Photochemistry of Bacteriochlorophylls in Human Blood Plasma: 2. Reaction Mechanism Investigated by Product Analysis and Deuterium Isotope Effect
Abstract
Transmetalated (Pd) bacteriochlorophyll derivatives are currently being clinically tested as sensitizers for photodynamic therapy. Protocols using short delay times between injection and irradiation generate interest in the photochemistry of these pigments in the blood. Using near-infrared irradiation where these pigments absorb strongly, we have studied the mechanism of photo-oxidation in two lipoprotein fractions, low- and high-density lipoproteins, derived from human blood plasma that preferentially accumulate these pigments (Dandler et al. [2009] Photochem. Photobiol., 85, in press). Using quenchers of reactive oxygen species, and chemical reporters, in particular peroxides generated from cholesterol as an inherent component of the lipoproteins, a Type II mechanism generating singlet oxygen has been demonstrated for Pd- and Zn-bacteriopheophorbides. In homogeneous systems, accelerated bleaching in D2O, compared with H2O, supports this mechanism. An unusual deuterium isotope effect was observed, by contrast, in heterogeneous amphiphilic-water systems. In the early phase, and under high oxygen concentrations, again a positive D-isotope effect is observed which later, in a second phase, is reversed to a negative D-isotope effect. The latter cannot be explained by heterogeneous pigment populations in the amphiphilic system; we, therefore, conclude a mechanistic switch, and discuss a possible mechanism.
Introduction
Photodynamic therapy (PDT) uses reactive oxygen species (ROS) to treat malignancies including cancer. ROS are generated by irradiating photosensitizing dyes with visible (VIS) or near-infrared (NIR) light. Pigments absorbing strongly in the NIR are particularly advantageous because tissue is relatively transparent to NIR light (1). Bacteriochlorophyll (BChl) derivatives are among such sensitizers (2–10). They show some favorable properties including low dark toxicity and relatively high accumulation in transformed tissue. In particular, Pd derivatives are chemically more stable (11,12) and give higher quantum yields for triplet generation than the parent Mg-complexes (13,14). The pigments are rapidly cleared from the body, which is probably due to a combination of factors including an active excretion system (15,16), the photolability of many such derivatives (11,12,17) and possibly dark degradations including oxidative ring opening (18,19). The effectiveness, in spite of the rapid clearance, supports evidence for indirect modes of action in PDT not involving a direct attack on the target cells; these indirect modes include apoptotic signaling by ROS and a vascular action (20–27). Pigments with rapid turnover times, like BChl derivatives, are particularly suited for vascular targeting. To design treatment protocols appropriate for this rapid clearance, the pharmacokinetics of photosensitizers becomes of considerable practical interest. This includes, in particular, their photochemistry in the blood where at least part of the primary action will take place when protocols are used involving short delay periods between injection and irradiation, or between subsequent irradiations (28,29).
Mechanistic details of the photodynamic action are currently only partly understood. Singlet oxygen in its 1Δg state (1O2) has been shown to be a major ROS in PDT, not only as a necrotic agent but also as a signal, for example, for apoptosis (22,23,30,31). It can be identified by chemical and physical characteristics such as the photo-oxidation products of cholesterol (8,32), β-carotene (33) or limonene (34), the phosphorescence at 1270 nm (35) or the pronounced H/D isotope effect on the lifetime of 1O2 (36). The latter is particularly pronounced in water, where replacement of H2O with D2O increases the lifetime of 1O2 by an order of magnitude (37,38).
While there is much research into the photochemistry of BChl related to photosynthesis (39,40), little is known about their photochemistry in blood. We have recently shown (41) that most of these pigments when added to blood are present in the blood plasma (BP) fraction, mainly in the low- (LDL) and high-density lipoproteins (HDL); the highly-polar taurinated Pd-complex, WST11 (structure see Fig. 1), accumulates also in the high-density protein (HDP) fraction (41). The better solubility in water is accompanied by a decreased photostability; it is, therefore, of interest if these two properties are linked. As it has been recognized that the generation of ROS species by these pigments depends on their microenvironment (42), we studied the photochemistry of three representative sensitizers in these BP fractions, namely WST11, and the Pd- and Zn-complexes of bacteriopheophorbide (BPheid), namely Pd-BPheid (WST09) and Zn-BPheid (for structures, see Fig. 1). Here, we report the photosensitization mechanism of the pigments in the different BP fractions, using chemical quenchers, product analysis of cholesterol, which is an inherent chemical reporter of lipoproteins, and deuterium isotope effects as mechanistic probes. A separate report (43) focuses on: the photostability of the pigments in the different BP fractions; the oxygen consumption during irradiation; the formation of hydro- and endoperoxides; and the light-induced cross-linking of BP proteins.
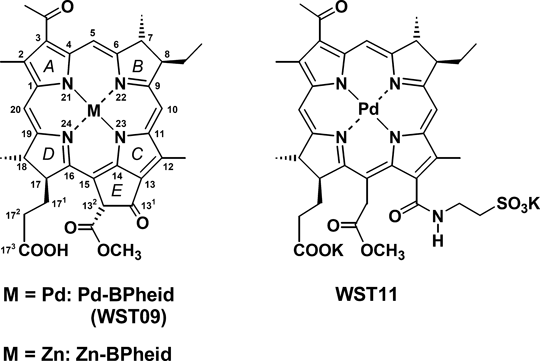
Molecular structures and abbreviations of pigments. Structures are numbered according to IUPAC-IUB (91). Pigments with an isocyclic ring are generally present as 132-epimer mixtures, but only the 132R-epimers are shown as they are usually present in large excess over 132S-epimers. The respective chlorins generated by oxidation have a Δ7,8 double bond while the respective porphyrins also have a Δ17,18 double bond.
Materials and methods
All experiments were carried out under green light (<1 μE m−2 s−1) and at ambient temperature unless stated otherwise.
