In vitro and in vivo characterization of silk fibroin/gelatin composite scaffolds for liver tissue engineering
Abstract
OBJECTIVE: To investigate the cytotoxicity of silk fibroin/gelatin (SF/G) composite scaffolds in vitro as well as their biocompatibility and degradation in vivo.
METHODS: The proliferation and relative growth rate of human hepatic QZG cells grown on different blends of two-dimensional (2-D) SF/G scaffolds were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Flow cytometry was used to evaluate apoptotic rate of QZG cells on different blends of 2-D SF/G scaffolds. The effect of silk protein materials on cell growth was observed by scanning electron microscopy. Three-dimensional (3-D) SF/G scaffolds of three different ratios (diameter 10 mm, thickness 1 mm) were implanted into subcutaneous pockets on male Sprague–Dawley (SD) rats. On the 7th, 14th and 30th day post-implantation, the rats were sacrificed. The scaffold area including the surrounding tissues was retrieved. Hematoxylin and eosin staining was performed for observation under a light microscope.
RESULTS: Significant cell attachment and proliferation on the SF/G scaffolds were observed. As the increased gelatin concentration, SF/G scaffolds became more amenable to cell adhesion. After the subcutaneous implantation of the SF/G scaffolds in SD rats, immunological rejection tests showed only slight inflammation, measured by the presence of inflamed cells on day 7 and 14. By day 30, each scaffold had been completely infiltrated and organized by fibroblasts and inflamed cells. The greater the gelatin concentration in the scaffold, the faster the degradation rate.
CONCLUSION: Composite SF/G scaffolds are a promising candidate matrix for implantable bio-artificial livers.
INTRODUCTION
Liver transplantation is the only proven effective therapy for acute liver failure, which is a challenging clinical syndrome. However, the shortage of donors and rapid disease progression limit the use of transplantation.1 Artificial and bioartificial systems support severe liver disease patients during recovery or transplantation.2 However, patients who accept this therapy may find it expensive and inconvenient. In recent years, attention has been focused on tissue engineering targeting the liver.3 Tissue engineering provides substitutes for the replacement of diseased tissues. To achieve this goal, the substitutes must mimic the organs in both histological organization and function.4
Finding a suitable matrix for tissue engineering is a key issue. Many biocompatible materials are available and have been made into scaffolds for cell attachment, proliferation and tissue-like shaping. Generally, scaffolds are designed to be porous,5 with good biocompatibility6 and must biodegrade at a suitable rate.7
Silk fibroin (SF) has high histocompatibility and mechanical characteristics, and has been widely used in the engineering of a variety of tissues including blood vessels and ligaments. SF can be processed relatively easily and has been molded into various shapes, such as meshwork, filaments and film.8 Because of the extensive hydrogen bonding which gives the material a hydrophobic character, silk is insoluble in solvents such as dilute acid, alkali and water.8,9 Therefore, pure natural SF is not useful for cell adherence due to its long degradation time.
Gelatin, derived from natural collagen, is a natural biopolymer. It can be manufactured for medical and pharmaceutical use,10 as a vector for drug release11 and wound dressing.12 There are many binding sites on gelatin that may promote cell differentiation and adhesion.13 As an extracellular matrix, gelatin has been shown to release growth factor enhancers and bioactive components. As a tissue engineering scaffold, it has been used for neural cell transplantation and corneal wound healing.14–16
Based on the characteristics of these two materials, SF and gelatin were made into two-dimensional (2-D) and three-dimensional (3-D) composite scaffolds. The aim of this study was to investigate the cytotoxicity of silk fibroin/gelatin (SF/G) composite scaffolds in vitro as well as their biocompatibility and degradation in vivo.
MATERIALS AND METHODS
Materials
Bombyx mori silkworm silk was purchased from Yi Xian Raw Silk Factory (Hebei Province, China). Gelatin was obtained from Sigma-Aldrich (St Louis, MO, USA). All other reagents were of analytical or higher grade. Ultrapure water was used throughout this study.
Preparation of 2-D and 3-D matrices of SF/G
SF purification and concentration
The raw silk was boiled for 1 h in an aqueous solution of 0.5 weight percent (wt%) sodium carbonate and washed with ultrapure water to remove the sericin proteins. The degummed silk was dissolved in a ternary solvent system of CaCl2/H2O/CH3CH2OH (mol ratio 1:8:2) at 80°C. Fibroin solution was filtered and dialyzed in ultrapure water for 3 days using a cellulose tube (MWCO 3500; Amresco Products, Solon, OH, USA) to yield an aqueous fibroin solution of 3–4 wt%, which was determined by weighing the solids after drying.
