Effect and Mechanism of a New Photodynamic Therapy with Glycoconjugated Fullerene
Abstract
When irradiated, fullerene efficiently generates reactive oxygen species (ROS) and is an attractive photosensitizer for photodynamic therapy (PDT). Ideally, photosensitizers for PDT should be water-soluble and tumor-specific. Because cancer cells endocytose glucose more effectively than normal cells, the characteristics of fullerene as a photosensitizer were improved by combining it with glucose. The cytotoxicity of PDT was studied in several cancer cell lines cultured with C60-(Glc)1 (d-glucose residue pendant fullerene) and C60-(6Glc)1 (a maltohexaose residue pendant fullerene) subsequently irradiated with UVA1. PDT alone induced significant cytotoxicity. In contrast, PDT with the glycoconjugated fullerene exhibited no significant cytotoxicity against normal fibroblasts, indicating that PDT with these compounds targeted cancer cells. To investigate whether the effects of PDT with glycoconjugated fullerene were because of the generation of singlet oxygen (1O2), NaN3 was added to cancer cells during irradiation. NaN3 extensively blocked PDT-induced apoptosis, suggesting that PDT-induced cell death was a result of the generation of 1O2. Finally, to investigate the effect of PDT in vivo, melanoma-bearing mice were injected intratumorally with C60-(Glc)1 and irradiated with UVA1. PDT with C60-(Glc)1 suppressed tumor growth. These findings indicate that PDT with glycoconjugated fullerene exhibits tumor-specific cytotoxicity both in vivo and in vitro via the induction of 1O2.
Introduction
Photodynamic therapy (PDT) has attracted attention as a less invasive method for treating cancer. In PDT, the photosensitizer, which is preferentially incorporated in the tumor, is administered to the patient and the tumor is irradiated with the appropriate wavelength of light. Reactive oxygen species (ROS) produced by excitation of the photosensitizer in the malignant tumor induce tumor cell apoptosis. The properties of an ideal photosensitizer are water solubility, low cytotoxicity in the dark, high stability against light, high tumor-specificity, high ability to produce ROS and rapid metabolism. Various photosensitizers with some of these properties were recently synthesized or extracted from plants (1,2).
Fullerenes are considered a new class of carbon molecules. General fullerene, discovered in 1985, is composed of 60 carbon atoms arranged in a soccer ball-like structure. Although fullerene is a well-known radical scavenger, it has excitation wavelengths in the ultraviolet region (340–400 nm) and generates ROS effectively upon excitation (3–5). Fullerene is not toxic in the dark (6–8). Therefore, PDT with various fullerene derivatives, such as C60 with polyethyleneglycol (9), C60 with porphyrin, which is a known photosensitizer (10) and lipid-membrane-incorporated C60 and C70 (11), has been reported, but these studies are still in the early stage.
Glucose is more effectively endocytosed by cancer cells than by normal cells. Glucose is intracellularly transported by glucose transporters (GLUT), which comprise a family of membrane proteins, and GLUT-1, in particular, is expressed more on tumor cells than on normal cells (12–15). Compared with normal cells, cells infected with the sarcoma virus take up approximately six times more glucose within 1 day after infection and ten times more within 2 days after infection (16).
We hypothesized that conjugating fullerene with glucose, i.e. glycoconjugation, would improve the water solubility and tumor specificity of fullerene, and that the glycoconjugated fullerene would generate high levels of ROS, thereby enhancing PDT. In the present study, we used a single d-glucose residue pendant fullerene (C60-(Glc)1), two d-glucose residue pendant fullerene (C60-(Glc)2), a maltohexaose residue pendant fullerene (C60-(6Glc)1) and two maltohexaose residue pendant fullerene (C60-(6Glc)2). PDT effects using C60-(Glc)1 or C60-(6Glc)1 were demonstrated in vitro and in vivo and the underlying mechanisms were investigated.