Materials. Chemicals were analytical grade or better. Unless otherwise noted, they were obtained from Sigma-Aldrich (Taufkirchen, Germany), Roth (Karlsruhe, Germany) or Merck (Darmstadt, Germany). Cremophor® EL (CrEL) was from Fluka (Taufkirchen, Germany) and D2O (99.6% D) from Merck. Diethyl ether was dried and peroxides removed using Alumina B-Super I columns (ICN Biomedicals, Eschwege, Germany). Doubly demineralized water was used for all aqueous solutions. Blood products were obtained from the Bavarian Red Cross (Munich, Germany) and stored at 4°C for up to 4 weeks. CE is a 1:1 (vol/vol) mixture of Cremophor® EL (CrEL) and ethanol, CES a 1:9 (vol/vol) mixture of CE and normal saline.
Pigments. All preparations were carried out under green light (<1 μE m−2 s−1) and argon. Solvents were gassed with argon prior to and during use. Reactions were followed by absorption spectroscopy. Pigments were stored dry, in the dark and under argon at −20°C. Pd-bacteriopheophorbide (Pd-BPheid; WST09; Tookad®) was a gift from Negma-Lerads (Magny-Les-Hameaux, France). Zn-bacteriopheophorbide (Zn-BPheid) was prepared as previously described (41). Palladium 31-oxo-15-methoxycarbonylmethyl-rhodobacteriochlorin 131-(2-sulfoethyl) amide dipotassium salt (WST11; Stakel®, Steba Biotech, Toussus-Le-Noble, France) was provided by A. Scherz (Weizmann Institute of Science, Rehovot, Israel). Synthetic all-trans-β-carotene was from Sigma (No. C-9750; Taufkirchen, Germany).
Spectroscopy. Static absorption spectra were recorded with a UV 2401 PC Spectrometer equipped with an ISR 240-A Integrating Sphere Assembly, controlled by Hyper UV 1.5 (all Shimadzu, Duisburg, Germany) based on Spectacle (LabControl, Cologne, Germany). Dynamic absorption spectra were recorded with a diode array detection (DAD) system consisting of a Tidas UVNIR/16–1024/100-1 diode array detector and a CLH 20 W lamp controlled by Spectralys 1.82 (all J&M, Aalen, Germany) based on Spectacle (LabControl). Fluorescence spectra and relative fluorescence units (RFU) were measured with a Spex Fluorolog 212 spectrofluorometer (Horiba Jobin Yvon, Munich, Germany) equipped with an SVX/LAX 1450 lamp (Müller Elektronik-Optik, Moosinning, Germany) and R374E photomultiplier (Hamamatsu Photonics, Hamamatsu City, Japan), controlled by Quantus (A. Pazur, Department Biology I—Botany, LMU München, Munich, Germany).
Sample preparation. Dialyzed fractions of human BP, loaded with various tetrapyrroles, were obtained by sequential density gradient ultracentrifugation (SDGU) as described previously (41). CES solutions were prepared by mixing a pigment solution in CrEL/ethanol (1:1 vol/vol) with H2O in a 1:19 (vol/vol) ratio. If necessary, samples were concentrated with Centricon® YM-30 centrifugal filter devices (Millipore, Schwalbach, Germany). For H/D-exchange of HDL samples, pigment-loaded HDL were diluted with HDL samples not containing pigments to an optical density of 1.0 at the Qy absorption maximum and dialyzed overnight at 4°C against H2O or D2O under otherwise identical conditions (Servapor® tubing, MWCO: 12,000–14,000; Ø: 16 mm; Serva, Heidelberg, Germany). Micellar solutions in CrEL were obtained by mixing a solution of WST09 (17.9 μm, resulting in an optical density of 1.0 at the Qy absorption maximum of WST09) in CrEL/ethanol (1:1 vol/vol) with either H2O or D2O in a 1:39 (vol/vol) ratio.
Irradiations. Samples (100 μL) were irradiated in an ultra-micro cell (105.202-QS; Hellma, Müllheim, Germany) from the top using a cold-light source (KL 1500 electronic; Schott, Mainz, Germany) equipped with either a RG630 or a RG695 low-pass filter (Schott). The photon flux at the position of the cuvette was determined with a photometer consisting of a SKP 200 display meter and a SKP 218/730 FR far-red sensor (Skye Instruments, Llandrindod Wells, UK); the NIR light intensity was maximally 530 μE m−2 s−1 at 730 nm (30 nm full width at half height). The absorption changes of the sample during irradiation were monitored with a DAD system. The samples were not stirred.
Oxygen determination. Oxygen consumption was measured with a Clark-type electrode consisting of a DW1 electrode chamber and an Oxylab control unit, controlled by Oxylab 1.10 (all Hansatech, Pentney, UK). The electrode was calibrated according to the manufacturer’s instructions. The sample (1.0 mL) was kept at 25°C and stirred with a power setting of the controller of 30%.
Extraction of tetrapyrroles and carotenoids. A lipoprotein sample (1 mL) was mixed (30 s vortexing) with 1 mL ice-cold ethanol. This mixture was mixed by vortexing (30 s) with 5 mL methylene chloride, and then centrifuged (1000 g, 3 min). The lower methylene chloride phase was completely removed and, after drying by rotary evaporation, the residue was extracted with acetone; the remaining solid on the wall of the flask (mainly insoluble lipids) should be colorless. The acetone solution was dried in a rotary evaporator, and the sample stored under argon at −20°C until HPLC analysis.
HPLC analysis. The HPLC system used consisted of a 600E multisolvent delivery system (Waters, Eschborn, Germany), a GROM-SIL 120 ODS-5 ST (5 μm) column (0.4 × 30 cm) (Alltech Grom, Rottenburg-Hailfingen, Germany) and a DAD system (see above). The binary system was composed of solvent A: 70% acetone, 30% H2O (pH 3.5 with acetic acid); and solvent B: 100% acetone; the flow rate was 1.2 mL min−1; using the following gradient: 0 min: 100% A; 4 min: 100% A; 19 min: 0% A; 20 min: 0% A; 24 min: 100% A; 30 min: 100% A. Chromatograms were extracted at 430 nm from the 3D data sets. All solvents were degassed before use. Tetrapyrroles were identified by co-chromatography with authentic samples and also by their absorption spectra. Carotenoids were identified by comparison with published HPLC analyses of BP carotenoids and/or by their absorption spectra (44–46).