Preparation of blended SF/G solutions
The aqueous SF solution was heated at 50–60°C with mild stirring to reach different concentrations. Gelatin (Sigma-Aldrich, St Louis, MO, USA) solutions of various concentrations were prepared by dissolving them in ultrapure water with stirring and heating. Glutaraldehyde solution (0.25 wt%) was incorporated into the gelatin solution at a ratio of 1:32 (v/v) to produce cross-linked gelatin solutions. SF and gelatin solutions were blended at a ratio of 1:1 (w/w) under agitation for 1 h at 37°C to form uniform solutions. The final concentrations (w/w) were SF: gelatin = 4:2, 4:3 and 4:4.
3-D scaffold fabrication
Fig. 1 summarizes the fabrication of liver tissue engineering scaffolds. Polydimethylsiloxane (PDMS) molds were prepared by mixing commercially available polymer and catalyzer at a ratio of 100:2 (w/w). The mixture was degassed under vacuum to eliminate bubbles and was poured into pre-prepared resin molds. The molds with mixture was then degassed under vacuum again and placed statically at room temperature for 24 h. PDMS mold was fabricated after demolding and washed with 75% ethanol. The SF/G mixture was poured into the PDMS molds and placed at −20°C for 24 h. The frozen mixture was lyophilized in a freeze-dryer (DTY-1SL; Detianyou Technology, Beijing, China) for 24 h and the porous scaffolds were treated with an aqueous solution of 95% methanol for 24 h and dried overnight in a vacuum hood. The 3-D SF and gelatin formed microporous sponges around silk cables. The porosity and pore size were 85.4% ± 2.1% and 250.0 ± 30.0 µm, respectively.

The fabrication process for silk fibroin/gelatin scaffolds. PDMS, polydimethylsiloxane.
2-D film fabrication
To prepare the film, the SF and gelatin solutions were added to the cell culture plates, and the film was dried in a fume hood overnight before treated with the aqueous solution of 95% methanol for 24 h and dried overnight again.
Maintenance of the human normal hepatic QZG cell line
The QZG cell line17 (donated by the Department of Pathology, The Fourth Military Medical University, Xi'an, Shaanxi Province, China) was cultured in a high-glucose Dulbecco's modified eagle's medium (DMEM; HyClone, Thermo scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Invitrogen-Gibco, Carlsbad, CA, USA) and 1% streptomycin-penicillin (Harbin Pharmaceutical Group, Harbin, Heilongjiang Province, China) in tissue culture flasks at 37°C with 5% CO2 in an incubator. The medium was changed every other day, and subculturing was performed at confluence. Cell viability was assayed using Trypan blue stain (Sigma-Aldrich, St Louis, MO, USA), and only cells with > 95% viability were used in the analysis.
Determination of cell viability and proliferation on 2-D film by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Assessments of cell viability and proliferation were carried out using the MTT assay (Sigma-Aldrich, St Louis, MO, USA), an indirect quantitative colorimetric assay method for determination of cell growth and proliferation. Briefly, QZG cells were cultured on 2-D SF/G films in 96-well plates for 7 days (5 × 103 cells/well). There were no films in 96-well control plates, and the culture medium was replaced with a serum-free culture medium containing MTT (0.5 mg/mL). After 4 h, the supernatant was aspirated and 200 µL dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA) was added into each well. The plate was micro-oscillated for 30 s, and then the samples were transferred to another 96-well plate and the absorbance at 490 nm was measured on a Bio-Rad microplate reader (Bio-Rad, Hercules, CA, USA).
