Oxygen Uptake in the Vitamin B2-sensitized Photo-oxidation of Tyrosine and Tryptophan in the Presence of Uracil: Kinetics and Mechanism
Abstract
Considering the significance of visible light-promoted reactions in complex biological media, the photo-oxidation of the amino acids (AAs) tyrosine (tyr) and tryptophan (trp) was studied in the presence of the naturally occurring oxidative scavenger uracil (ur). The involved photoprocesses, studied at pH 7 and 9, are driven through the reactive oxygen species (ROS) singlet molecular oxygen (O2(1Δg)), superoxide radical anion (O2?−) and hydrogen peroxide (H2O2). The effect on the effectiveness of the overall photo-oxidation process due to the presence of an added electron-donating substrate such as ur is not straightforwardly predictable. The addition of the pyrimidine compound, a much lesser photo-oxidizable substrate than the AAs themselves, produced different results: (1) antioxidative for tyr at pH 9, decreasing the overall rate of oxygen uptake; (2) synergistic for tyr at pH 7, increasing the oxidation rate more than the corresponding addition value of the respective individual rates and (3) no effect for trp at both pH values. The final result depends on the respective abilities of the substrates as quenchers of both the long-lived riboflavin triplet excited state and the generated ROS and the pH of the medium. An interpretation for the different cases is attempted through a kinetic and mechanistic analysis.
Introduction
Reactions between biologically significant compounds are being increasingly investigated in relation to relevant chemical/biochemical events in several fields, such as biology, pharmacology and medicine (1–3). This is the case of thermal and photochemical interactions between amino acids (AA) and nucleic bases, which strongly attracted scientific attention (4–6). The addition of AAs to uracil (ur), upon photoirradiation with ultraviolet light is known and has been investigated for several decades (4). The relevance of these reactions was related to the mechanism by which DNA and proteins are cross-linked in vivo, stimulated by ultraviolet light. Similarly, the formation of cross-links between ur-substituted nucleic acids and tryptophan-containing (trp) proteins was detected and studied by the characteristic fluorescence of the ur-trp fluorophore (5). In addition, energetic aspects of charge- and proton-transfer reactions between nucleobases and AAs, that can produce DNA and RNA damage, have been recently studied by means of computational calculations for several nucleoside models including ur (6).
Turning to potential light-induced processes involving ur derivatives and AAs, and in the frame of naturally driven photoreactions, a first observation arises: both families of compounds are transparent to daylight. Nevertheless it is well known that ur and AAs can be affected by visible light irradiation if some compound, namely a photosensitizer, able to absorb visible light and to generate reactive species, is present in the same environment (7). A daylight-absorbing pigment of particular interest is the naturally occurring vitamin B2, riboflavin (Rf). The vitamin is endogenously present in living organisms, and has been postulated as a possible sensitizer for the in vivo photo-oxidative degradation of proteins, puric bases and fatty acids, among other biologically relevant substrates (8–13).
Riboflavin was found to generate reactive oxygen species (ROS) upon photoexposure. Studies with puric bases showed that Rf-photosensitized processes could be responsible for photodamaging of DNA (14). Recently we reported kinetic studies on the Rf-sensitized photo-oxidation of ur, uric acid, xanthine and hypoxanthine in aqueous solution, mediated by ROS (9,15). The aerobic light-induced interaction of Rf with proteins is well known to occur through ROS-mediated oxidation of one or several of the five photo-oxidizable AAs: tyr, trp, methionine, histidine and cysteine (16–19). A typical example is the photo-oxidation of milk proteins, sensitized by the vitamin (20–22). Also, it is well established that photo-promoted reactions can occur in the human body, especially in regions directly exposed or transparent to environmental light, in the presence of Rf (23,24).
Recently, the photolysis of several flavins was studied in air-saturated aqueous solution in the presence of appropriate electron donors, including aromatic AAs (25). The overall reaction observed was conversion of oxygen via the hydroperoxyl/superoxide radical.
As Rf, ur-containing biomolecules and proteins can occupy common locations in complex biological systems, elucidating the kinetics of tyr and trp photo-oxidation, in the presence of the vitamin and ur, will allow: (1) a greater understanding of the chemical and physical behavior of ROS involved; (2) the interpretation of the actual photochemical reactions of ur and AAs; and (3) a greater insight into the influence of different substrates on tyr and trp oxidation.