Materials and Methods
Chemicals. The glycoconjugated fullerenes C60-(Glc)1, C60-(Glc)2, C60-(6Glc)1 and C60-(6Glc)2 were synthesized by Dr Hiromi Ohi (Endowed Research Section, Photomedical Science, Innovative Collaboration Center, Kyoto University, Kyoto, Japan), Ms. Yuki Yoshida and Ms. Masami Naemura (Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, Japan). The detailed synthetic methods of these glycoconjugated fullerenes were described previously (17).
Cells and cell culture. Several human cancer cell lines, 5637 (human bladder cancer cells), HGC-27 (human stomach cancer cells), COLO679 (human melanoma cells) and DU145 (human prostate cancer cells), were obtained from the Riken Bioresource Center. HaCaT (human cancer keratinocyte cells) were a kind gift from Dr Norbert Fusening (Institute of German Cancer Research Center, Heidelberg, Germany), and F113 (normal human fibroblast cells) were from Dr Peter Schroder (Institute for Environmental Medicine Research, Dusseldorf, Germany).
The 5637 cells, COLO679 cells and DU145 cells were cultured with RPMI-1640 (Sigma-Aldrich, St Louis, MO). HGC-27 cells, HaCaT cells and normal fibroblast cells were cultured with Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) supplemented with 5% fetal bovine serum (Invitrogen, Carlsbad, CA), 1% antibiotic antimycotic (Invitrogen) and 1%l-glutamine (Sigma-Aldrich) in 5% CO2 at 37°C in a humidified incubator.
PDT treatment and cell viability assays. Cells were cultured in 96-well plates at a density of 5 × 103 cells per 100 μL per well (cancer cells) and 2 × 104 cells per 100 μL per well (normal cells) for 24 h and then coincubated with the glycoconjugated fullerenes for 24 h at 37°C in the dark. After removing the culture medium, the cells were rinsed with Dulbecco’s phosphate-buffered saline (Sigma-Aldrich) and subsequently irradiated with a UVA1 light source (SELLAMED® 3000/System; Dr. Sellmeier, Gevelsburg, Germany) at 5 J cm−2 (intensity: 40 mW cm−2) and cultured further for 24 h at 37°C in the dark. Cytotoxicity was evaluated using a WST-8 assay with a Cell Counting Kit-8 (CCK-8; DOJINDO, Kumamoto, Japan). The absorbance value at 450 nm was read with a 96-well plate reader (SPECTRA MAX 300; Molecular Devices, Silicon Valley, CA) to determine the viability (% of control) = (ODtre−ODmedium)/(ODcon−ODmedium) × 100%, where ODtre was the absorbance value at 450 nm of treated cells, ODcon was that of control cells and ODmedium was that of the medium.
Detection of apoptosis (18). Cells were cultured in a six-well plate at a density of 2 × 105 cells per 2 mL per well for 24 h and treated by PDT with 10 μm of C60-(Glc)1 or C60-(6Glc)1. The cells were then stained with Annexin V (MBL, Nagoya, Japan) and 7-amino-actinomycin D (7-AAD; BD, Franklin Lakes, NJ), and analyzed by flow cytometry (FACS Calibur, BD).
Detection of ROS (19). Cells were cultured in a six-well plate at a density of 2 × 105 cells per 2 mL per well for 24 h and treated by PDT with 1 μm of glycoconjugated fullerene. After irradiation, the cells were stained with 5-(and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate (carboxy-H2DFFDA; Invitrogen) and analyzed by fluorescence-activated cell sorting (FACS).
Inhibition of singlet oxygen generation (20,21). Cells were cultured in a 96-well plate and treated by PDT with C60-(Glc)1 and C60-(6Glc)1. Cells were irradiated in phosphate-buffered saline containing 50 mm of sodium azide (NaN3; Sigma-Aldrich). Cytotoxicity was evaluated with the WST-8 assay.