HPTLC of cholesterol oxidation products. Lipid extraction: Lipids were extracted from the irradiated samples (0.5 mL) with a mixture of methylene chloride and methanol (1:1 vol/vol) (1 mL). The methylene chloride phase was concentrated to 50 μL under a stream of argon, and diluted with 50 μL of methanol. If reduced peroxides were assayed, a methanolic solution of NaBH4 (50 mm) and NaOH (10 mm) was used for dilution, and the samples were incubated for 1 h at ambient temperature. In both cases, an aliquot (16 μL) was applied to HPTLC plates (Kieselgel 60 on aluminum sheets; Merck), which were developed with a mixture of n-hexane and ethyl acetate (1:1 vol/vol) in a horizontal chamber. After development, hydroperoxides were stained dark blue within a few minutes at ambient temperature with N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD, 6 mm) in H2O, methanol and acetic acid (50:50:1 vol/vol/vol, modified from Ref. [47]). Lipids were stained with CuSO4 (0.32 m) in H2O, concentrated H3PO4 and methanol (85:10:5 vol/vol/vol, modified from Ref. [48]). Upon heating the wet plates with a heat gun, the lipid spots gave characteristic colors depending on their structures: Chol, purple; (O)OH-Chol, light blue; 7-keto-Chol, yellow; sodium cholate, olive; most other lipids, brown.
Cholesterol esterase reaction: 2.5 mg cholesterol esterase (Porcine pancreatic CholEase; 33.1 U mg−1; Fluka, Taufkirchen, Germany) and 20 mg sodium cholate were dissolved in 15 mL phosphate buffer (50 mm; pH 7.2). For each assay 400 μL of this solution and 100 μL of the respective sample were incubated for 1 h at 37°C, and subsequently analyzed by HPTLC (see above). The enzymatic activity was checked by a solution of cholesterol oleate (12.3 mm) in 2-propanol.
Preparation of standards: Primary and secondary cholesterol oxidation products were identified by co-chromatography against freshly prepared standards (49,50). A mixture of 3β-hydroxycholest-5-ene-7α-hydroperoxide (7α-OOH-Chol) and 3β-hydroxycholest-5-ene-7β-hydroperoxide (7β-OOH-Chol) was produced by overnight incubation of an LDL or HDL lipoprotein fraction with the same volume of aqueous CuSO4 (20 mm) and citric acid (50 mm; adjusted to pH 4.0 with NaOH) at 37°C. A mixture of the respective alcohols (7-OH-Chol) was produced by treatment of 100 μL 7-keto-cholesterol (12.5 mm) in methanol with 100 μL of a methanolic solution of NaBH4 (50 mm) and NaOH (10 mm). The solvent was evaporated with a stream of argon, and the residue containing 7αβ-OH-Chol was dissolved in a mixture of methylene chloride and methanol (1:1 vol/vol). 3β-hydroxy-5α-cholest-6-ene-5-hydroperoxide (5α-OOH-Chol) was produced by irradiating a mixture of 100 μL Chol (12.9 mm) in methanol with 100 μL rose bengal (50 mm) in methanol for 1 h with white light from a cold-light source (see above) at maximum light intensity. The respective alcohol (5α-OH-Chol) was produced by chemical reduction of 5α-OOH-Chol with NaBH4 in methanol, under the conditions used for the reduction of 7-keto-Chol to 7-OH-Chol (see above).
Results
Effects of ROS quenchers
Type I reactions involving radicals and Type II reactions initiated by singlet oxygen (1O2) can be distinguished by quenchers (see Bonnett [8] for definition). For initial tests, d-mannitol (51) was used as a quencher for Type I reactions and l-histidine (52) for Type II reactions while sodium ascorbate (53,54) was used as a nondiscriminating antioxidant. The photostability of the sensitizer, Zn-BPheid, was not increased by the presence of mannitol, but was enhanced by histidine and, even more so, by ascorbate, indicating that photobleaching is due to 1O2. The same is true for Pd-BPheid: although it bleached much more slowly (43), the effects of the quenchers were comparable. There was also no significant difference between LDL and HDL. Experiments with a larger set of ROS quenchers were undertaken with the more rapidly bleaching Zn-BPheid, and only in HDL (for supporting information, see Table S1). Again, the photostability was not increased by quenchers for Type I reactions (sucrose, glycerol, DMSO). The response to quenchers for Type II reactions was heterogeneous. D2O, which enhances the lifetime 10-fold relative to H2O (36), slightly increased photostability. Sodium azide was ineffective, probably because of its ionic nature; like many other ROS quenchers, it is too polar to interact with membranes (55) and, therefore, with membrane-bound pigments. As quenching requires physical contact with the ROS (55), 1,4-diazabicyclo[2.2.2]octane and β-carotene were tested as lipophilic 1O2 quenchers. The former only slightly increased photostability while the latter was more effective even considering that CrEL, used for solubilizing β-carotene, was partly responsible for this protection. As the natural carotenoid concentration in human BP is only about 2 μm (56), it would only marginally protect pigments (and other molecules) from this level of ROS attack. Among the other 19 standard amino acids (see Table S1), photostability was also increased by cysteine, methionine, tyrosine and tryptophan that react with 1O2 (57,58) and, therefore, are quenchers of Type II reactions (52). Proline had no significant effect, although it can quench 1O2 under certain conditions (59).
Cholesterol oxidation products
A more rigorous approach to distinguish photo-oxidation mechanisms was the analysis of oxidation products of cholesterol (Chol) and its esters (CholE) (60) by HPTLC, either directly using TMPD staining, or indirectly, after reduction with sodium borohydride to the corresponding more stable hydroxyl derivatives, using acidic CuSO4 staining. Type I reactions generate 7-OOH-Chol (Fig. 2), predominantly in the 7β-configuration, together with traces of 7-OH-Chol, 5,6-epoxy-Chol and 7-keto-Chol. Type II reactions generate mainly 5α-OOH-Chol, accompanied by traces of 6-OOH-Chol (both epimers).
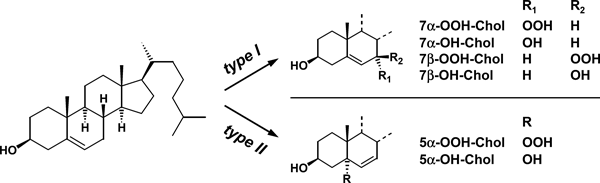
Structures of cholesterol (left) and its main oxidation products generated by Type I (top right) and Type II photoreactions (bottom right). Alcohols are obtained from the respective peroxides by reduction with sodium borohydride.