Analysis of apoptosis by flow cytometry
QZG cells were seeded on 2-D SF/G films which had been precoated onto 6-well plates and cultured for 5 days (1 × 106 cells/well). There were no films in 6-well control plates. On the first and fifth day after seeding, the QZG cells were digested by a trypsin-ethylenediaminetetraacetic acid solution (Sigma-Aldrich, St Louis, MO, USA) and then collected. After neutralization with 6 mL fetal bovine serum (Invitrogen-Gibco, Carlsbad, CA, USA) for 5 min, the cell suspension was centrifuged at 110 ×g for 5 min and then resuspended in cold phosphate buffered saline (PBS, pH 7.4; Sigma-Aldrich, St Louis, MO, USA). The suspension was centrifuged at 110 ×g for 5 min again, and the supernatant was discarded. The cell pellet was mixed with 70% chilled ethanol while shaking, and the mixture was then incubated for 45 min at 4°C. PBS was used to wash the cell pellets twice and then the pellets were resuspended in 250 µL 1 X binding buffer. Cell concentration was adjusted to 1 × 106/mL, then 100 µL of the cell suspension was mixed with 5 µL Annexin V-fluorescein isothiocyanate (V-FITC) (Annexin V-FITC Kit; Beijing 4A Biotech Co., Ltd, Beijing, China) and 10 µL propidium iodide (20 µg/mL) in a 5-mL tube. After 15 min of incubation at 4°C in the dark, 400 µL PBS was added to the mixture and the sample was analyzed with a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Evaluation of cell proliferation on 3-D scaffolds by scanning electron microscopy (SEM)
QZG cell morphology and proliferation were investigated using SEM (Hitachi S-3000N; Hitachi, Tokyo, Japan). After culturing on the 3-D SF/G scaffolds (diameter 10 mm, thickness 1 mm; 5 × 104 cells/scaffold), cells were taken out of the culture plates on the third and seventh day and immediately rinsed with 0.2 mol/L sodium cacodylate buffer. Critical drying was performed after 2.5% glutaraldehyde treatment. The specimens were coated with gold particles in a sputter coater and the cross-sectioned scaffolds were examined using SEM at 5 kV.
Implantation into subcutaneous pockets in Sprague–Dawley (SD) rats
The experiments were conducted in accordance with Chinese legislation on the protection of animals and the National Guidelines for the Care and Use of Laboratory Animals. The 3-D SF/G scaffolds were sterilized by cobalt-60 irradiation and incubated in sterile PBS for 1 h before implantation. Forty-five male SD rats (supplied by the Forth Military Medical University, Xi'an, Shaanxi Province, China, weighing 200–250 g) were anesthetized by an intraperitoneal injection of sodium pentobarbital for subcutaneous pocket creation and scaffold implantation. A total of 90 scaffolds were implanted in the rats (three groups with three blends of SF/G, for scaffold retrieval on day 7, 14 and 30, respectively), and all rats were monitored for signs of infection by observing the food intake, activity level and wound healing. On day 7, 14 and 30 post-implantation, three rats from each group were sacrificed. The scaffold area including the surrounding tissues was retrieved, chemically fixed with 10% formalin for 4 h, paraffin embedded and cut into 5-µm sections for staining. For light microscopy, 5-µm sections were prepared by hematoxylin and eosin (HE) stain for analysis of the degradation of the scaffolds, inflammation and angiogenesis.
Statistical analysis
Differences between data sets were analyzed by one-way anova using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered as statistically significant.
RESULTS
QZG cell line viability and proliferation on 2-D SF/G films
MTT assay showed that cell proliferation on 4:2, 4:3 and 4:4 SF/G films (w/w) was comparable to the control plates (Fig. 2). As the gelatin concentration increased, cell proliferation rates also increased. The survival of QZG cells on the 4:4 SF/G films was significantly higher than that on 4:3 and 4:2 films (P < 0.05). These results indicated that the matrix was suitable for cell culture applications and gelatin promoted cell viability and proliferation significantly.

Optical density (OD) value of 3-(4, 5-dimethylthiazol −2-yl)-2,5-diphenyltetrasodium bromide (MTT) assay with human normal hepatic QZG cells cultured for 7 days on 2-dimensional (2-D) films of the following blends of silk fibroin/gelatin (SF/G) 4:2 ( ), 4:3 (
), 4:3 ( ), 4:4 (
), 4:4 ( ) and control (
) and control ( ).
).
Analysis of apoptosis in QZG cells cultured on 2-D SF/G scaffolds
The results of QZG cell apoptosis by flow cytometry analysis are shown in 3, 4. On the first day after seeding, the apoptosis rate (population Q2 + Q4) of the QZG cells cultured on 4:2, 4:3 and 4:4 SF/G films (w/w) was approximately 8%, which was not significantly different from that of the control (Fig. 3, P > 0.05). These results suggested that the cell viability after culture on all the ratios of SF/G films was identical. After 5 days of culture, the apoptosis rates were 25.4% ± 2.0% at SF/G rate of 4:2, 19.0% ± 0.6% at SF/G rate 4:3 and 13.1% ± 0.3% at SF/G rate 4:4, while the control group had an apoptosis rate of 10.4% ± 0.3% (Fig. 4, P < 0.05). The decrease in the apoptosis rate might be due to the increase of gelatin content, which was better for cell attachment. This result was in accordance with the results of the MTT analysis described earlier.