PDT in vivo (22,23). Nude mice (CAnN.Cg-Foxn1nu/CrlCrlj; Charles River, Wilmington, MA) were bilaterally transplanted with COLO679 cells (1 × 106) via subcutaneous injection into the flank. At 12 days after COLO679 transplantation, C60-(Glc)1 (0.1 mg in 100 μL of physiologic saline; Otsuka Pharmaceutical, Tokyo, Japan) containing 40% PEG400 (Kanto Chemical, Tokyo, Japan) was injected into the tumor on the right side. Four hours after injection, the right-side tumor was irradiated with UVA1 laser (Aicure LED SPOT TYPE ANUJ5010; Panasonic, Osaka, Japan) (24) at 10 J cm−2 (intensity: 128 mW cm−2; day 0). As a control, physiologic saline containing 40% PEG400 was injected into the tumor on the left side. The size of the tumor was measured and we calculated the relative volume using the following equation: relative volume (v/v) = volume on day measured/volume on day 0. The formula for computing tumor volume applies to the standard volume of an ellipsoid, where volume = 4π/3(length/2 × width/2 × depth/2). Assuming that depth equals width and π = 3, volume = 1/2 × (major axis)2 × (minor axis).
Statistics. To determine cell survival, the statistical significance of differences was calculated by Student’s t-test and the values were expressed as mean ± the standard deviation (SD). To determine the relative tumor volume in mice the statistical significance of differences was calculated by Wilcoxon’s rank sum test and the values were expressed as mean ± standard error (SE). A P-value less than 0.05 was considered statistically significant.
Results
Phototoxicity of PDT with C60-(Glc)1 or C60-(6Glc)1 against cancer cells
To investigate the effect of PDT against cancer cells and normal cells, an in vitro viability assay was performed and the half maximal inhibitory concentration (IC50) was estimated. Cell viability was not decreased by incubation with glycoconjugated fullerenes without irradiation. The IC50 of PDT with C60-(Glc)1 and C60-(6Glc)1, however, were significantly lower than the IC50 without irradiation. The IC50 of these drugs were significantly higher in normal fibroblast cells than in cancer cells (Table 1). These findings suggest that the effects of PDT with C60-(Glc)1 and C60-(6Glc)1 were preferentially induced in cancer cells compared with normal fibroblast cells.
| UVA1 | (Glc)1 [μm] | (Glc)2 [μm] | (6Glc)1 [μm] | (6Glc)2 [μm] | |
|---|---|---|---|---|---|
| 5637 (bladder cancer) | (−) | 96.4 | 106.2 | 106.7 | 72.4 |
| (+) | 2.5 | 61.4 | 7.8 | 60.3 | |
| HGC-27 (stomach cancer) | (−) | 99.1 | 122.7 | 105.4 | 116.1 |
| (+) | 2.9 | 70.8 | 13 | 120.2 | |
| COLO679 (melanoma) | (−) | 128.2 | 140.6 | 140.9 | 141.9 |
| (+) | 2.2 | 71.1 | 4.5 | 125.9 | |
| DU145 (prostate cancer) | (−) | 86.7 | 94.4 | 100.5 | 101.2 |
| (+) | 2.5 | 37.6 | 7.1 | 67 | |
| HaCaT (cancer keratinocytes) | (−) | 139 | 151.4 | 137.7 | 123.6 |
| (+) | 1.6 | 80 | 6.9 | 126.8 | |
| F113 (normal fibroblasts) | (−) | >190 | 187.5 | >190 | 174.6 |
| (+) | 19.4 | 156 | 22.5 | 168.3 |
PDT with C60-(Glc)1 or C60-(6Glc)1 induces apoptosis in cancer cell lines
To evaluate the apoptosis induced by PDT using C60-(Glc)1 or C60-(6Glc)1, the influence of the incubation duration on apoptosis after irradiation was analyzed. To detect apoptosis induced by PDT with C60-(Glc)1 or C60-(6Glc)1, cells were incubated with the compounds for various time periods and then irradiated. At 24 h after irradiation, the cells were stained with Annexin V and 7-AAD and analyzed by FACS. Irradiation after 6–12 h of incubation effectively induced apoptosis of cancer cells, whereas no significant apoptosis of normal fibroblast cells was induced, even if the cells were incubated for 24 h (Fig. 1). Based on these results, cells were incubated in the presence of C60-(Glc)1 or C60-(6Glc)1 for 24 h in subsequent experiments. To detect when apoptosis occurred after irradiation, cells were stained with Annexin V and 7-AAD at various time points after irradiation and analyzed by FACS. The cells underwent apoptosis 1 h after irradiation in a time-dependent manner (Fig. 2). A previous report suggested that apoptosis in the early process, which is called “immediate apoptosis,” is induced by singlet oxygen (20). Therefore, to examine the relationship between the effect of PDT with glycoconjugated fullerene and singlet oxygen, we inhibited the generation of singlet oxygen.
