-based study, we found that 43.6% NAFLD patients remained unchanged, 34.2% improved, and 22.2% worsened, including one patient (0.9%) who developed cirrhosis. The NAFLD patients with MS simultaneously had a poorer prognosis than those without MS. Our results suggest that, in the general population, NAFLD has a relatively benign prognosis, as most of our NAFLD patients were mild by ultrasonographic diagnosis (70/117). In addition, NAFLD scores evaluated by ultrasonography might reflect NAFLD severity.12–14,20
| Study | Diagnosis | n | Follow-up (years) | Improved (%) | No change (%) | Worsened (%) | Cirrhosis (%) |
|---|---|---|---|---|---|---|---|
| Adams et al. (2005)22 | NASH | 103 | 3.2 | 30 (29) | 35 (34) | 38 (37) | 9 (9) |
| Lindor et al. (2004)23 | NASH | 107 | 2.0 | 22 (21) | 56 (52) | 27 (25) | 0 |
| Fassio et al. (2004)24 | NASH | 22 | 4.3 | 4 (18) | 11 (50) | 7 (32) | NA |
| Harrison et al. (2003)25 | NASH | 22 | 5.7 | 4 (18) | 11 (50) | 7 (32) | 2 (9) |
| Ekstedt et al. (2006)26 | NAFLD | 70 | 13.7 | 11 (16) | 30 (43) | 29 (41) | 2 (2.9) |
- Patients were divided into groups according to changes in fibrosis stage between biopsies as either ‘improved’ (decreased in fibrosis stage), ‘no change’ (stable) or ‘worsened’ (increased in fibrosis stage). Fibrosis change calculated by dividing the difference in fibrosis stage by the time interval (in years) between biopsies.
- NA, not available; NASH, nonalcoholic steatohepatitis.
Some studies have described the prognosis of NAFLD. Most found that the survival rate and cause of death of SFL patients were not significantly different from those of the general population.1–3 The main causes of death were not liver-related, but were CVD and malignancy of other organs. The mortality rate of NASH patients increased significantly with more liver-related deaths than that was found in SFL patients.27,28 Matteoni et al.28 divided patients into SFL and NASH groups by histology, during a mean follow-up of 8.3 years, 22% of NASH patients developed cirrhosis and 10% died of liver-related diseases, compared with 4% developing cirrhosis and 2% liver-related deaths in the SFL group. Rafiq et al.29 followed-up 173 biopsy-proven NAFLD patients, in which 72 with NASH and 101 with SFL for a minimum of 5 years, the most common causes of death were CVD, malignancy and liver-related disease. Although the overall mortality rate did not differ between the NAFLD subtypes, liver-related mortality was significantly higher in NASH patients. In our study, NAFLD patients recruited from the general population was mainly mild, therefore overall mortality rate was low. NAFLD patients had a significantly higher mortality rate than patients without NAFLD, although the causes of death were not significantly different, as most died of CVD. Patients with MS components (obesity, type 2 diabetes, dyslipidemia and high blood pressure) were associated with NAFLD susceptibility.
MS confers a 4-fold to 11-fold increase in risk for NAFLD development.1–4,7,8,30 However, longitudinal studies investigating the risk factors relating to NAFLD incidence and its natural course have not been well documented. Hamaguchi et al.17 found that patients met the criteria for MS at baseline were more likely to develop NAFLD (OR 4.0) and NAFLD was less likely to regress in MS patients. In Guangzhou, Lu et al.31 reported that NAFLD is a common condition among MS patients with type 2 diabetes and obesity. Because our study excluded patients with concomitant diseases, alcohol intake and those on medications at baseline, we focused on the risk factors of patients with primary NAFLD. Several variables involved in MS showed a trend for the development of NAFLD, but only WC, LDL-C, TG and DBP reached significance in this small sample-size study. These results were consistent with the study of Hamaguchi et al.17 In our previous cross-sectional study of the same population, we found that hypertension, high BMI, WC, WHR, serum TG and FBG were independent risk factors for fatty liver disease (FLD)7 and that genetic polymorphisms influenced NAFLD susceptibility.32,33 In the present cohort study, we found that people with MS components were not only at risk for the development of NAFLD, but also for its progression.
The present study had some limitations. First, the NAFLD diagnosis was based on an abdominal ultrasound. Although this method is commonly used in epidemiological surveys and its scoring system provides valuable information about hepatic steatosis,9,12–14 it is not the gold standard for NAFLD diagnosis and cannot distinguish NASH from SFL.34 Second, our results might not adequately represent long-term outcomes because of the short follow-up period. Third, the large number of drop-outs from the new survey group might have caused a selection bias. In a new survey group of 624 patients, the standardized prevalence of NAFLD in 2005 was 10.5%,7 but it increased to 22.6% in 2009 in the present study. This suggests that those who thought they might have a medical problem were more likely to participate in the new survey. In spite of these limitations, this study still reflects the main features of the natural course of NAFLD in Chinese group. To our knowledge, there have been few population-based surveys investigating both the incidence and natural course of NAFLD. Longitudinal population data using liver enzymes as markers reported the incidence, but not the natural course of the disease,18 and studies using ultrasound included only patients receiving health checkups.17 This study based on the natural course of NAFLD in the population of southern China, adds new information to the existing knowledge and may have an impact on future research.
ACKNOWLEDGMENT
This study was supported by the Foundations from Guangzhou Health Bureau, China (No. 2009-ZDi-03 and 2008-Zdi-1).