This was the goal of the present study, driven through the evaluation of the rates of oxygen uptake by the AAs and uracil, in the simultaneous presence of visible light and vitamin B2 as dye-sensitizer. The relative values of oxygen uptake rates were taken as a measure of overall photo-oxidability by the substrates. The AAs trp and tyr were chosen as typical protein oxidizable targets, under visible light photoirradiation in the presence of ROS generators.
Materials and methods
Materials. Uracil, l-tryptophan, l-tyrosine, Rf, superoxide dismutase (SOD) from bovine erythrocytes and catalase from bovine liver, were purchased from Sigma Chem. Co. (St. Louis, MO). Rose bengal (RB) and sodium azide (NaN3) were from Aldrich (Milwaukee, WI). All these chemicals were used as received. Water was triply distilled. All the measurements were carried out at room temperature and with freshly prepared solutions. Buffered aqueous solutions were prepared, with 0.025 m KH2PO4/0.025 m Na2HPO4 (pH 7), and 0.01 m Na2B4O7.10H2O (pH 9) (26).
Methods. Absorption spectra were registered with a Hewlett Packard 8452A or an Agilent 8453 diode-array spectrophotometer. Continuous photolysis was performed in a home-made photolyzer with a 300 W quartz-halogen lamp and a cutoff filter at 360 nm, using RB (A(530) = 0.5) or Rf (0.04–0.05 mm) as sensitizer. The relative rate of Rf- and RB-sensitized photo-oxidation of each system (AAs, ur and their mixtures) was determined by evaluation of the initial slope of oxygen consumption as a function of the irradiation time, with an Orion 97-08 or an Orion 810A+ specific oxygen electrode. Normalized oxygen uptake rates for each sensitizer and for each pH value were the quotient between the rate value for a given AA or mixture AA + ur and the respective rate value for ur.
Results
The visible light irradiation of air-equilibrated pH 7 and pH 9 aqueous solutions of individual 0.15 mm trp, 0.22 mm tyr and 0.16 mm ur, all in the presence of 0.04 mm Rf as a dye sensitizer, modified the absorption spectra of the substrates. See Fig. 1 as an example for the case of trp at pH 7. It shows typical absorbance decrease in the absorption band centered at 270 nm, already described in the literature (19), due to the dye-sensitized photo-oxidation of the amino acid. The same was observed for the case of tyr with spectral changes at 275 nm at pH 7 and at 240 and 290 nm at pH 9.
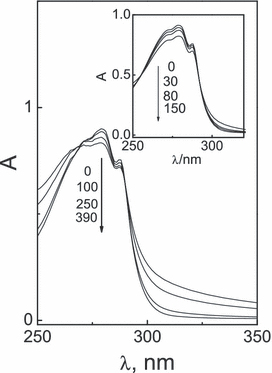
Changes in the UV absorption spectra of a pH 7 aqueous solution of Rf (A(446) = 0.40) plus 0.17 mm trp, taken vs Rf (A(446) = 0.40). Inset: changes in the UV–Vis absorption spectra of a pH 7 aqueous solution of RB (A(549) = 0.55) plus 0.17 trp, taken vs RB (A(549) = 0.55). Irradiation (λirr > 360 nm) under air-saturated conditions. Numbers on the spectra represent photoirradiation time in seconds.
In separate experiments, oxygen consumption was observed upon Rf (0.05 mm)-sensitized photoirradiation of similar solutions containing individually trp, tyr and ur, all at a concentration of 0.5 mm, and mixtures trp–ur and tyr–ur, both 0.5 mm, again at pH 7 and 9. The relative rates of oxygen uptake are presented in Table 1. Typical runs are shown in Fig. 2. Both spectral modifications and oxygen uptake are due to a Rf-sensitized photo-oxidation mediated by ROS (7,9,15). The respective rates of oxygen uptake can be considered as a relative measure of the overall photo-oxidability of the substrates. Prolonged photolysis of Rf alone produced a slight oxygen consumption that can be considered negligible in relative terms within the typical irradiation times employed in the presence of ur and/or the AAs (Fig. 2).