Lipids extracted from nonirradiated HDL contained no peroxides; those from LDL contained trace amounts of several peroxides (data not shown). In all irradiated samples, 5α-OOH-Chol was found (see supplementary material, Fig. S1). The majority of the peroxides, however, were derivatives of different CholE that could not be separated using the HPTLC system that was optimized for analysis of Chol peroxides. We found, however, that the CholE peroxides can be hydrolyzed nearly quantitatively (compare Fig. 3a and Fig. S1) by cholesterol esterase (CholEase) to form the corresponding Chol peroxides, yielding 5α-OOH-Chol as the main oxidation product (Fig. 3a). In addition to traces of nonhydrolyzed CholE (peroxides), two further peroxide bands of unknown nature were detected in each sample. These may be peroxides derived from fatty acids, but they were not identified. Phospholipid peroxides remain at the origin in the chromatography system used (50). In the context of investigating the photo-oxidation mechanism, it was important that they contained no 7-OOH-Chol as shown by co-chromatography of the corresponding alcohols (see below) against an authentic sample obtained by reduction of 7-keto-Chol. The unknown product II is formed in small amounts only in LDL, but approaches or even exceeds 5α-OOH-Chol in HDL, irrespective of the sensitizer used. Using the summed intensities of the stained peroxide bands as an estimate for peroxide generation, their concentrations decreased in the following order: LDL + Pd-BPheid ? HDL + Pd-BPheid ≅ LDL + Zn-BPheid > HDL + Zn-BPheid. Although this is only a rough estimate, it conforms with the results from a quantitative test assay based on the formation of 2′,7′-dichloro-fluorescein (43).
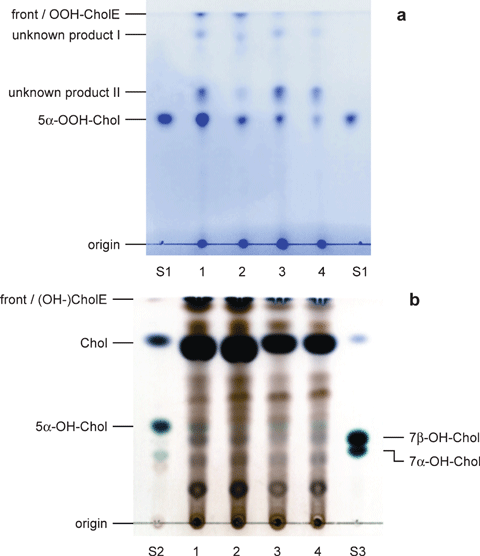
HPTLC of lipids extracted from irradiated, pigment-loaded lipoprotein fractions after treatment with CholEase (a, TMPD staining) and after treatment with CholEase and NaBH4 (b, CuSO4 staining). Lipoprotein fractions were obtained by SDGU fractionation of BP incubated with the respective pigment and irradiated with NIR light (low-pass filter RG695; 530 μE m−2 s−1; 60 min). 1: LDL + Pd-BPheid; 2: LDL + Zn-BPheid; 3: HDL + Pd-BPheid; 4: HDL + Zn-BPheid; S1: 5α-OOH-Chol standard; S2: 5α-OH-Chol standard; S3: 7-OH-Chol standard. Top bands (esters) in lanes 1–4 could contain traces of (5α-)OH esters in (b).
HPTLC of the peroxides gives only poor separations of the epimers of 7-OOH-Chol and 5α-OOH-Chol (see supplementary material, Fig. S2). The resolution of the HPTLC system and the stability of the products could be considerably improved if the peroxides were reduced with NaBH4 prior to chromatography: the resulting alcohols (5α-, 7α- and 7β-OH-Chol) can be clearly identified (61). Furthermore, staining with CuSO4 permits a clear distinction between hydroxylated Chol derivatives by their blue color from other Chol derivatives. The results are compatible with a Type II reaction: after reduction, all four irradiated samples contained 5α-OH-Chol, the largest amount being present in LDL loaded with Pd-BPheid (Fig. 3b). There is also an unidentified band with the rf value of 7β-OH-Chol (assignment of the two 7-OH-Chol epimers by their relative rf values [49,50]), but it lacks the characteristic light blue color that is clearly visible in the corresponding standard. Furthermore, in no case was there an accompanying band of 7α-OH-Chol that would have also been generated by a Type I reaction.
The Type II mechanism also dominates reactions of Pd-BPheid in many organic solvents. If the pigment was irradiated together with Chol, 5α-OOH-Chol (5α-OH-Chol after reduction) was formed in most solvents, and 7-OOH-Chol (7-OH-Chol after reduction) was never detected (see Figs. S3 and S4). In some cases, the oxidation products apparently reacted with the solvent, thereby forming nonassignable Chol derivatives. No oxidation products were found in DMSO, which is not only a quencher of hydroxyl radicals (62), but also of 1O2 (63,64).
Carotenoid oxidation products
Carotenoids were extracted from irradiated lipoprotein samples loaded with either Zn- or Pd-BPheid, and investigated by HPLC analyses for oxidation products. The three most abundant carotenoids in both LDL and HDL were β-cryptoxanthin (tr: 21.2 min), lycopene (tr: 23.0 min) and β-carotene (tr: 24.0 min). No additional carotenoids could be detected after irradiation in untreated lipoproteins (data not shown). In LDL (Fig. 4a) and HDL (see supplementary material, Fig. S5a) loaded with Zn-BPheid, one additional carotenoid band was detected. The product (tr: 22.7 min) had a typical three-banded carotenoid absorption (λmax = 409 [shoulder], 430 and 454 nm, Fig. 4b). The blueshift of all bands is indicative of a shortened conjugation system as is present, for example, in 7,8-dihydro-β-β-carotene (λmax = 410 [sh], 429 and 454 nm) (65). Two oxidation products of β-carotene have a similar absorption spectrum: 5,8-epoxy-5,8-dihydro-β-β-carotene (mutatochrome) and 5,8-endoperoxy-5,8-dihydro-β-β-carotene (5,8-endoperoxy-β-carotene, Fig. 5); the latter was identified by several groups as a main reaction product of β-carotene with 1O2 (33,66–68).
