Flagellar Motions in Phototactic Steering in a Brown Algal Swarmer
Abstract
Using infrared high-speed video microscopy, we observed light-triggered transitory flagellar motions in flagellate reproductive cells (swarmers) of a brown alga, Scytosiphon lomentaria, under primary helical swimming conditions before and during negative phototactic orientation to unilateral actinic light. The posterior flagellum, which is autofluorescent and thought to be light-sensing, was passively dragged in the dark and exhibited one to several rapid lateral beats during orientation changes for phototactic steering. Notably, a brief cessation of anterior flagellar beating was occasionally observed concomitantly with rapid beats of the posterior flagellum. This behavior caused a pause in helical body rotation, which may contribute to the accuracy of phototactic steering. Thus, coordinated regulation of the movement of the two flagella plays a crucial role in phototactic steering.
Introduction
Flagella are a universally distributed extracellular organelle among eukaryotes and bring about cell movement through beating. Heterokont flagellates such as brown and chrysophycean algae have two heterogeneous flagella that are easily distinguishable in morphology as well as in motility (1). The anterior flagellum bears many tubular mastigonemes on its surface (2,3) and provides progressive force through undulatory beating (4). The smooth posterior flagellum is usually inactive but occasionally bends sideways. This flagellum might serve a rudder-like function (5).
In brown algal swarmers (flagellate reproductive cells), physicochemical stimuli such as light and sex pheromones (6) cause changes in their swimming behavior by modulating flagellar motion; the responses to these stimuli are known as phototaxis and chemotaxis, respectively. Various modes of modulation have been observed in the pheromone-induced flagellar responses (reviewed by Maier [7]). In Ectocarpus, the posterior flagellum, which is usually motionless, shows rapid lateral beats upon stimulus, which causes an abrupt turn by the swarmer (5). In Laminaria (8) and Hormosira (9), rapid lateral beats of the posterior flagellum were also observed upon a sudden decrease in pheromone concentration. The anterior flagellum is also involved in pheromone-induced directional swimming. In Ectocarpus, asymmetry of the anterior flagellum is correlated with the curvature of the swimming track (5). In Laminaria, however, the anterior flagellum keeps up a regular undulation during the change in chemotactic direction caused by the posterior flagellum (8).
On the other hand, roles of the flagella in light perception and phototactic orientation of brown algal swarmers are understood on the basis of the eyespot apparatus model (reviewed in Refs. [10,11,12,13]). Localization of green fluorescent substances on the posterior flagellum or the flagellar swelling was observed by epifluorescence microscopy (14,15). Reflected light from the concave side of the eyespot was found to be focused on the flagellar swelling of the posterior flagellum (16,17). Two independent action spectra for phototaxis of the swarmers suggested the involvement of flavin-like chromophore(s) in blue light perception; one spectrum was obtained from gametes of a primitive brown algal member (Ectocarpus) (16), the other was from zoospores of a highly developed member (Laminariales) (18). Microspectrofluorometry of the green autofluorescent substances in the posterior flagellum provides additional support for the involvement of flavin chromophores in light perception (19). These findings support the idea that the posterior flagellum is involved in light perception during phototaxis in brown algal swarmers.
It seems natural to expect that the posterior flagellum plays a crucial role in phototactic steering as it does in the pheromone-induced flagellar response (5,8). However, pheromone-induced behaviors are considerably different from phototactic ones. Chemotactic behaviors are related to thigmotaxis, which keeps the swarmer on the surface of substratum by eliminating the helical rotation. For example, in Ectocarpus (5) and Laminaria (8) but not Hormosira (9), thigmotaxis was enhanced by addition of pheromone. In contrast, primary helical motion during swimming was thought to be needed for phototaxis (12). In previous studies on chemotactic behaviors, observations were made by visible light illumination via a microscope. However, in photobehavioral studies, such an intensity of observation light might artificially influence photosensitivity of the swarmer. From this point of view, it can be said that no reliable observations of flagellar motion that causes phototactic orientation have yet been made.
The purpose of this report is to precisely describe the motions of flagella during phototactic orientation. We employed high-resolution and high-speed video microscopy under infrared light, which is inactive in light perception by the algae. In addition, we used a deep sample chamber in order not to prevent helical movement of the swarmers during swimming.