| Substrates | Rel. rate (Δ[O2]/Δt) in RB—pH 9 | Rel. rate (Δ[O2]/Δt) in Rf—pH 7 | Rel. rate (Δ[O2]/Δt) in Rf—pH 9 | k q3 × 109 (m−1 s−1) in H2O | k r × 107 (m−1 s−1) | k t × 107 (m−1 s−1) | k r/kt |
|---|---|---|---|---|---|---|---|
| ur | 1.00 ± 0.03 | 1.00 ± 0.03 | 1.00 ± 0.03 | 0.025* | 0.003* (pH 7)0.03* (pH 9) | 0.05* (pH 7)0.2* (pH 9) | 0.06 (pH 7)0.15 (pH 9) |
| trp | 34 ± 1 | 36.1 ± 1.1 | 2.0 ± 0.06 | 1.80† | 4.70‡ (pH 7) | 7.2‡ (pH 7) | 0.65 (pH 7) |
| tyr | 14.1 ± 0.4 | 13.0 ± 0.4 | 1.7 ± 0.05 | 1.30† | <10−4 (pH 7)§3.8 (pH 10)‡ | 1.5 (pH 7)||∼20¶ | ∼0∼0.2 |
| gly | NC** | ∼0.05 | ∼0.05 | <10−4 | <10−4 | ||
| trp + ur | 34.7 ± 1 | 36.6 ± 1.1 | 2.3 ± 0.07 | ||||
| tyr + ur | 14.2 ± 0.4 | 16.1 ± 0.5 | 1.5 ± 0.05 | ||||
| gly + ur | 1.02 ± 0.03 | 1.00 ± 0.03 | 1.00 ± 0.03 |
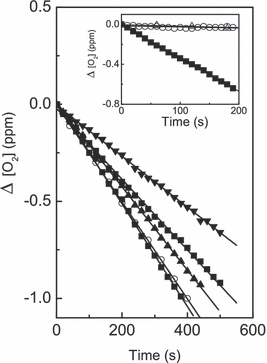
Oxygen uptake by a pH 9 aqueous solution of Rf (A(446) = 0.40) in the presence of () 0.5 mm ur; () 0.5 mm tyr + 0.5 mm ur; () 0.5 mm tyr; (○) 0.5 mm trp and () 0.5 mm trp + 0.5 mm ur. Inset: Oxygen uptake by a pH 7 aqueous solution of RB (A(549) = 0.55) in the presence of (Δ) 0.5 mm ur; (O) 0.5 mm tyr; and () 0.5 mm trp. Irradiation (λirr >360 nm).
Rate values of oxygen uptake for each system in Table 1 represent the mean value of a set of six runs under identical conditions. All rate values of the set did not differ by more than 3% of the mean value. Standard deviation values for the individual runs gave values lower than 1%. Nevertheless, we included ±3% as the error bar for the rates of Table 1, a more realistic estimation that assists in the interpretation of the actual magnitude of the observed effects.
It is well known that under aerobic photoirradiation, and in the presence of adequate electron donors, such as aromatic AAs (25) or ur (9), Rf generates the ROS O2(1Δg), O2?− and H2O2. As RB exclusively generates O2(1Δg) under visible light photoirradiation (7,27), photolysis experiments similar to those performed with Rf were made for comparative purposes, replacing the vitamin by the xanthenic dye sensitizer (A(549) = 0.52 for RB), and keeping constant the remaining experimental conditions. For tyr, trp and ur both typical photo-oxidative-spectral modifications and oxygen consumption (Table 1) were observed at pH 9 whereas this behavior was only detected for trp at pH 7 (Fig. 2, inset). In other words, O2(1Δg) was practically unreactive toward tyr and ur at pH 7, confirming that the nonionized form of these compounds is only a physical quencher of the oxidative species (16). The pKa values of 9.5 (26,28,29) and 10.1 (26) for the ionization of the OH group in ur and tyr respectively indicate that a considerable proportion of their ionized species is present at pH 9. Regarding trp, it is known that the kinetics of O2(1Δg)-photo-oxidation of this AA is not significantly affected by pH changes (16).
The photoirradiation of RB alone did not produce oxygen consumption. Besides, the absorption spectrum of the sensitizer remained within the irradiation time employed in typical runs.
Spectral changes for trp at pH 7 are shown in Fig. 1, inset. It can be seen that these changes are practically the same as those observed in the Rf-sensitized process at the same pH value (Fig. 1, main). This fact indicates the presence of structurally similar photoproducts in both cases, being formylkynurenine (30,31) and pyrroloindole-like compounds (32) the reported oxidation products.
The available literature data on kt and kr rate constants and the corresponding kr/kt values are included in Table 1. The rate constants kr and kq account for the respective reactive and physical quenching processes of O2(1Δg) by the substrates, being kt = kr + kq. The quotient kr/kt can be envisaged as the fraction of the overall interaction O2(1Δg) substrate that leads to effective chemical transformation (18).
The presumable unreactivity of the nonoxidizable AA gly (16) was confirmed by means of an oxygen uptake experiment, under identical conditions employed for tyr and trp, in the individual presence of RB or Rf as dye sensitizers. No oxygen consumption was observed at any pH value, as stated in Table 1. This result can be considered as a blank for the common AA moiety of the aromatic AAs.