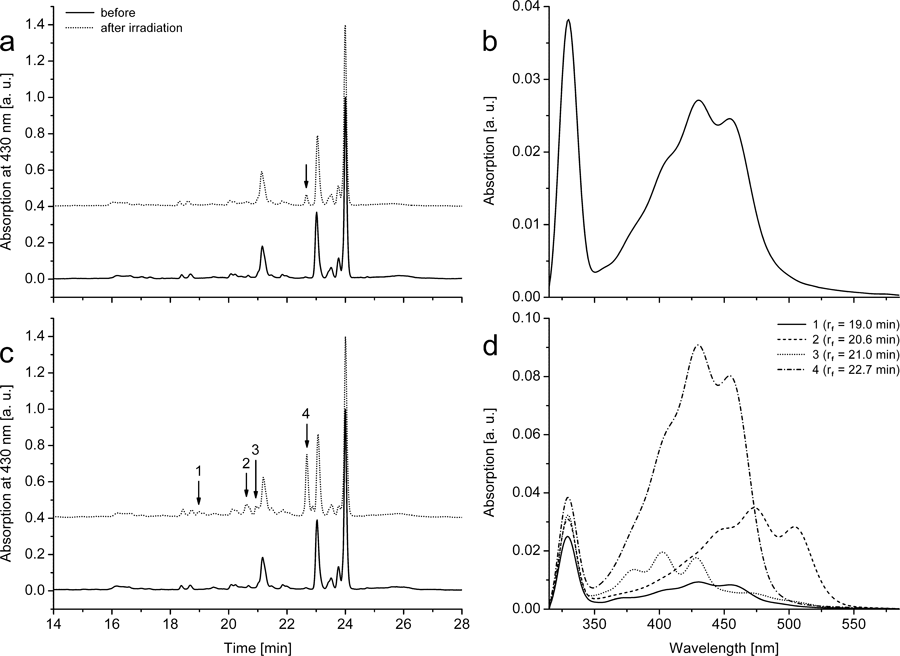
Photosensitized products of endogenous carotenoids of blood plasma. HPLC of extracts of LDL loaded with (a) Zn-BPheid (12 μm) or (c) Pd-BPheid (13 μm) before and after irradiation with NIR light (low-pass filter RG695; 530 μE m−2 s−1) for 10 min (a) or 180 min (c), and in situ absorption spectra (b, d) of the respective main oxidation products (retention times are marked with arrows in the HPLC chromatograms). BP was pigment-loaded prior to LDL preparation by SDGU. Chromatograms of the irradiated samples were shifted up by 0.4 units. See Fig. S5 for the respective data obtained in HDL.
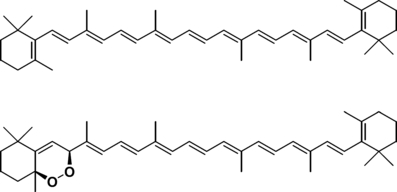
Structures of β-carotene (top) and 5,8-endoperoxy-5,8-dihydro-β-carotene (bottom).
The concentration of this product increased seven-fold if lipoprotein samples were irradiated in the presence of Pd-BPheid instead of Zn-BPheid: three additional oxidation products were found in low concentrations (Fig. 4d) and all had carotenoid-like absorption spectra (Figs. 4c and S5b). The pigment eluting at 19.0 min had an absorption spectrum (λmax = 409 [sh], 430 and 455 nm) that is almost identical to that of the 5,8-endoperoxide; judged from its mobility, however, it is possibly a more polar derivative with further modifications outside the conjugation system. The pigment eluting at 21.0 min had an even shorter conjugation system (λmax = 381, 402 and 428 nm), resembling that of 7,8,7′,8′-tetrahydro-ψ,ψ-carotene (ζ-carotene; λmax = 378, 401 and 426 nm) (46) or 5,8,5′,8′-diendoperoxy-β-carotene; the latter was identified as a photo-oxidation product of β-carotene with 1O2 in solution (33). The third minor product had an absorption spectrum (λmax = 453 [sh], 473 and 504 nm) indicative of a longer conjugation system that is present, for example, in lycopene (λmax = 449 [sh], 473 and 504 nm), but the product is more polar than the latter judged from the retention time of 20.6 min. It is possibly a lycopene oxidation product in which the π-electron system has not been shortened, but which is instead modified at an end group without affecting the π-system.
Chlorophyllous oxidation products
When irradiating LDL (Fig. 6a) or HDL fractions (data not shown) loaded with Pd-BPheid (λmax = 760 nm), the corresponding chlorin derivative, [3-acetyl]-Pd-pheophorbide ([3-acetyl]-Pd-Pheid; λmax = 660 nm), was the only detectable oxidation product with significant absorption in the VIS/NIR range. It was identified by co-chromatography with an authentic sample obtained from Pd-BPheid by oxidation with 2,3-dichloro-5,6-dicyano-p-benzoquinone (69). The reaction is analogous to the oxidation of BChl a to [3-acetyl]-chlorophyll a (70); dehydrogenation of ring B is favored over dehydrogenation of ring D for steric reasons (69,71,72). Based on the absorption changes and the known extinction coefficients, less than 50% of Pd-BPheid was photo-oxidized to [3-acetyl]-Pd-Pheid, while the remainder formed products with little absorption in the VIS/NIR range. This is similar to results obtained with BChl-derivatives in organic solvents (12). There was, in particular, no spectroscopic or HPLC indication of a further photo-oxidation of the chlorin to the respective porphyrin derivative. This is not only due to the aforementioned preferential reaction at ring B but also to the NIR irradiation conditions (695 nm low-pass filter) that provide only little light in the absorption range of [3-acetyl]-Pd-Pheid (λmax = 660 nm). There was also no indication of the formation of cation radicals, which have a broad, low-intensity NIR absorption band (73).
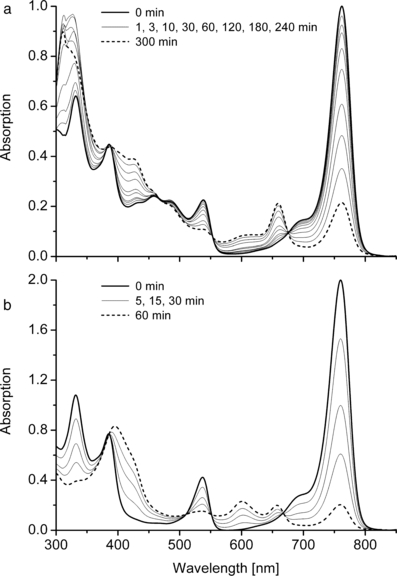
Photoreaction of WST09 in a dialyzed LDL fraction (a) and in CES (b). Absorption spectra before and during irradiation with NIR light (low-pass filter RG695; 530 μE m−2 s−1).