Materials and methods
Organism. Scytosiphon lomentaria (Phaeophyceae) was collected along the northern coast of Awaji Island of the Inland Sea (Hyogo, Japan) during the spring tide between late March and early June. Mature thalli were washed with seawater to remove epiphytic microorganisms, then put between absorbent paper to keep them in a semi-dried condition at 4°C. Swarmers were discharged by bathing the thalli in ice-cooled artificial seawater for several minutes under sunlight. Details for collection and handling of the samples have been described elsewhere (20). The freshly harvested swarmers were put into a hand-made sample chamber (16 mm × 16 mm × 0.15 mm) for microscopy.
Tracking of swimming paths. The locomotion of the swarmers was recorded for 6 s by infrared dark-field video microscopy under a 4× objective at a video rate of 10 frames per second (fps). For the first 2 s, the swarmers were kept in darkness, and for the following 4 s were exposed to a unilateral stimulus of light (437.1 nm, 17 μmol m−2 s−1).
The unilateral stimulus light was provided from a xenon short-arc lamp (driving power 500 W; Ushio Electric, Inc., Tokyo, Japan) equipped with a heat-cut filter (CF-B; Vacuum Optics Corp. of Japan, Tokyo, Japan), a 437.1 nm interference band-pass filter (full width at half maximum of 8.9 nm; Vacuum Optics Corp. of Japan) and neutral density filters. Photon fluence rate of the unilateral stimulus light was measured by hand-made quantum sensor with a calibrated silicon photodiode (S1337-66BQ; Hamamatsu Photonics Co., Ltd., Hamamatsu, Japan). The timing of the stimulus was controlled by an electromagnetic shutter (Chuo Precision Ind., Co., Ltd., Tokyo, Japan), which was synchronously operated by an external trigger connected to the computer-aided video recording system.
In each video frame, X–Y coordinates corresponding to center of gravity of each cell were obtained by computer-aided image analysis using NIH Image software (http://rsb.info.nih.gov/nih-image/, developed at the U.S. National Institutes of Health) running on a Macintosh computer (Power Macintosh 7600; Apple Computer, Inc., Cupertino, CA); details have been described elsewhere (21).
Infrared video microscopy for observation of flagellar motion. In order to observe flagellar motion in the absence of a light stimulus, we employed an IR-optimized microscope (BX51/IR; Olympus Optics Corp., Tokyo, Japan) equipped with an infrared light source (U-LH100-IR; Olympus Optics) and a high-resolution objective, for which chromatic aberration was corrected at infrared wavelengths (UPlanSApo, 100× N.A. of 1.40; Olympus Optics). Observation light was filtered by an infrared band-pass filter (BP 775, Olympus Optics, maximum wavelength of 775 nm, full width at half maximum of 20 nm). The microscope was equipped with a fast, computer-aided digital video recording system (Fastcam-PCI-R2; Photoron Ltd., Tokyo, Japan). The camera had high spectral sensitivity in the infrared region and was enabled to capture video frames (512 × 240 pixels) as fast as 500 fps.
Stimulus light was given through the 100× objective using an epi-illumination system with a high-pressure mercury lamp as the light source. The stimulus light was passed through a heat-cut filter (CF-B; Japan Vacuum Optics Corp.), a short-cut filter (L-42; Hoya Glass Corp., Tokyo, Japan), neutral density filters and a micro diaphragm of 100 μm in diameter, which restricted the stimulus light projection area within the microscopic field. The photon fluence rate was 32 μmol m−2 s−1 and the beam size was ∼60 μm in diameter. The photon fluence rate of the micro projection was calculated as that measured total photon number per second of the whole beam was divided by the projection area.
Results
General swimming behaviors
Swarmers of S. lomentaria in a sample chamber were observed by video microscopy under infrared light for determining the fundamental features of their swimming behaviors.
Tracking analysis Traveling of swarmers was observed in darkness followed by unilateral actinic light. In one microscopic field, 34 swarmers were traced, of which 22 showed negative phototaxis while 10 exhibited spiral revolution with continuously curved paths and the remaining two transformed from spiral to negative phototaxis (Fig. 1a).
