Characterization of an Unusual LOV Domain Protein in the α-Proteobacterium Rhodobacter sphaeroides
Abstract
The facultatively phototrophic purple bacterium Rhodobacter sphaeroides 2.4.1 harbors a LOV (light, oxygen and voltage) domain protein, which shows a particular structure. LOV domains perceive blue light by a noncovalently bound flavin and transmit the signal to various coupled output domains. Proteins, that harbor a LOV core, function e.g. as phototropins or circadian clock regulators. Jα helices, which act as linker between the LOV core and the output domain, were shown to be involved in the light-dependent activation of the output domain. Like PpSB2 from Pseudomonas putida, the LOV domain protein of R. sphaeroides is not coupled to an effector domain and harbors an extended C-terminal α helix. We expressed the R. sphaeroides LOV domain recombinantly in Escherichia coli. The protein binds an FMN as a cofactor and shows a photocycle typical for LOV domain containing proteins. In R. sphaeroides, we detected the protein as well in the cytoplasm as in the membrane fraction, which was not reported for other bacterial LOV domain proteins.
Introduction
Light is an important environmental factor that can be utilized as energy source by many organisms. At high intensities, light can be harmful to the cell, therefore distinct adaptation mechanisms have evolved to sense this stimulus and to respond appropriately to it. The role of blue light receptors is well analyzed in plants and is also defined recently in a growing number of cases in bacteria (1).
Light, oxygen and voltage (LOV) domains are small blue light sensitive parts of proteins, which form a subset of the PAS (PER–ARNT–SIM) family that is an important module in diverse signaling processes in bacteria, archaea and eukarya (2). LOV domains noncovalently bind a flavin chromophore, which, upon blue light illumination, forms a covalent adduct with the conserved cysteine residue (3,4). This signal is transferred to a variety of coupled output domains (5). Depending on the effector domain, proteins that harbor a LOV core fulfill different functions. Equipped with two LOV domains and a serine/threonine kinase the phototropins phot1 and phot2 of Arabidopsis thaliana regulate e.g. phototropism, chloroplast movement and stomatal opening (5,6). The White Collar 1 protein of Neurospora crassa contains a LOV, PAS and a zinc-finger domain and is involved in circadian clock regulation (7). In the Bacillus subtilis YtvA, the LOV domain coupled to a sulfate transporter/anti-sigma factor antagonist domain act as inhibitor of an anti-sigma factor (8). Further, it is supposed to be involved in sporulation by binding the signaling components ATP and GTP in a light-dependent manner (9,10). LovK from Caulobacter crescentus harbors a histidine kinase domain as effector domain. By interacting with LovR it is supposed to be important for the light-dependent cell attachment (11). A similar LOV-histidine kinase in Brucella abortus seems to be crucial for the light-dependent virulence of this bacterium in mammal macrophages (12).
Besides other blue light receptors, like the AppA protein (13) and three cryptochrome-like proteins (14), a LOV domain protein is encoded by the genome of Rhodobacter sphaeroides 2.4.1. This LOV protein exhibits no additional functional domain, raising the question, how a blue light-dependent signal is transmitted. So far, a bacterial protein consisting just of the LOV domain was characterized in Pseudomonas putida (PpSB2), but the physiological role remained unknown (15). The Short-LOV protein Vivid (or VVD) in the fungus N. crassa was shown to be involved in photoadaptation (16). Vivid undergoes an N-terminal conformational change upon illumination (17). We expressed the LOV domain protein of R. sphaeroides 2.4.1 recombinantly in Escherichia coli and in R. sphaeroides. We purified the protein, defined its chromophore and light-dependent absorbance spectrum and analyzed its cellular localization.