Further oxidation to the porphyrin, however, is also dependent on the environment: it did occur if Pd-BPheid was irradiated under otherwise identical conditions in CES, a mixture of CrEL, ethanol and (normal) saline that is used as a solubilizing system for tetrapyrroles into BP. The oxidation product, [3-acetyl]-Pd-protopheophorbide ([3-acetyl-Pd-PPheid; λmax∼600 nm) (Fig. 6b), was previously reported as a photoproduct of Pd-BPheid in 3% Triton X-100/chelexed PBS (42).
Deuterium isotope effect
WST09 is very stable in NIR light. When an HDL fraction loaded with WST09 was dialyzed against H2O and irradiated in an open cuvette with the maximum light intensity of our apparatus (NIR light intensity: 530 μE m−2 s−1), 50% bleaching was observed after 25.5 min (Fig. 7a). The high stability of this Pd-complex is similar to that reported for Pd-bacteriopheophytin (Pd-BPhe) in organic solvents (11,12). WST11 is, by contrast, much more photolabile: at the same light intensity it was 50% bleached within only 2.1 min (Fig. 7b).
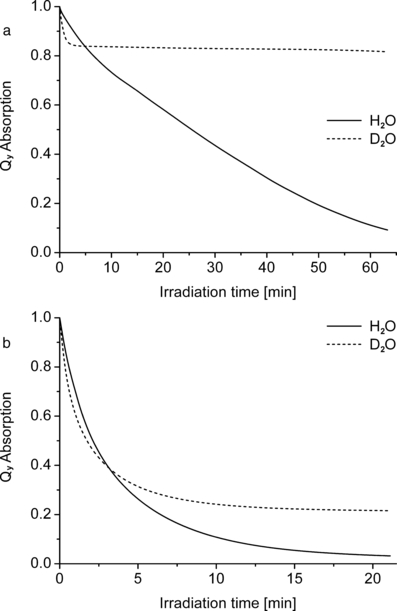
Photobleaching kinetics of WST09 (a) and WST11 (b) in lipoproteins (HDL). Pigment-loaded HDL was dialyzed against H2O or D2O, and irradiated with NIR light (530 μE m−2 s−1). Note the different time scales in (a) and (b).
When the same experiments with WST09 and WST11 were performed in D2O, there was, in both cases, initially a more rapid bleaching than in H2O, but this effect was reversed after longer periods when the bleaching was inhibited in D2O compared with H2O. This reversal effect is more pronounced with the less polar WST09: at the onset (<3 min) the bleaching in D2O was much faster than in H2O (kD/kH = 2.25; all given kD/kH values are initial rate constants extrapolated to t = 0), but was then almost arrested in D2O at a level of ∼83% of the original pigment content (Fig. 7a). This reduced kinetics is not due to the depletion of oxygen, because in H2O the pigment is fully bleached after approximately 1 h. The effect is qualitatively similar with WST11 (kD/kH = 1.75), but less pronounced (Fig. 7b). Care was taken that the samples were comparable, except for the replacement of H2O and D2O. For isotope exchange, HDL samples were dialyzed against D2O. The H2O-controls were dialyzed in exactly the same way against H2O, which ensured that the concentrations of natural, water-soluble antioxidants were reduced to a similar extent in both samples.
Some lipophilic antioxidants will remain in the HDL after dialysis. To test if the unusual isotope effect is inherent in the HDL fraction, WST09 was irradiated in micelles of CrEL. This polyethylene glycol derivative of castor oil is frequently used in medicine as a substitute for DMSO, although it is not a completely inert substance (74). WST09 was first dissolved at very high concentration (∼720 μm) in a mixture of CrEL and ethanol (1:1 vol/vol), and this solution was subsequently diluted (1:39 vol/vol) with either H2O or D2O. In this artificial system that contains unsaturated fatty acids but is otherwise devoid of antioxidants, the same two phases of the illumination kinetics were observed (Fig. 8), albeit less pronounced than in HDL. The initial bleaching (<3 min) was still faster in D2O than in H2O (kD/kH = 1.95), and it leveled off in D2O at ∼52% of the original pigment content.
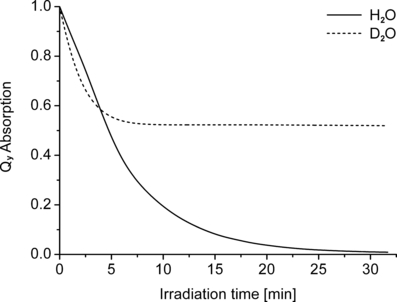
Photobleaching kinetics of WST09 in a micellar environment. WST09 was dissolved in a mixture of CrEL and ethanol (1:1 vol/vol), subsequently diluted with H2O or D2O (1:39 vol/vol) and irradiated with NIR light (530 μE m−2 s−1).
Both the lipoprotein and the CrEL systems are heterogeneous; it was, therefore, of interest to study the isotope effect on photobleaching also in a homogeneous system, such as in water. The high solubility of WST11 allowed such studies, while WST09 is only poorly soluble in water. Photobleaching of WST11 gave a positive deuterium isotope effect throughout the reaction (kD/kH = 1.50), and the pigment was eventually completely bleached in both D2O and H2O (Fig. 9). Thus, these results indicate that the unusual biphasic deuterium isotope effect is related to the heterogeneous amphiphile-in-water reaction medium.
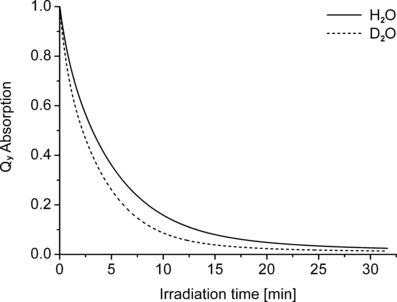
Photobleaching kinetics of WST11 in H2O and D2O. Solutions were irradiated with NIR light (12 μE m−2 s−1).