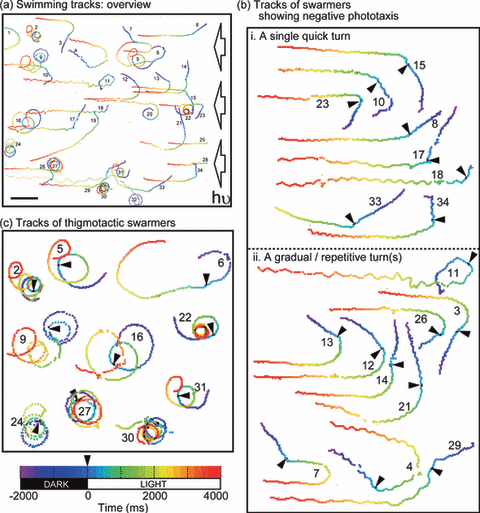
Swimming tracks. Each line shows the swimming track of an individual swarmer. Time sequence (total 6000 ms) of the swimming tracks is indicated by the color-coded scale (lower left); the initial 2000 ms in the dark and the following 4000 ms were under unilateral irradiation. Arrowheads indicate onset of the light stimulus. (a) Swimming tracks: overview: Thirty-four swarmers observed in a microscopic field of view were tracked. The right-hand side of the view (indicated by arrows) was unilaterally illuminated by actinic light. Individuals were numbered sequentially. Scale bar: 100 μm. (b) Tracks of swarmers showing negative phototaxis: Swarmers showing directional change upon light stimulus were selected from (a) and categorized into two patterns (i and ii) depending on response. The number of each individual is identical to that in (a). (i) A single quick turn (<200 ms): swarmers oriented in negative phototaxis by a single turn within 200 ms after onset of the light stimulus. (ii) Gradual or repetitive turns: Swarmers oriented in negative phototaxis by gradual or repetitive turns after onset of the light stimulus. (c) Tracks of thigmotactic swarmers: Swarmers showing spiral revolution were selected from (a). The number of each individual is identical to that in (a).
A set of 18 typical tracks showing negative phototaxis is shown in Fig. 1b. Such movement is thought to be due to free-swimming individuals with helical rotation. Swarmers in this state strictly responded to the unilateral actinic light, which in most individuals resulted in negative phototaxis (21 individuals were phototactic among the 22 free-swimming individuals). The 18 examples presented in Fig. 1b were sorted into two categories depending on the manner of turning, either via (i) one quick turn or (ii) gradual or repetitive turns. In every case, the initial directional change occurred within 200 ms after onset of the light stimulus.
Another 10 individuals that swam in circular paths are shown in Fig. 1c. These individuals were in thigmotactic conditions (5,8), under which once a swarmer comes near the surface of the cover slip, it tends to maintain its position. Among 12 individuals that were initially thigmotactic, 10 maintained a spiral revolution even at the dark/light boundary. Only two individuals reverted to phototaxis from the thigmotactic state (20 and 32 in Fig. 1a). Therefore, we determined to focus only on the swarmers that showed forward swimming with helical rotation in the following experiments.
Distinct cellular movements, helical rotation and thigmotaxis During 210 ms of swimming, the appearance of the anterior flagellum repeatedly altered between a wavy shape (0–30 and 150–210 ms) and a straight one (60–120 ms), although bending waves must occur continuously (Fig. 2a). This observation indicates a helical rotational behavior of a free-swimming swarmer. On the other hand, Fig. 2b shows thigmotactic behavior of a swarmer during 210 ms of swimming. The wavy form of the anterior flagellum was evident in every frame. This means that the beating plane of the anterior flagellum always stayed parallel to the cover slip without any helical rotations. Note that the swarmer gradually changed its swimming direction because it traveled along an arc-shaped path (part of a spiral revolution).
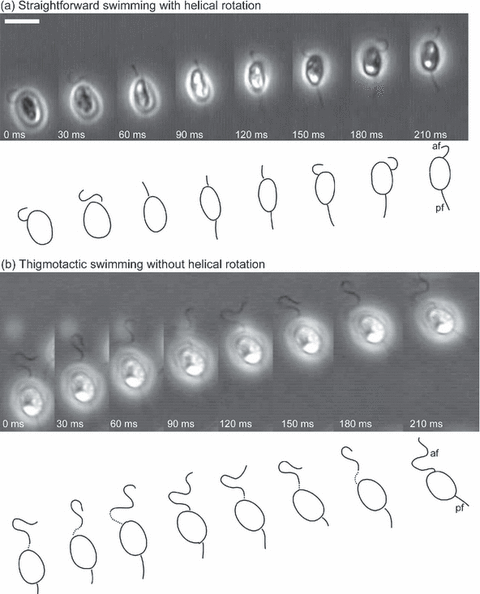
Two types of swimming behaviors. Two series of video micrographs obtained at an interval of 30 ms are presented. Each series shows a distinct type of swimming behavior. A line drawing of the cell is shown below each micrograph. af = anterior flagella; pf = posterior flagella. Scale bar = 5 μm. (a) Straightforward swimming with helical rotation (free swimming). (b) Thigmotactic swimming without helical rotation.