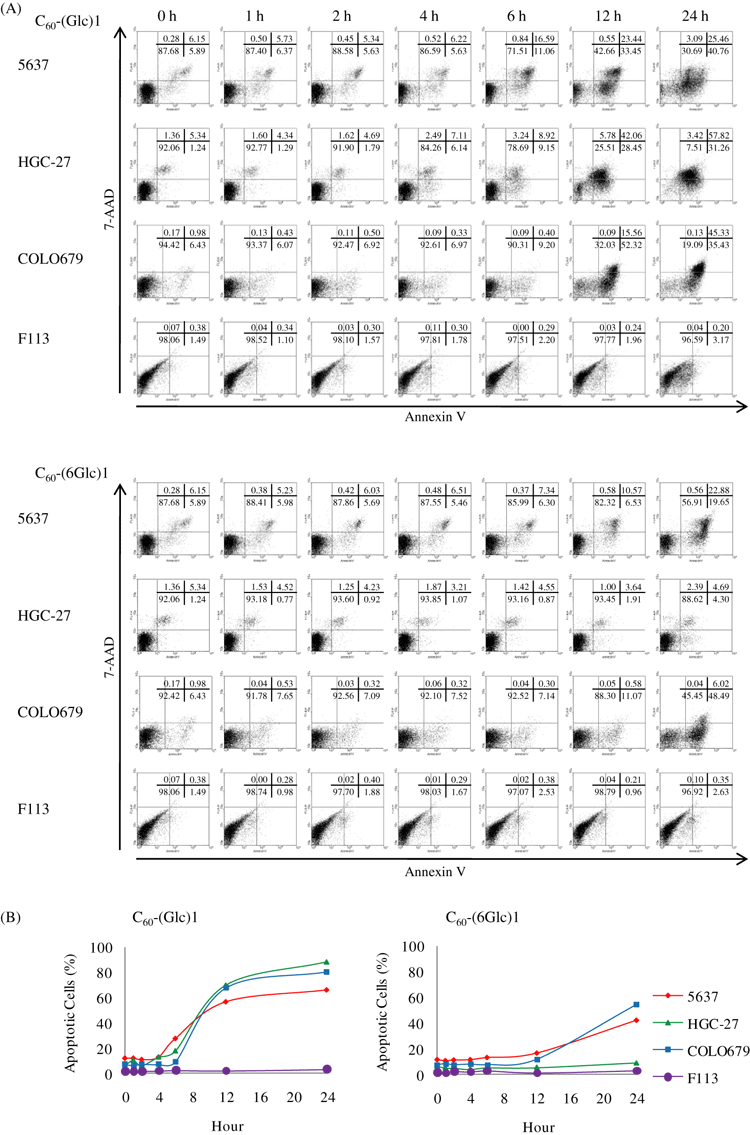
Apoptosis induced by incubation of cells with glycoconjugated fullerenes for various time periods before irradiation. (A) Cells were stained with Annexin V and 7-amino-actinomycin D (7-AAD) for fluorescence-activated cell sorting analysis: cells negative for both markers (viable cells), cells positive for only Annexin V (early apoptosis), cells positive for both Annexin V and 7-AAD (late apoptosis) and cells positive for only 7-AAD (necrosis). (B) The percentage of apoptotic cells is shown. Data are representative from one experiment.