Materials and methods
Purification of the recombinant LOV domain protein from Escherichia coli. To analyze the LOV domain protein of R. sphaeroides in vitro, we expressed the protein recombinantly in E. coli. The coding region rsp2228 of LOV was amplified by polymerase chain reaction (PCR) using the oligonucleotides lovpQE32SphIup (5′-ACATGCATGCTGGACCAAAAACAGTTCGAG-3′) and lovpQE32HindIIIdown (5′-CCCAAGCTTGTCAGGTCCGGCGCTGCC-3′). The fragment was subcloned in a pDrive cloning vector (Qiagen, Hilden, Germany) and afterwards into the overexpression vector pQE32 (Qiagen). The resulting plasmid pQElov was transferred into E. coli JM109 (New England Biolabs, Frankfurt am Main, Germany); 35 mL of overnight grown E. coli JM109 (pQElov) was inoculated in 1 L Luria-Bertani broth medium and shifted to 17°C. Expression was induced at an OD600 of 0.5 by adding 0.1 mm isopropyl-β-1-thiogalactopyranoside (IPTG). Cells were grown for 24 h and harvested by centrifugation. The LOV protein carrying the N-terminal His-tag was purified by Ni–nitrilotriacetic acid (NTA) affinity chromatography following the manufacturer’s recommendation (18). One liter of E. coli JM109 (pQElov) yielded more than 20 mg of the LOV protein. The presence of the LOV protein heterologously expressed in E. coli was confirmed by Western blot analysis by using a penta-His antibody (Qiagen).
Gel filtration analysis and production of polyclonal antibodies. The protein was further purified to homogeneity by gel filtration (Fig. 3) using a HiLoad 16/60 Superdex 75 prep grade column (GE Healthcare, Munich, Germany) in buffer containing 0.3 m NaCl, 10 mm Na2HPO4, 2.7 mm KCl and 1.8 mm NaH2PO4. Gel-purified protein was used to raise polyclonal antibodies in rabbit (Biogenes, Berlin, Germany). To detect the oligomeric state, gel filtration was performed under constant light and in the dark by isolating the protein under safety light and wrapping the gel filtration column in aluminum foil.
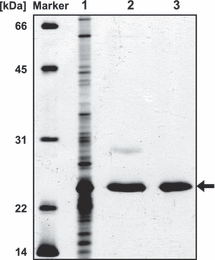
Silver stained 12% SDS–PAA gel with cell extract of Escherichia coli JM109 (pQElov) after induction with 0.1 mm IPTG (lane 1), LOV purified by Ni–NTA affinity chromatography (lane 2) and LOV further purified by gel filtration (lane 3).
HPLC and spectroscopic analyses. HPLC analysis was performed as described in (19) with some modifications in buffer system and separation protocol. A continuous linear gradient of 0.1% formic acid in acetonitril (13.5–18%) mixed with 0.1% formic acid in water was used for separation and elution of the cofactors. Excitation and emission spectra of the holoprotein and the released chromophore were carried out as previously described (20) using an RF-5301 PC spectrofluorophotometer (Shimadzu, Kyoto, Japan). The absorbance spectrum of illuminated (100 μmol m−2 s−1 provided by a 60 W light lamp; Osram) or dark stated LOV protein solution at room temperature was recorded in a range of 300 to 600 nm (Photometer Specord50; AnalytikJena, Jena, Germany).
Expression of the LOV domain protein in Rhodobacter sphaeroides. The LOV protein expression plasmid for R. sphaeroides was generated by cloning the strong oxygen dependent puf promoter (21) in front of the LOV protein coding region. The region −428 bp upstream of the puf genes coding region including the initiation codon was amplified by PCR using the oligonucleotides pufpRK415HindIIIup (5′-CCCAAGCTTGCGGAACTGAAAGCCGGTCA-3′) and pufpRK415XbaIdown (5′-GCTCTAGATGGTTCTCTCCCTTCCTCTCG-3′). The fragment was subcloned in a pDrive cloning vector (Qiagen) and afterwards into the plasmid pRK415 (22). For the expression of LOV in R. sphaeroides, rsp2228 was amplified by PCR using the oligonucleotides lovHispRKpufup (5′-GCTCTAGACATCATCACCATCACCATGACCAAAAACAGTTCGAGAAGATCCG-3′) and lovpRKEcoRIdown (5′-GGAATTCTCAGGTCCGGCGCTGCCAGG-3′) adding an N-terminal 6xHis tag to the protein. For the expression of the LOV construct without the predicted α helix (1–126 amino acid; aa) the respective coding region was amplified by PCR using the oligonucleotides lovHispRKpufup and lovΔαpRKEcoRIdown (5′-GGAATTCAACTCCCAGACCGCCCAAGCTC-3′). The PCR products were cloned in a pDrive cloning vector, respectively. The XbaI/EcoRI inserts from this plasmid were ligated behind the puf promoter in the plasmid pRKpuf. The resulting plasmids pRKpuflov and pRKpuflovΔα were transferred into E. coli JM109 and further into R. sphaeroides 2.4.1 (23) by triparental conjugation (24), respectively. Rhodobacter sphaeroides strains were cultivated at 32°C in a malate minimal salt medium (25) by continuous shaking at 140 rpm. Expression of LOV or LOVΔα in R. sphaeroides was achieved by growing R. sphaeroides (pRKpuflov) or (pRKpuflovΔα) over night under aerobic conditions and shifting them at an OD660 of 0.2 to semiaerobic conditions at 17°C. Cells were harvested when an OD660 of 1 was reached. Cells were broken by sonication.