Discussion
Photodynamic action of bacteriopheophorbides in lipoproteins is dominated by the Type II mechanism
Investigation of the mechanism, by addition of quenchers, indicated a Type II reaction mechanism and this was supported by a more rigorous test relying on the formation of mechanism-dependent reaction products of a probe substrate. A particularly convenient substrate, in the present case, was cholesterol: as a natural component of lipoproteins (55) it does not require any additions to the samples, and its photoproducts have been established (32).
Identification of 5α-OOH-Chol after irradiation of both LDL and HDL gave strong confirmatory support for a Type II mechanism using Pd-BPheid or Zn-BPheid as photosensitizer. The HPTLC analysis of the reaction mixtures was, nonetheless, unsatisfactory: the separation of the peroxides is poor and the products are relatively labile. Free Chol is furthermore accompanied by Chol-esters, and these as well as their photo-oxidation products are not included in the standard analysis. We therefore modified the assay in two ways. First, the esters were hydrolyzed (nearly) quantitatively to the respective free Chol derivatives by treatment with CholEase, and second the product peroxides were reduced with borohydride to the respective alcohols, 5α-OH-Chol and the two epimers of 7-OH-Chol. The HPTLC separation of these products was much clearer, and the products can be identified more rigorously by co-chromatography with stable authentic samples that are accessible by established procedures (49,50). Of particular importance was the resolution of the 7-OH-Chol epimers. The 5α-OH-Chol standard contained small amounts of 7α-OH-Chol, while 7β-OH-Chol is absent. It is also known that 5α-OOH-Chol can be transformed into 7α-OOH-Chol via an allylic rearrangement, while the 7β-epimer is formed much more slowly by subsequent epimerization to 7β-OOH-Chol (75,76). 7-OOH-Chol generated by a Type I reaction is, by contrast, an epimer mixture that yields, after reduction, the two epimeric alcohols, as does reduction of 7-keto-Chol (49,50). The consistent lack of spots at the position of 7β-OH-Chol is, therefore, the unequivocal evidence for a Type II reaction of both sensitizers.
In principle, 1O2 can also be generated by disproportionation of peroxyl radicals (32,77) but, in this case, formation of some 7-OOH-Chol is expected by a competing direct action of the latter. The consistent absence of 7β-OH-Chol, the reduction product of 7β-OOH-Chol, also argues, therefore, against such an indirect production of 1O2. Thus, we conclude that only 5α-OOH-Chol is formed in the lipoprotein samples upon irradiation and that Chol in lipoproteins is therefore attacked by Type II reactions, irrespective of the use of Pd- or Zn-BPheid as photosensitizer.
While these results agree with the action of many photosensitizers, a more complicated mechanism has been suggested by Vakrat-Haglili et al. (42). Using sodium azide as quencher, the authors concluded that Pd-BPheid is oxidized to the chlorin level by superoxide or peroxide radicals, and only the subsequent oxidation to the porphyrin level involves 1O2. It should be noted, however, that photobleaching of the sensitizer, or its chlorin oxidation product, are only minor reactions in plasma lipoproteins that are rich in oxidizable components that compete for the oxidant. In a recent study, it has been estimated that the concentration of peroxides at the end of the irradiation (160 μm) is ca 18-fold higher than the initial concentration of the sensitizer (9 μm) (43). The chlorin, [3-acetyl]-Pd-Pheid which, under our conditions, amounts to about half the concentration of Pd-BPheid originally present therefore constitutes only about 3% of the products.
Such a model also explains the much higher stability of (monomeric) BChl derivatives in lipoproteins, compared to the situation in detergent micelles or in solution where such competitors are missing (43). In pure detergent environments (CES; Triton X-100) that are poor as competing substrates, the bacteriochlorin sensitizers are much more rapidly oxidized to chlorins (and other products), and the chlorins are further photo-oxidized to porphyrins. The relevance of competing target molecules, such as Chol(esters) (see above), unsaturated fatty acids and reactive amino acids, is further supported by the observation that the oxygen consumption upon irradiation increases with increasing lipoprotein concentrations (data not shown).
In summarizing these results, we suggest that lipoproteins have a considerable capacity for quenching Type II photoreactions, and that at least the peroxides of Chol and its esters are exclusively formed by reaction with 1O2. In cells, 1O2 is mainly quenched by proteins (78) but this may be true only for quite hydrophilic photosensitizers and environments. For more hydrophobic photosensitizers including Pd-BPheid that are embedded in membranes, a higher participation of lipids and other nonpolar substances can be expected. Last but not least, the resulting photo-oxidation products may be still quite reactive and may require further attention regarding their participation in photodynamic action. Such a complication has previously also been suggested for carotenoids: β-carotene can bind up to eight oxygen atoms (33). Absorption spectra of the major and of two of the three minor photoproducts match with those of endoperoxides reported in the latter work.
A mechanistic switch at low oxygen concentration
Notwithstanding the evidence for Type II as the main photosensitizing mechanism in lipoproteins, the H/D isotope experiments point to a mechanistic switch at low oxygen concentrations. For many porphyrin sensitizers, the isotope effect on the 1O2 lifetime is not an important limiting factor for photobleaching (kD/kH = 1.2–1.6) (79,80). These data indicate that the unusual biphasic deuterium isotope effect is related to the heterogeneous amphiphile-in-water reaction medium; in agreement with this is its absence in water.
The unusual isotope effect is more pronounced with WST09 than with the more hydrophilic WST11; that is, with WST09 there is a much smaller amplitude than with WST11 of the phase with the normal, positive D-isotope effect that is expected for a 1O2-induced bleaching (and indeed observed in water). Therefore, this part of the photobleaching process seems to be related to a fraction of the pigment that resides in a largely aqueous environment near the surface of the HDL particles, or even free in solution. The presence of substantial amounts of free pigment is unlikely, because there was no loss of pigment during dialysis. We therefore conclude that the pigment fraction showing the positive isotope effect is located near, or exposed to the aqueous phase. The second pigment fraction exhibiting the negative D-isotope effect of photobleaching would then be located in the more hydrophobic interior. This is more readily accessible for WST09 than for the more polar WST11: while >80% of WST09 is located in the lipoprotein fractions (LDL and HDL) of human BP, ∼80% of WST11 is bound to HDP, most probably human serum albumin (41).