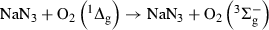 ((I))
((I))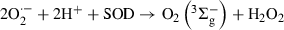 ((II))
((II))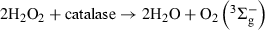 ((III))
((III))
Oxygen uptake by a pH 9 aqueous solution of Rf (A(446) = 0.52) in the presence of (•) 0.5 mm ur plus 2 mm NaN3; (○) 0.5 mm ur plus 1 mg 100 mL−1 SOD; () 0.5 mm ur plus 1 mg 100 mL−1 catalase and () 0.5 mm ur. Irradiation (λirr >360 nm).
Results in Table 2, expressed as the ratio between the respective rates of oxygen uptake in the presence (R) and in the absence (RO) of the additives, individualize the ROS that are involved in the overall oxygen uptake process observed in the Rf-sensitized photoirradiation of ur, tyr and trp. A value R/R0 = 1 indicates that a given additive does not affect the oxygen uptake rate. A delay in the rate of oxygen uptake in the presence of the additive (R/R0 < 1) indicates that a given ROS is involved in the photo-oxidation of the substrate.
| Compound | ROS quencher | R/R0 | |
|---|---|---|---|
| pH 7 | pH 9 | ||
| ur | NaN3 | 1 | 0.12 |
| SOD | 1 | 0.17 | |
| Catalase | 1.4 | 0.33 | |
| tyr | NaN3 | 1 | 0.11 |
| SOD | 1 | 1 | |
| Catalase | 0.42 | 1 | |
| trp | NaN3 | 0.18 | 0.14 |
| SOD | 1 | 0.19 | |
| Catalase | 0.46 | 1 | |
Discussion
The accepted general mechanism for a RB- or Rf-sensitized photo-oxidation of a given substrate can be depicted by Scheme 1 (9,15):
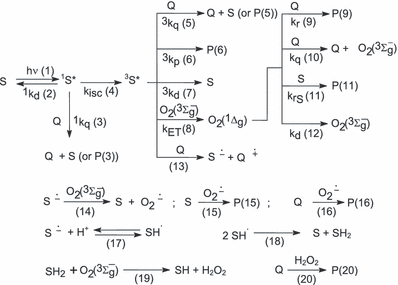
Possible pathways in a dye-sensitized photo-oxidation. S = sensitizer; Q = quencher; 1S* and 3S* = electronically excited singlet and triplet states of Rf; O2 (3Σg−) = ground state triplet oxygen; O2(1Δg) = singlet molecular oxygen; O2?− = superoxide radical anion; H2O2 = hydrogen peroxide. P(n): eventual photoproduct for step (n).
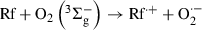 ((IV))
((IV))Hence, the generation of the species Rf˙+ and O2?− is negligible in kinetic terms. Rf is also a moderate quencher of O2(1Δg), with an overall rate constant, ktRf = kqRf + krRf = 6 × 107 m−1 s−1 (13). Nevertheless, in the presence of an adequate substrate Q, 3Rf* can give rise to O2?− (process 14), via Rf?− (process 13). At pH 7, in the presence of proton-donating species, the generation of Rf neutral radical (RfH?, step 17) should occur, with a pKa value of 8.4 (25,40,41).
It is known that the reaction of ground state oxygen with RfH? is much slower than the corresponding one with Rf?− (process 14). Besides, the bimolecular decay of RfH? through a disproportionation reaction can yield Rf and fully reduced Rf (RfH2) (process 18). The reduced flavin can be reoxidized in the presence of O2(3Σ−g) producing Rf and H2O2 (step 19) (40). As an overall result, reactions (14) and (18) constitute pathways for O2?− and H2O2 production and Rf regeneration, as the oxygenated species are good candidates for reacting with compounds Q.
Given the pKa value for process (17), the study carried out also at pH 9 illustrates the hypothetical case of a prevailing concentration of the species O2?− that could be achieved if the reaction would occur in a less-proton-donating environment than pH 7 water.