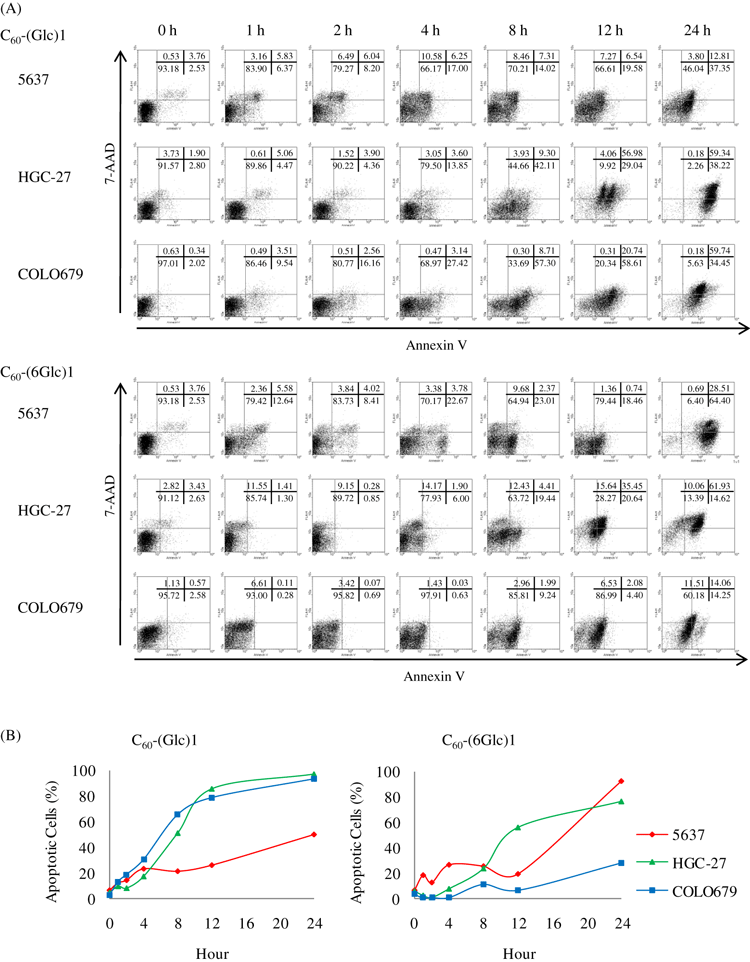
Apoptosis induced by photodynamic therapy (PDT) of cells incubated with glycoconjugated fullerenes at various time points after irradiation. (A) Cells were stained with Annexin V and 7-amino-actinomycin D (7-AAD) for fluorescence-activated cell sorting analysis: cells negative for both markers (viable cells), cells positive for only Annexin V (early apoptosis), cells positive for both Annexin V and 7-AAD (late apoptosis) and cells positive for only 7-AAD (necrosis). (B) The percentage of apoptotic cells is shown. Data are representative from one experiment.
PDT with C60-(Glc)1 or C60-(6Glc)1 induces ROS
In PDT, ROS generated by irradiating the photosensitizer induce cell death (1). We examined whether PDT with the glycoconjugated fullerenes generated ROS. Cells were stained with H2DFFDA, which detects general ROS, including singlet oxygen and superoxide, hydroxyl radical, peroxide, and hydroperoxide. In all cancer cell lines, a large amount of ROS was generated in the presence of C60-(Glc)1 and C60-(6Glc)1. In particular, COLO679 cells generated more ROS. Few ROS were detected in cells treated with C60-(Glc)2 and C60-(6Glc)2, except in HGC-27 cells treated with C60-(6Glc)2. In contrast, in normal fibroblast cells, little ROS was detected, even in the presence of C60-(Glc)1 or C60-(6Glc)1 (Fig. 3). These findings suggested that cell death in cancer cell lines was induced via the generation of ROS by PDT with C60-(Glc)1 and C60-(6Glc)1.