Purification of Rhodobacter sphaeroides membrane fraction. The cell lysates of R. sphaeroides (pRKpuflov), (pRKpuflovΔα) and App11 (pBBRpucappA) were ultracentrifuged (100 000 g) to separate the cytoplasmic and the membrane fraction. To further purify this fraction, it was resuspended in PBS and loaded on a discontinuous sucrose density gradient (26). The gradient consisted of 1.5, 1.2, 1.0 and 0.6 m sucrose in PBS. Ultracentrifugation was performed in a swinging-bucket rotor at 200 000 g for at least 16 h. The two resulting red colored fractions contain the light and heavy membrane fraction harboring the photosynthetic complexes. The different fractions have previously been characterized in detail (27). The fractions were mixed with SDS–PAGE buffer, boiled and loaded on a SDS–PAGE. The LOV domain protein, expressed in R. sphaeroides, was detected using the anti-LOV antibody and the AppA protein by using the anti-AppA antibody.
Results and discussion
Primary and predicted secondary structure of the rhodobacter sphaeroides LOV domain protein
The open reading frame rsp2228 of R. sphaeroides 2.4.1 encodes a 176 aa LOV domain protein (1, 2). Alignments with other LOV domains show that it bears the highly conserved GXNCRFLQ motif (7), including the cysteine residue, essential for the blue light dependent formation of a photoadduct with the flavin chromophore (3,4). The R. sphaeroides LOV domain further harbors putative flavin binding residues (not shown). The protein lacks the C-terminal output domain (Fig. 1), present in other LOV domain containing proteins (7). This was also shown for PpSB2–LOV from P. putida (15) and is the case for 13% of all predicted bacterial LOV domain proteins, named Short-LOV (28). The unusual N-terminal cap region, previously described for the LOV domain protein Vivid of N. crassa (29) (Fig. 1) is also missing. Instead, an extended 46 aa α helix is present at the C-terminus, which was detected by using a secondary prediction program (30) (Fig. 1). In comparison, the predicted α helix of PpSB2 of P. putida has a length of 31 aa (15), the Jα helix in YtvA of B. subtilis is only 21 aa (10) (Fig. 1) and that of phot1 LOV2 of Avena sativa is about 20 aa long (31). Jα helices act as linker between the LOV core and the output domain and were shown to be involved in the light dependent activation of the output domain (31–33).
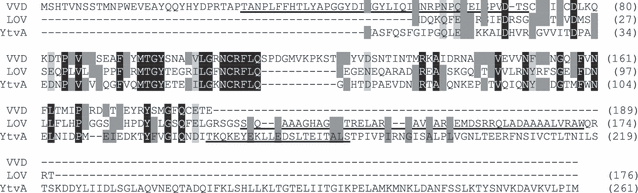
Amino acid alignment of Vivid (VVD) from Neurospora crassa, LOV from Rhodobacter sphaeroides and YtvA from Bacillus subtilis. The underlined amino acids in Vivid indicate the N-terminal cap (29), the putative α helix in R. sphaeroides LOV annotated with a secondary prediction program (30) and the Jα helix in YtvA (36). The comparison was performed using MultAlin software (http://www.prodes.toulouse.inra.fr/multalin/multalin.html).
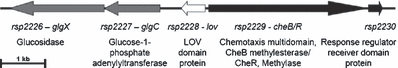
Genetic context of lov (rsp2228). The arrows indicate the direction of transcription.