According to the respective lifetimes of Rf electronically excited states (42), and to the values for the rate constants kq3 shown in Table 1, 3Rf* can be intercepted by substrates in the sub-mm concentration range. This is the case of ur, trp and tyr, all three at a concentration of 0.5 mm under work conditions and O2(3Σ−g) with a concentration of ca 0.4 mm in aerated aqueous solutions (43). In principle, the dominant mechanism, either energy transfer (reaction 8) or electron transfer (step 13), will depend on the respective reaction rates of the substrates with 3Rf*. It is known that reaction (8) occurs with a rate constant kET in water of 7 × 108 m−1 s−1, equivalent to 1/9 of the diffusional value (44). On this basis, and according to literature values for reaction (13) (Table 1), the kinetic balance indicates that processes (8) and (13) are competitive for tyr and trp whereas process (8) highly prevails in the case of ur. Hence, O2(1Δg) is always produced. Besides, in the presence of tyr or trp, relatively efficient interceptors of 3Rf* (Table 1), and according to the pKa value for process (17) (25,40), the concentration of the species RfH?, at pH 7, is ca 25-fold higher than that of Rf?. At pH 9 this proportion is inverted, being the concentration of Rf?ca four times greater than that of RfH?. As a consequence in neutral solution H2O2 is highly prevalent, formed through process (19) and at pH 9 the predominating ROS is O2?−, generated through process (14). This observation agrees with experimental results recently published, where the respective transient absorption spectra of the neat species RfH? at pH 5, and Rf? at pH 11, are shown (25).
On these grounds, results of oxygen uptake in Tables 1 and 2 and the kinetic data on O2(1Δg)-oxidation and quenching of 3Rf*, collected in Table 1 can be conjunctively analyzed.
For the Rf-sensitized experiments at pH 7, the overall photo-oxidation rate of tyr + ur is higher than that expected for the simple addition of both individual rates, reflecting a sort of synergistic effect. According to the results shown in Table 2, at this pH both tyr and ur react exclusively through H2O2 mechanism (step 20). The O2?− component, due to the pKa value for step (17), constitutes only a minor contribution to the overall oxygen consumption. The rate constant for the quenching of 3Rf*, the process responsible for H2O2 generation, is much higher for tyr than for ur. This fact could indicate that the efficiency of H2O2 generation and hence the stationary concentration of the oxidative species available for ur oxidation in the mixture ur + tyr will be also higher than that available for ur alone. As a result, an increase in the overall rate of oxygen consumption by the mixture should be expected.
For the case of trp at pH 7, oxygen uptake occurs through processes (9) and (20). The contribution of the O2(1Δg)-mediated step to the overall oxygen consumption is apparently high, as indicated by the reported oxidative efficiency, with a kr/kt ratio of 0.65 (19) (Table 1). The rate for the mixture trp + ur is practically the same as the corresponding one for the AA alone. In this case oxygen uptake by trp is ca 40-fold faster than that exerted by ur, and the rate for the mixture only reflects the massive O2(1Δg)-mediated contribution of the AA.
The relative rate values of oxygen uptake for tyr, trp, ur and their mixtures at pH 9 are very close. Nevertheless, the respective behaviors of the photo-oxidation rates for the mixtures AA + ur do not parallel. Again for trp, as occurred at pH 7, the photo-oxidation of the mixture ur + AA seems to be the addition of the individual rates, possibly dominated by a O2(1Δg) process.
Regarding the case of tyr at pH 9, it can be seen that the rate of oxygen consumption by tyr + ur is much lower than the addition of the respective individual rates. It is known that tyr, in the alkaline pH range, is easily oxidized by O2(1Δg) producing unstable endoperoxides via [1,4]-cycloaddition (7). The endoperoxides could generate radical intermediates, strong reactants that could favorably interact via ur, without additional oxygen consumption, in a competitive pathway with the O2?− route (process 16), possibly the prevailing source of oxygen consumption by ur at this pH. This argument for the additional radical mechanism has been already employed to explain a similar situation in the photo-oxidation of ascorbic acid in the presence of AAs (45).
Conclusions
The effect of the electron-donating substrate ur on the effectiveness of the overall Rf-sensitized-photo-oxidation process of the AAs trp and tyr is not straightforwardly predictable. In the presence of ur, much lesser photo-oxidizable than the AAs, the observed effect was either antioxidative, synergistic or even null. It depends on several connected factors, such as the respective abilities of the substrates as quenchers of both the long-lived Rf triplet excited state and the generated ROS. The pH of the medium constitutes an important factor, as it regulates the nature and reactivity of the ROS. An interpretation of the different cases may be attempted through a kinetic and mechanistic analysis.
Acknowledgments
Acknowledgements— Financial support from the following institutions is gratefully acknowledged: Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and Secretarías de Ciencia y Técnica of the Universidad Nacional de Río Cuarto (UNRC), Universidad Nacional de la Patagonia Austral (UNPA) and Universidad Nacional de San Luis (UNSL).