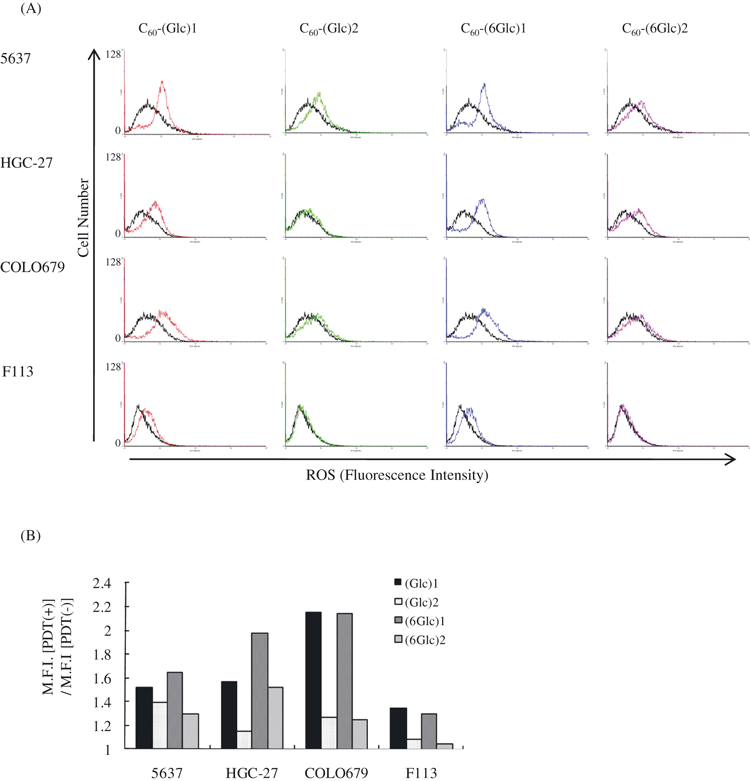
Reactive oxygen species (ROS) induction by photodynamic therapy (PDT) with glycoconjugated fullerene. (A) Cells were incubated with glycoconjugated fullerene and irradiated or not. Subsequently, the cells were stained with 5-(and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate for fluorescence-activated cell sorting analysis. Black lines, non-irradiated cells; colored lines, PDT-treated cells. (B) Comparison of mean fluorescence intensity between PDT (−) and PDT (+). Data are representative from one experiment.
PDT with C60-(Glc)1 or C60-(6Glc)1 induced cytotoxicity via the generation of singlet oxygen
In PDT, photosensitizers excited to a triplet state undergo two kinds of reactions, Type I (light absorption) and Type II (energy transfer). In Type II, the generation of singlet oxygen induces cell death (1). To investigate whether the efficacy of PDT with C60-(Glc)1 or C60-(6Glc)1 depends on the generation of singlet oxygen, 50 mm of NaN3, an inhibitor of singlet oxygen generation, was added during irradiation (20,21). NaN3 significantly decreased PDT-induced cell death in 5637 and HGC-27 cells. The effect could not be measured in COLO679 cells because of activation by NaN3 (Fig. 4). These results suggested that PDT with C60-(Glc)1 and C60-(6Glc)1 induces phototoxicity against cancer cells through the induction of singlet oxygen.
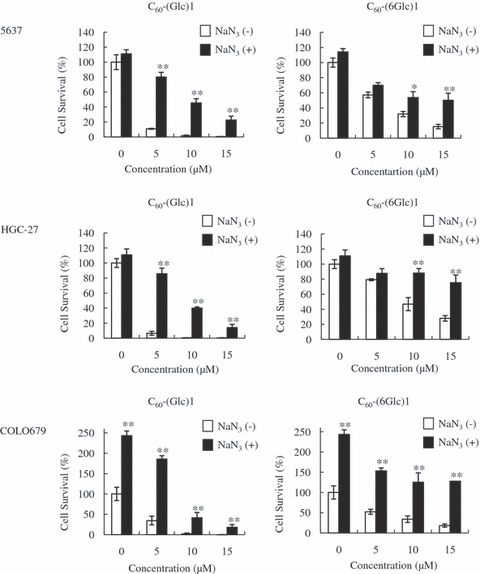
Photodynamic therapy (PDT) with glycoconjugated fullerene induces cytotoxicity via the induction of singlet oxygen. Cells were treated with PDT in the presence or absence of NaN3. The statistical significance of differences was calculated using Student’s t-test. Values are expressed as mean ± SD. **P < 0.01; *P < 0.05.