Analysis of apoptosis in QZG cells on day 1 of culture on 2-dimensional (2-D) films of the following blends of silk fibroin/gelatin (SF/G): (a) 4:2 ( ), (b) 4:3 (
), (b) 4:3 ( ), (c) 4:4 (
), (c) 4:4 ( ) and (d) control (
) and (d) control ( ). (e) Results in each group were representative of three independent experiments (mean ± standard deviation, P > 0.05). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
). (e) Results in each group were representative of three independent experiments (mean ± standard deviation, P > 0.05). FITC, fluorescein isothiocyanate; PE, phycoerythrin.

Analysis of apoptosis in QZG cells on day 5 of culture on 2-dimensional (2-D) films of the following blends of silk fibroin/gelatin (SF/G); (a) 4:2 ( ), (b) 4:3 (
), (b) 4:3 ( ), (c) 4:4 (
), (c) 4:4 ( ) and (d) control (
) and (d) control ( ). (e) Results in each group were representative of three independent experiments (mean ± standard deviation, P < 0.05). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
). (e) Results in each group were representative of three independent experiments (mean ± standard deviation, P < 0.05). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
QZG cell attachment and proliferation on 3-D SF/G scaffolds
On day 3 and 7, the morphology of QZG cells which had been attached to the scaffolds was observed under SEM. Fig. 5 shows the spherical shape of QZG cells and the abundant extracellular secretions. Feelers from the QZG cells were attached to the surface of SF/G scaffold. QZG cells which were cultured for 3 days adhered to the surface of the scaffolds and had low rates of proliferation (Fig. 5a, c, e); on day 7, the cells associated with each scaffold showed excellent growth, including extracellular secretions and intercellular connections (Fig. 5b, d, f). The results also showed a better cell condition on the scaffolds with higher gelatin ratios.

Scanning electron micrographs showing sequential steps of cell attachment and growth on silk fibroin/gelatin (SF/G) 3-dimensional (3-D) scaffolds: (a, b) SF/G = 4:2; (c, d) SF/G = 4:3; (e, f) SF/G = 4:4; Culture time of a, c, e are 3 days, b, d, f are 7 days. White arrows: QZG cells and extracellular matrix.
3-D SF/G scaffolds implanted into SD rat subcutaneous pockets
Histological analysis showed both degradation and tissue response within and surrounding the scaffold. Observation of the recruitment of inflamed cells gave a gross indication of the scaffold histocompatibility. Neutrophils and acidophilic leukocytes migrated from the blood to the scaffolds on day 7 (6-8), inflamed cells were at low levels, indicating minimal immunological reaction. On day 14 the framework of each scaffold was complete (6-8) and the recruitment of inflamed cells increased but was still at a low level. The scaffolds were fully degraded on day 30 (6-8), as observed by the fragmentation of the framework. The entire scaffold was organized by a surrounding fibrotic layer and interior inflamed cells. Fibroblasts around the scaffold fragments filled all the vacant spaces. As the ratio of gelatin increased, scaffold degradation accelerated (6-8), Fig. 8c1 and 8c2 clearly show the massive degradation. Only a small amount of the scaffold remained. Fibrosis encasement of the global scaffold in Fig. 8b and 8c is clearly thinner, demonstrating that the gelatin portion accelerated the degradation and prevented encapsulation and the formation of granulomas. In summary, the SF/G 3-D scaffolds could be implanted into the subcutaneous pockets of SD rats, resulting in only slight inflammation. The degradation rate was influenced by the proportion of gelatin.