Rhodobacter sphaeroides contains five chemotaxis loci, two of them are essential for chemotaxis (34). Directly adjacent to the LOV protein coding region on the other strand of the R. sphaeroides 2.4.1 genome one gene for a chemotaxis-related protein is located (Fig. 2): a CheBRA fusion protein, which encodes the fourth chemotaxis locus with so far unknown function (34,35). This may hint to an original role of LOV in phototaxis, but phototaxis is not observed in strain 2.4.1.
Purification of the recombinant LOV domain protein from Escherichia coli
The LOV domain protein expressed in E. coli was purified by Ni–NTA affinity chromatography and gel filtration (Fig. 3). The protein has a calculated molecular mass of 21 kDa. Under constant light it has an apparent molecular mass of 20.3 kDa as detected by gel filtration (Fig. 4), which is the monomeric form of the protein. The size of the dark state protein differs only slightly (18.5 kDa, Fig. 4), which could be due to the different running behavior of LOV after its conformational change in the dark. Vivid was shown to dimerize in the light, dependent on certain residues in the N-terminal cap (17). Whereas an elongated monomer (1.56 times larger than the theoretical molecular mass) is mainly present in full-length YtvA (1–261 aa; Fig. 1) (10), a light-independent dimerization was also shown for YtvA without the Jα helix (1–126 aa) (10) and the LOV domain of YtvA comprising the Jα helix (20–147 aa) (36). The Short-LOV protein PpSB2 from P. putida with its predicted α helix was also shown to dimerize, when the protein was expressed in E. coli (15). It is conceivable that the 1.5-fold extended α helix of the R. sphaeroides LOV protein (Fig. 1) hinders its dimerization or that the conformation of the protein as isolated from E. coli does not favor dimerization.
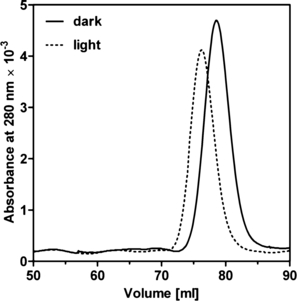
Gel filtration of LOV expressed in Escherichia coli with a HiLoad 16/60 Superdex 75 prep grade column. The protein elutes as a monomer with a molecular mass of 20.3 kDa in the light and 18.5 kDa in the dark.
Rhodobacter sphaeroides LOV binds FMN as chromophore
The LOV1 and LOV2 domains of plant phototropins bind flavin mononucleotide as chromophore (5), and so does e.g. YtvA of B. subtilis (8), whereas Vivid from N. crassa needs flavin adenine dinucleotide for activity (29). The purified R. sphaeroides LOV protein expressed in E. coli had a bright yellow color, indicating the presence of a flavin chromophore. We identified and quantified the chromophore by HPLC analysis. The LOV protein exclusively binds FMN (Fig. 5B) with a molar ratio of FMN to LOV protein of 0.5:1. Absorbance spectra further confirmed the identity of the released and separated cofactor, which corresponded to that of the FMN standard (Fig. 5A). Excitation and emission spectra of the holoprotein (not shown) and the released chromophore (Fig. 5C) gave further evidence for the binding of FMN.
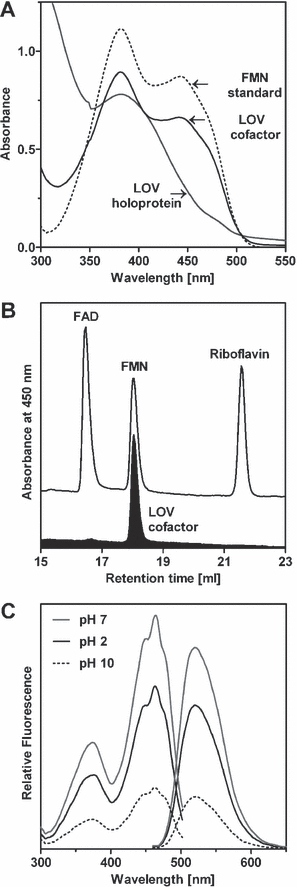
(A) Absorbance spectrum of the LOV holoprotein (at neutral pH), expressed in Escherichia coli and the chromophore compared to FMN (both at acidic pH). (B) HPLC analysis and (C) fluorescence excitation spectra recorded for emission at 520 nm (left spectra) and emission spectra recorded for excitation at 450 nm (right spectra) of the released chromophore of LOV expressed in E. coli.