Tumor growth is suppressed by PDT with C60-(Glc)1
Among the four glycoconjugated fullerenes, C60-(Glc)1 had the highest phototoxicity in vitro. Therefore, to investigate the effect of PDT with C60-(Glc)1 in vivo, COLO679 cells were subcutaneously transplanted into both the left and right flanks of nude mice. When the major axis of the tumor became greater than 5 mm, C60-(Glc)1 (0.1 or 0.2 mg in 100 μL of physiologic saline containing 40% PEG400) was injected into the tumor on the right side. Four hours later, the right-side tumor was irradiated with UVA1 laser at 10 J cm−2 (day 0). In the left-side tumor, 100 μL of physiologic saline containing 40% PEG400 was injected as a vehicle control. Twenty days after PDT, tumor growth on the right was significantly decreased compared with tumor growth on the left in several mice. No differences were observed in five mice receiving PDT with 0.1 mg of C60-(Glc)1, however, and the relative volume of the tumor on the right reached that of the tumor on the left (Fig. 5A). In contrast, PDT with 0.2 mg of C60-(Glc)1 had statistically significant effects and the relative volume of the tumor on the right did not reach that of the tumor on the left, even 20 days after PDT (Fig. 5B). These results suggested that PDT with the glycoconjugated fullerene effectively suppressed tumor growth in an in vivo tumor model.
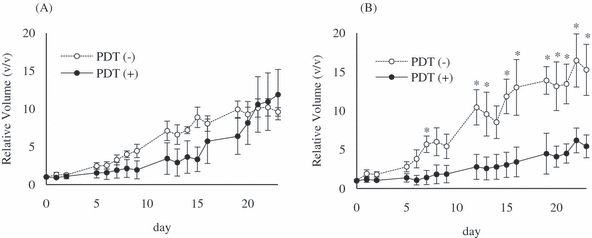
Effect of Photodynamic therapy (PDT) with glycoconjugated fullerene in vivo. Twelve days after transplantation of COLO679, C60-(Glc)1 (A; 0.1 mg [1 × 10−7 mol], n = 5) or (B; 0.2 mg [2 × 10−7 mol], n = 4) in 100 μL of physiologic saline containing 40% PEG400 was injected into the tumor on the right side. The right-side tumor was irradiated with UVA1 laser at 10 J cm−2 4 h after the injection (day 0). As a vehicle control, physiologic saline containing 40% PEG400 was injected into the tumor on the left side. The statistical significance of differences was calculated by Wilcoxon’s rank sum test. Values are expressed as mean ± SE. *P < 0.05.
Discussion
In the present study, we examined the effect of PDT with glycoconjugated fullerene. When porphyrin is excited, the energy is expended in the production of fluorescence. Because fullerene produces little fluorescence and phosphorescence when excited, however, energy is efficiently transferred to oxygen and ROS are generated. Thus, we thought that a fullerene derivative would produce a greater effect in PDT than a porphyrin derivative. PDT with C60-(Glc)1 and C60-(6Glc)1 induced phototoxicity against various cancer cells, but not against normal fibroblast cells. Even if glycoconjugated fullerene was applied to normal fibroblast cells at a concentration equal to that applied to cancer cells, few apoptotic cells were detected. Therefore, the difference in endocytotic capacity between cancer cells and normal cells might account for the difference in the cytotoxicity of PDT. Expression of GLUT-1, which endocytoses glucose, is higher on cancer cells than on normal cells, indicating that cancer cells have increased glucose utilization (12–17). Therefore, the difference in GLUT-1 expression might be responsible for the difference in endocytotic capacity and phototoxicity between cancer cells and normal cells.