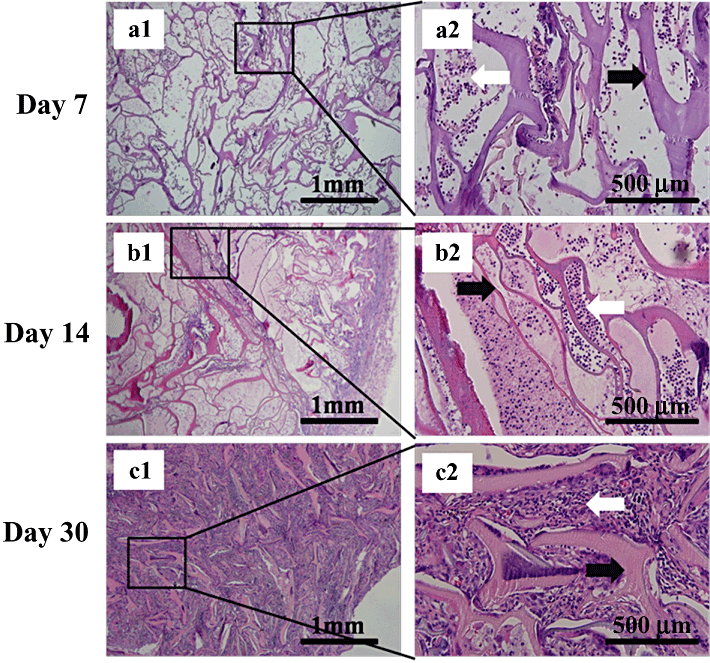
Morphologies of subcutaneously implanted silk fibroin/gelatin (SF/G) scaffolds (4:2) in Sprague–Dawley rats on day 7 (a1–2), day 14 (b1–2) and day 30 (c1–2). a2, b2 and c2 are enlarged views of the boxed areas in figure parts a1, b1, c1. HE stain (left: ×40, right: ×200). Black arrows in a2, b2 and c2 show remaining scaffolds; white arrows in a2 and b2 show inflamed cells; white arrow in c2 shows fibroblasts and inflamed cells.
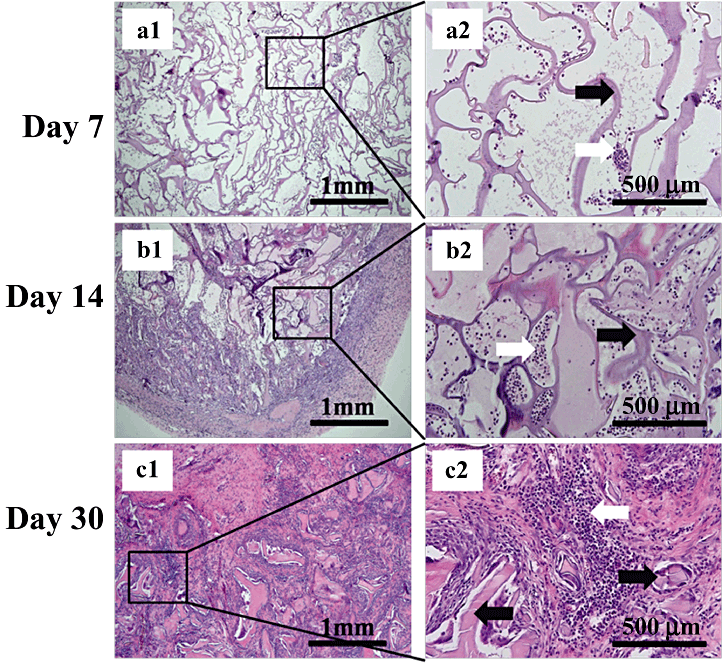
Morphologies of subcutaneously implanted silk fibroin/gelatin (SF/G) scaffolds (4:3) in Sprague–Dawley rats on day 7 (a1–2), day 14 (b1–2) and day 30 (c1–2). a2, b2, and c2 are enlarged views of the boxed area in figure parts a1, b1, c1. HE stain (left: ×40, right: ×200). Black arrows in a2, b2 and c2 show remaining scaffolds; white arrows in a2 and b2 show inflamed cells; white arrow in c2 shows fibroblasts and inflamed cells.