Observation of flagellar motions leading to turning behavior
The swarmer shown in Fig. 1b performed a quick directional change showing phototaxis within 200 ms after onset of the light stimulus, then soon reverted to the usual forward locomotion. This suggests that phototactic steering is transient and should be observed within 200 ms after the onset of actinic light. To capture such quick motion at higher resolution, we prepared a microscopic setup, in which stimulus light was projected on the central part of the microscopic field using epi-illumination (see Materials and Methods). Swarmers traveling in the peripheral dark region randomly encountered the actinic light at the boundary of the spot. The flagellar motions of light-stimulated swarmers were observed under infrared illumination coupled to high-speed video microscopy.
Light-triggered rapid beats of the posterior flagellum The posterior flagellum of most heterokont flagellates does not work actively during locomotion (see Fig. 2 as an example). However, the posterior flagellum was found to play a role in phototactic steering via one to several rapid sideward bends as described below.
The travel of a swarmer after onset of actinic light was recorded in 80 video frames (duration of 160 ms). Figure 3a shows the directional profile during travel. An obvious change in direction appeared between 100 and 160 ms after onset of the light stimulus, while in other periods it moved almost directly forward. A series of video images acquired during the obvious directional change (Fig. 3bI, corresponding to images at 122–130 ms in Fig. 3a) revealed that the posterior flagellum made a rapid sideward bend followed by a stretch. In contrast, the posterior flagellum was passively dragged while swimming straight forward (Fig. 3bII, corresponding to images at 220–228 ms in Fig. 3a). This result represents the observation that the posterior flagellum, which was usually motionless, was temporarily activated by a light stimulus and made rapid beats concomitant with a directional change. The number of beats induced by a single light stimulus varied from one to several in other observations. The presented data of the rapid beat of the posterior flagellum are typical of the population. Similar examples are provided as movies in the online supplemental materials (Movies S1–3). Additionally, Movie S4, an example of straightforward swimming is also supplied as a comparison. More detailed information on the rapid beats of the posterior flagellum is presented in the next paragraph.
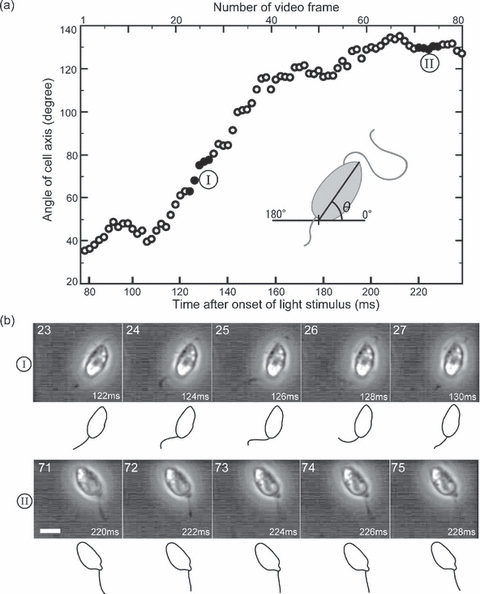
Swimming direction and motion of posterior flagellum. (a) Diagram of cellular orientation during phototactic steering: The angle of the cell axis (θ in inset) was measured for a swarmer captured in 80 consecutive video frames obtained between 78 and 238 ms after onset of the light stimulus. The time interval between each frame is 2 ms. The measured angles are plotted against time after onset of the light stimulus. Solid circles (indicated by I, II) correspond to micrographs shown in (b). (b) Motion of posterior flagellum: Two series of video micrographs, both of which are among the 80 video frames used in (a), are shown. The frame number and time indicated in each micrograph are identical to those indicated in (a). A line drawing of the cell is shown below each micrograph. The time interval of each series is 2 ms. Scale bar = 5 μm. (I) Series corresponding to the 23rd to 27th frames. A swarmer is showing a directional change. (II) Series corresponding to the 71st to 75th frames. A swarmer is showing straightforward locomotion.