While this model can rationalize the extent and properties of the first phase of bleaching with a positive D-isotope effect, we are currently unable to conclusively explain the negative isotope effect in the later phase. We first considered several possible, but ultimately unsatisfactory, explanations: (1) First, that oxygen is more rapidly depleted in D2O than in H2O because the larger lifetime means a larger range of actions, thus allowing access to more target molecules like cholesterol (esters), unsaturated fatty acids, oxidizable amino acids and lipophilic antioxidants. Such target molecules are absent, however, or at least much less abundant in the CrEL-system, while the same biphasic behavior is observed. (2) Second, that the duration of residence of oxygen near the sensitizer is decreased in D2O to such an extent that it compensates the increased lifetime of 1O2. This seems unlikely even if it involves H-bonding. While this cannot be excluded, it would still not explain the mechanistic switch. (3) Third, the change from H2O to D2O may induce a phase transition, or accelerate one that is induced by ROS. However, in membranes containing unsaturated fatty acids and cholesterol no isotope effect on the molecular organization of the lipid membranes could be detected (81), and it is unlikely that a similar effect would operate in lipoproteins and in the CrEL system. (4) Fourth, it is conceivable that the dehydrogenation reaction is affected. As only a fraction of the pigments is oxidized to chlorins, an isotope effect of the observed size also seems unlikely. (5) Finally, bleaching during the late phase, where oxygen concentration is low, may relate to a mechanism that is independent of 1O2, but rather relies on a Type I or other mechanism (82). Although isotope effects have been reported not only for the lifetime but also for the yield of triplets (and 1O2) (83–85), there is, to our knowledge, no report on a negative D-isotope effect on these processes.
Because none of the above five hypotheses can satisfactorily explain the negative deuterium isotope effect, we propose, therefore, an alternative explanation that involves two factors: first, the second phase of bleaching is not due to the action of 1O2, and second, the equilibrium concentration of 1O2 during irradiation is so high with respect to the total oxygen concentration that the amount of triplet oxygen (3O2) is significantly reduced. A mechanistic switch has also been previously reported for hematoporphyrin: at low concentrations (∼1 μm) Type II reactions prevail, while at higher pigment concentrations (∼15 μm) Type I photoreactions dominate. Aggregation has been invoked for this mechanistic switch (86), which does not, however, apply to all the BChl derivatives studied in this work (41). Alternatively, Type I photoreactions are favored at low pigment/oxygen ratios, because of the higher probability for primary reactions with molecules other than oxygen (82).
The second precondition is that the reduction of 3O2 in D2O relative to that in H2O at low oxygen concentrations can be met when 3O2 is more rapidly excited to 1O2 than when the latter relaxes back to 3O2. The extended lifetime of 1O2 in D2O would, in this case, reduce the amount of 3O2 present further than is the situation in H2O, thus generating a negative isotope effect for reactions depending on the latter. At a quantum flux of 530 μE m−2 s−1, and a pigment concentration of 18 μm, each pigment molecule is excited ∼3 times s−1. With a quantum yield of 99%, this could generate ∼3 molecules of 1O2s−1. At saturating O2 conditions (250 μm), this excitation rate would be far too low because O2 is present at an approximately 14-fold molar excess over the sensitizer; however, O2 is depleted in the case of WST09 in HDL within ∼2.5 min (measured by a Clark-type electrode; data not shown) and can only be restored by diffusion, which is further inhibited in the heterogeneous lipid system. A reduction to values of 1% and less is also compatible with in vivo reports using a bacteriopheophorbide-serine as sensitizer (87). Assuming an 1O2 lifetime of 60 μs in D2O, a reduction in the oxygen concentration by a factor of 104 would suffice to maintain a substantial fraction of the total oxygen in the singlet state. This model, therefore, seems to be compatible with the experimental results.
Concluding remarks
A Type II mechanism was previously established for photosensitization by transmetalated BChl derivatives in solution (11). This has now been extended to the two lipoprotein fractions, LDL and HDL, that carry the majority of these pigments in the bloodstream (41). The only exception to this rule is the water-soluble taurinated derivative, WST11, for which a Type I mechanism has been proposed (42,88). This difference in mechanism may be a direct result of the chemical modification, but can also be due, indirectly, to the hydrophilic microenvironment preferred by the latter pigment that is also responsible for its enrichment in the HDP fraction of BP (89). The mechanistic switch seen for WST09 and WST11 at low O2 concentrations is compatible with such a dominant microenvironmental effect. Such a switch at low oxygen concentrations, from a Type II reaction involving 1O2 to one involving other ROS, may be of practical significance for PDT and thus warrants further investigation.
For mechanistic studies in blood, we have shown that the use of Chol as an intrinsic reporter of lipoproteins for distinguishing the different mechanisms of ROS action (60) can be extended to the more abundant Chol esters. Peroxides of these esters can be hydrolyzed without further modification to the free Chol peroxides, which can subsequently be reduced with borohydride to the more stable hydroxides. In addition to Chol and its esters, carotenoids, and even the photosensitizers, can yield information about the sensitizing mechanism. They show, again, a distinct difference between the highly water-soluble WST11 and the more hydrophobic BChl derivatives. However, this current evidence may be less reliable because neither the carotenoid oxidation products nor, in particular, the BChl-derived oxidation products have as yet been fully characterized: the chlorins and porphyrins derived from the latter only account for a fraction of the photo-oxidation products (11,12). Without a full characterization of all the other products, and their correlation with specific photosensitizing mechanisms, Chol and its esters remain the internal probes of choice. Last but not least, it is important to remember that the discrimination between Type I and Type II photoreactions may become blurred by subsequent radical reactions of the peroxides formed by both photoreaction types (90).
Acknowledgments
Acknowledgements— This work was supported by the Hans-Fischer-Gesellschaft e. V. München (Munich, Germany), by a Ph.D. grant to J.D. We are indebted to A. Scherz (Weizmann Institute of Science, Rehovot, Israel) for providing WST11, and to R.J. Porra (CSIRO, Canberra, Australia) for help in preparing this manuscript. WST09 was provided by Steba N.V. through collaboration with Negma-Lerads.