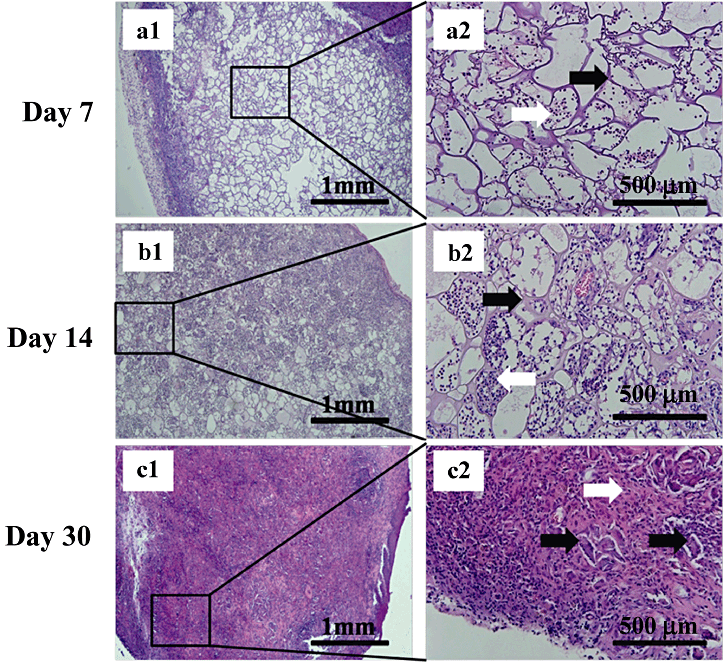
Morphologies of subcutaneously implanted silk fibroin/gelatin (SF/G) scaffolds (4:4) in Sprague–Dawley rats on day 7 (a1–2), day 14 (b1–2) and day 30 (c1–2). a2, b2 and c2 are enlarged views of the boxed area in figure parts a1, b1, c1. HE stain (left: ×40, right: ×200). Black arrows in a2, b2 and c2 show remaining scaffolds; white arrows in a2 and b2 show inflamed cells; white arrow in c2 shows fibroblasts and inflamed cells.
DISCUSSION
Many biomaterials are suitable for the development of tissue engineering scaffolds that specifically target the liver, and many methods are available for determining the scaffold quality. However, a main concern is cytotoxicity and biocompatibility. Silk has a long history of clinical applications and many advantages over other materials. It has better mechanical properties than other natural fibrins, can easily be chemically modified on surfaces as cell binding sites and has better structural properties and performance when cross-linked or composited with other biomaterials. However, there are still some concerns that cannot be neglected. For example, to avoid incompatibility, sericin must be sufficiently eliminated from silkworm silk. Silk also has a slower rate of degradation both in vitro and in vivo due to its extensive hydrogen bonding and hydrophobic nature; on the other hand, the activity of macrophages can result in aborted proteolysis, leading to encapsulation and granuloma formation. Furthermore, there is also a possibility of potential allergic response.8
Gelatin has high hydrophilicity, which can be cross-linked with glutaraldehyde in aqueous solution to produce biodegradable hydrogels, which can be made into porous hydrogels after freeze-drying.14 Several reports have shown that when cultured on 3-D porous scaffolds in vitro, hepatocytes may maintain liver specific functions, such as albumin secretion and urea synthesis.18–22 Moreover, gelatin is degraded relatively quickly and has better biocompatibility than silk. These properties indicate that a gelatin composite with silk fibroin may have appropriate properties for tissue engineering. Our composite 3-D scaffolds showed the expected effects on both cell proliferation and histocompatibility. We also demonstrated that SF-scaffold degradation was controllable through composition with gelatin. These scaffolds have an organized architecture with the potential to enable various liver cells to secrete extracellular matrix and easily establish cell junctions, thus guiding the organ regeneration in a controlled manner.
These materials show a good cytocompatibility in vitro as well as biocompatibility and controllable degradation in vivo. The ratio of the two ingredients must be optimized, and other ratios should be investigated, not only with cell culture experiments but also with biocompatibility tests in vivo. The time of degradation must match the growth time of the cells inoculated onto the scaffolds.
Conclusion
This study systematically investigated the cytotoxicity in vitro as well as the biocompatibility and degradation in vivo of scaffolds made of blends of SF/G. The results show that in vitro, the obtained SF/G scaffolds supplied a good surface for the attachment and proliferation of QZG cells. Within a certain range, increasing the ratio of gelatin resulted in improved cell growth. In addition, in vivo implantation indicated that SF/G scaffolds had low immunogenicity, encapsulation and granuloma formation were prevented by increasing the proportion of gelatin in the composite scaffolds. The scaffold began to degrade after 14 days; the more gelatin in the mixture, the faster the degradation. The gelatin ratio must still be optimized and further cell culture and in vivo experiments are required to verify the efficiency of the 3-D scaffolds for liver tissue engineering.
ACKNOWLEDGMENT
The study was approved by the National 863 Program of China (No. 2009AA043801).