Figure 4 shows three consecutive light-elicited beats of the posterior flagellum that brought about a directional change. Each beat was accomplished within 10 ms (i.e. 5 frames); thus, the frequency of these beats was ∼100 Hz. In each beat, it took 6–8 ms for bending and 2–4 ms for a recovery by stretching. The directional change progressed during the bending process. Thus, our observations suggest that a steering force is generated through the bending process, i.e. an effective stroke (Fig. 4).
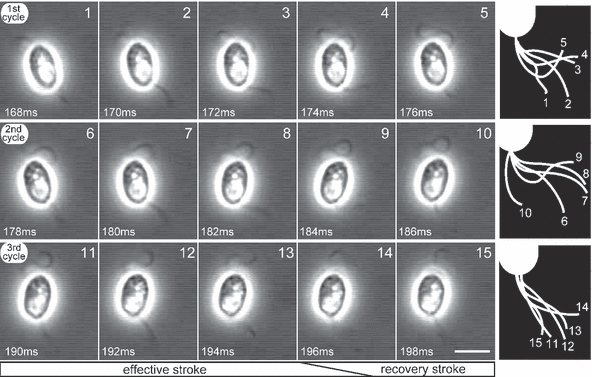
Rapid beats of posterior flagellum. Fifteen consecutive video micrographs at a 2 ms interval show three cycles of beats of the posterior flagellum. These micrographs were obtained between 168 and 198 ms after onset of the light stimulus. Frame number and time after onset of light stimulus are indicated in each micrograph. Each row of micrographs shows a single beat cycle of the posterior flagellum. Illustrations of the motion of the posterior flagellum in each cycle are shown on the right-hand side. Scale bar = 5 μm.
A brief cessation of the anterior flagellum during phototactic steering In the heterokont algae, force for forward locomotion is generated by the tripartite tubular mastigonemes attached to the anterior flagellum (4). Therefore, the anterior flagellum keeps working during locomotion. However, in our observations, a brief cessation of the undulation of the anterior flagellum was found during a directional change (Fig. 5).
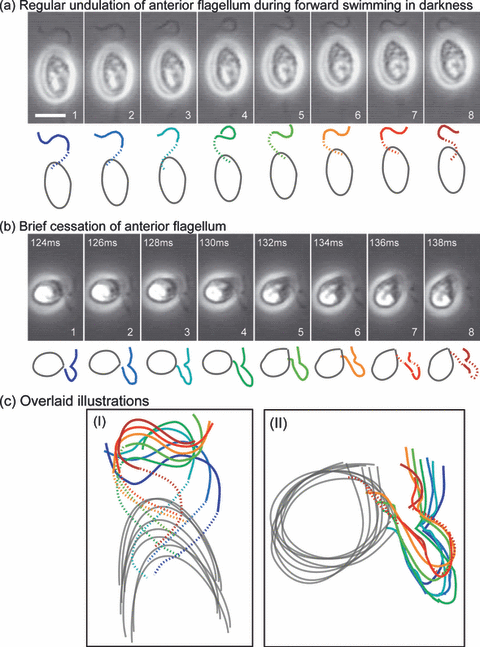
A brief cessation of the anterior flagellum. The motion of the anterior flagellum is shown in eight consecutive video micrographs obtained at a 2 ms interval. Line drawings of the cell are shown below each micrograph. The hidden part of the anterior flagellum was extrapolated (dotted line). Scale bar = 5 μm. (a) Regular undulation of the anterior flagellum during forward swimming in darkness. (b) Brief cessation of the anterior flagellum: The motion of the anterior flagellum is shown during a directional change that appeared 124–138 ms after onset of the light stimulus. (c) Magnified overlaid illustrations. The colors used in the illustrations are identical to the ones in (a) and (b).