The light-induced photocycle of Rhodobacter sphaeroides LOV
When we analyzed the absorbance spectrum of the LOV protein, which was expressed in E. coli, in the dark, the absorbance spectrum shows one major peak at 452 nm, one minor peak at 477 nm and one shoulder at 425 nm (Fig. 6). The same spectrum could be shown for the LOV protein, which was expressed in R. sphaeroides. After illumination with light for 1 min, the spectrum changed completely and a new maximum at 390 nm was obtained (Fig. 6). The complete recovery of LOV to the dark state took 4 h, which is slower than the 2 h described for YtvA (8) and comparable to the 5 h which Vivid needs for recovering (16). We also were able to identify the three isosbestic points (Fig. 6), which are characteristic for the formation of a flavin-cysteinyl-adduct (6). The presence of a photocycle proofs that the R. sphaeroides LOV protein is photoactivable and could perhaps function as a photoreceptor. We created an R. sphaeroides mutant that does no longer express the LOV protein, but did not observe a change of the phenotype. The biological function of LOV needs to be elucidated further in the future.
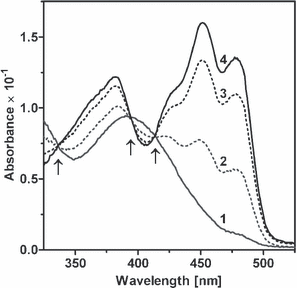
Absorbance of LOV in the dark (spectrum 4), after 1 min of illumination (spectrum 1) and after incubation in the dark for 10 min (spectrum 2), 1 h (spectrum 3) and 4 h (spectrum 4). The three isosbestic points are indicated by arrows.
Localization of Rhodobacter sphaeroides LOV in the cell
The plant phototropins were shown to be associated with the plasma membrane (37,38), but to our knowledge this is not the case for the known LOV domain containing proteins in bacteria. We wanted to check the localization of LOV in R. sphaeroides but we were not able to detect the protein in extracts of wild type cells. According to microarray data, the gene is constitutively expressed but only at a low level (39). For the analysis in a homologous system, two expression plasmids for R. sphaeroides were generated, coding for the full LOV domain region and the region without the α helix. We detected the LOV protein in the cytoplasmic (Fig. 7A), in the light (not shown) and in the heavy membrane fraction (Fig. 7A), concluding that at least part of the protein is membrane bound. We suggested that the C-terminal extended α helix could mediate the membrane association of LOV in R. sphaeroides. Therefore the LOV protein without the α helix was expressed from a plasmid in R. sphaeroides and analyzed for its localization in the cell. Unfortunately, the protein could not be observed with the anti-LOV antibody in any of the analyzed fractions (not shown). We also used an antibody against the N-terminal His-tag of both proteins, but were only able to detect the full-length LOV domain protein and not the shortened version, suggesting that the α helix in the LOV domain protein of R. sphaeroides is needed for stabilization of the protein. In order to proof the clean separation of the cytoplasmic and membrane fraction and to exclude the possibility that the membrane association is caused by the His-tag, we also analyzed the fractions of strain App11 (pBBRpucappA), which expresses the AppA protein with a His-tag from the pBBRpuc plasmid and has the appA gene deleted from the chromosome. AppA harbors a BLUF (blue light sensing using flavin adenine dinucleotide) domain (40) and a heme binding domain (41,42) and is involved in redox-light-dependent regulation of photosynthesis genes in R. sphaeroides (13,43). An antibody directed against AppA detected the protein in the cytoplasmic fraction but not in the membrane fraction (Fig. 7B).
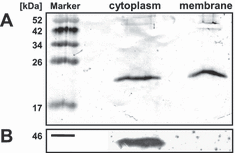
Cytoplasmic and membrane fraction of (A) Rhodobacter sphaeroides (pRKpuflov) analyzed by western blotting with the antibody anti-LOV against the LOV domain protein and (B) R. sphaeroides (pBBRpucappA) analyzed by western blotting with the antibody anti-AppA against the AppA protein.
The presence of a photocycle suggests that LOV functions as a photoreceptor in R. sphaeroides. Elucidation of its biological functions and of the mechanisms of light-dependent signaling requires further investigations.
Acknowledgments
Acknowledgements— We thank Anne Konzer for the construction of plasmid pRKpuflov. This work was supported by grant Kl563/15-5 from the Deutsche Forschungsgemeinschaft.