The effect of PDT with C60-(Glc)1 was stronger than that of PDT with C60-(6Glc)1. The amount of generated ROS differed not only between normal fibroblast cells and cancer cells, but also among cancer cell lines. The effects of PDT were not correlated, however, with ROS generation rates. Moreover, because PDT with C60-(Glc)2 and C60-(6Glc)2 generated small amounts of ROS, it is possible that these two glycoconjugated fullerenes were not incorporated into the cell. Therefore, the difference in the effects of PDT with glycoconjugated fullerenes may be owing to factors other than endocytotic capacity.
Cell survival after PDT was increased by the addition of NaN3, which inhibits the generation of singlet oxygen. In PDT with glycoconjugated fullerene, singlet oxygen might have been generated by UVA1 irradiation, resulting in cell death. The two pathways of PDT (Types I and II) are light absorption and energy transfer (1,2). A ground-state photosensitizer is excited to a singlet state. Because this state is short-lived and loses energy, the photosensitizer in this state undergoes intersystem crossing to form an excited triplet state. Subsequently, in the Type I reaction, the triplet state photosensitizer reacts directly with the physiologic tissue and transfers a proton or an electron to form a radical anion or cation. These radicals react with oxygen to produce ROS. In contrast, in the Type II reaction, the triplet state photosensitizer transfers its energy directly to oxygen, thereby generating singlet oxygen (1,3–5). In PDT with glycoconjugated fullerene, the Type II pathway is thought to be dominant, generating singlet oxygen that induces apoptosis.
Fullerene has poor solubility, and thus cannot be dissolved and incorporated into the cell. Therefore, we could not directly compare ROS generation between fullerene and glycoconjugated fullerene in the present study.
Moreover, the cell localization of the glycoconjugated fullerene could not be specified in the present study. Various photosensitizers localize in the lysosomes, mitochondria, endoplasmic reticula, Golgi bodies, plasma cell membrane, etc. (1). Determining the localization is very important, however, to understand the apoptotic pathway of PDT with glycoconjugated fullerene. It is also necessary to establish a method to detect glycoconjugated fullerene in the cell. GLUT-1 might be the specific receptor for the efficacy of glycoconjugated fullerenes; therefore, further analysis is required to identify the endocytosis pathway.
Moreover, it is necessary to examine the metabolism of glycoconjugated fullerene in more detail. Intravenous injection of 14C-labeled fullerene into female Sprague–Dawley rats is rapidly cleared from the circulation and the majority of the 14C-labeled fullerene accumulates in the liver (25). The liver does not metabolize or eliminate fullerene (6). Therefore, we considered administration of glycoconjugated fullerene by intratumoral injection safer than that by intravenous injection.
Glycoconjugated fullerene is excited mainly by UVA, and not by a longer wavelength of visible light. Based on the efficacy and the selectivity of glycoconjugated fullerene, its use is applicable for relatively superficial lesions such as carcinoma in situ. It would be highly useful for skin cancers, especially, for example, actinic keratosis and Bowen’s disease. For certain types of gastric and bladder cancers, an endoscopic approach would be necessary.
We investigated the in vivo effect of PDT with C60-(Glc)1, which showed the highest phototoxicity in vitro among the four glycoconjugated fullerenes. PDT with C60-(Glc)1 effectively suppressed tumor growth in mice. Because the tumor growth rate rapidly increases after suppression by PDT, repeated PDT with glycoconjugated fullerene might be necessary.
In this report, PDT with glycoconjugated fullerene had cytotoxic effects in various cancer cells, which was thought to be a result of the generation of singlet oxygen by a photochemical reaction between the glycoconjugated fullerene and UVA1. Moreover, PDT with glycoconjugated fullerene induced cytotoxicity in vivo as well as in vitro, suggesting that glycoconjugated fullerene may be an excellent candidate PDT-enhancing drug.
Acknowledgements— This work was financially supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and partially supported by Dr Masamichi Ipponmatsu (Renaissance Energy Investment Co., Ltd., Kyoto, Japan).