Regular undulation of the anterior flagellum was observed during forward locomotion in the dark (Fig. 5a), where the swarmer progressed ∼2.6 μm during the 16 ms. A bending wave was transferred from the base to the tip of the flagellum; the phase of the wave was just inverted between the first and the sixth frames, as well as between the second and seventh frames. It took 12 ms for the phase inversion; thus, the undulation frequency was 42 Hz. In other observations, the frequency ranged between 40 and 60 Hz (data not shown).
A brief cessation of undulation of the anterior flagellum was detected concomitantly with a light-induced directional change (Fig. 5b). The swarmer executed flagellar undulation before and after the directional change (data not shown). In this period, the swarmer made a noticeable turn by ca 80° without forward progress. Unfortunately, the posterior flagellum was out of focus, but this directional change was thought to be caused by the rapid beats of the posterior flagellum. It was difficult to observe both flagella simultaneously because the swarmer tended to move vertically during the directional change. The brief cessation of beating was unequivocally confirmed by overlay of the illustrations (Fig. 5c). In other observations, such a brief cessation during a directional change appeared frequently but not in every case. When the brief cessation occurred, the change in direction tended to be greater (96 ± 18°, n = 13). Similar examples are provided as movies in the online supplemental materials (Movies S5–8).
The cessation of beating of the anterior flagellum brought about a brief stop of the helical rotation. This might be useful for correct phototactic steering because the eyespot should be kept in the same plane during steering.
Discussion
Unilateral light stimulus did not induce phototaxis in thigmotactic swarmers despite resulting in negative phototaxis of the free-swimming swarmers (Fig. 1). In contrast, ectocarpen and lamoxirene, the sex pheromones of Laminaria, were able to induce chemotactic behavior in thigmotactic swarmers (8). Moreover, thigmotactic behavior was enhanced by the addition of pheromones (8). Therefore, a positive relationship is postulated between thigmotaxis and pheromone-induced behavior, while phototactic behavior seems to be in conflict with thigmotaxis.
The phototactic steering was caused by the rapid beats of the posterior flagellum (3, 4). This behavior was similar to pheromone-induced behaviors but the details seemed different, i.e. the forward stroke took longer (6–8 ms) than the recovery stroke (2–4 ms) in photo-orientation of Scytosiphon while the forward stroke took less time than the recovery in observations of chemotactic directional change in Laminaria (8). But this comparison may not be adequate, because the beat in Scytosiphon was considerably faster than the one in Laminaria, which has a posterior flagellum that is quite long.
More significant differences between pheromone-induced behaviors and phototactic behavior are found in motion of the anterior flagellum. In Ectocarpus, asymmetrical undulation of the anterior flagellum was induced by pheromone (5), while in Laminaria, the anterior flagellum kept its usual undulation during chemotactic orientation (8). During photo-orientation of Scytosiphon, the anterior flagellum occasionally ceased its undulation (Fig. 5). Such brief cessation of beating of the anterior flagellum was frequent when the photo-orientation lasted over a longer period (∼200 ms). What significance does the brief cessation of the anterior flagellum have?
A brief stop of the helical rotation is postulated to be accompanied by the brief cessation of the anterior flagellum. At the beginning of phototactic steering by the rapid beats of the posterior flagellum, the eyespot probably faces the incident light. If the helical rotation ceased during phototactic steering, the cell could steer in the primary plane where incident light is passing through. This seems to confer a high degree of accuracy in phototactic steering. However, the physiological mechanism for synchronized control of the anterior and the posterior flagella is unknown.
As a physiological effect of ectocarpen, extracellular Ca2+ influx was suggested in Ectocarpus via two pharmacologically distinct channels (22). Signaling effects of calcium on flagellar activities are well known in other organisms, e.g. conversion of flagellar beating modes in Chlamydomonas (23,24) and hyperactivation of flagellar motion in mammalian sperm (25). Recently, a transient increase in Ca2+ in the flagellum was directly visualized during chemotactic orientation in ascidian sperm using a fast Ca2+ imaging system (26). Application of such a technique might provide a clue to understand the mechanism for simultaneous control of the two heterogeneous flagella in the brown algal swarmer during photo-orientation.
Acknowledgments
Acknowledgements — This work was supported in part by the Research Program of Hayama Center for Advanced Studies of Sokendai (S.M., M.I.). This work was also supported in part by grants from the Japan Society for the Promotion of Science (JSPS) to M.W. (18077003), M.I. (19614004) and A.M. (17GS0314 and 17657018).




