Depth Profile of Protoporphyrin IX Fluorescence in an Amelanotic Mouse Melanoma Model
Abstract
Protoporphyrin IX (PpIX) fluorescence was measured at different depths in a subcutaneous amelanotic melanoma model (LOX) in mice. PpIX was induced by topical application of 5-aminolevulinic acid (ALA) and two of its derivatives, the methylester (MAL) and hexylester (HAL) onto the normal skin covering the tumor. The PpIX fluorescence intensity on the surface of the tumors was the highest for HAL, followed by ALA and MAL. Using equimolar concentrations (0.5 mmol g−1), HAL induced nearly twice as much fluorescence as ALA did. The depth profile of PpIX fluorescence was measured at different layers of the tumor, which was carefully sliced and controlled in situ ex vivo. The PpIX fluorescence was mainly localized within the upper 2 mm of the tissue for ALA and within 1 mm for MAL and HAL. There were no significant differences in the shape of the fluorescence excitation spectra, but the long wavelength excitation peak (633 nm) was so weak that these results are unreliable for depth estimation. When considering the low fluorescence intensity (around 5% of the intensity at the tumor surface), the actual penetration depth of HAL was comparable to that of ALA. The fluorescence after topical application of ALA and HAL was significantly above the background level down to a depth of around 6 mm, and there were traces of PpIX fluorescence even at the tumor base (10 mm). The fluorescence after topical application of MAL was detectable down to 1 mm. In the depth of 2–6 mm, the fluorescence was slightly higher for HAL than for ALA. Using the estimated diffusion coefficients for topically applied ALA (0.16 ± 0.03 mm2 h−1), MAL (0.045 ± 0.005 mm2 h−1) and HAL (0.037 ± 0.003 mm2 h−1), the behavior of the drugs after different application times could be estimated in this tumor model.
Introduction
Photodynamic therapy (PDT) is becoming an established treatment modality of malignant and non-malignant disorders (1–3). It is based on systemic or topical administration of photosensitizing agents (photosensitizers) that are preferentially retained in tumors (4–6). After exposure of the photosensitized lesion to light, a number of phototoxic reactions are initiated, leading to the death of cancer cells and elimination of the tumor (7,8).
During the last decade a new approach, taking advantage of the process of heme biosynthesis, is becoming widely used in PDT. Exogenous administration of 5-aminolevulinic acid (ALA), a natural precursor of heme, or one of its derivatives induces accumulation of protoporphyrin IX (PpIX), which is the last intermediate before heme is produced (2,3,9,10).
It has been claimed that the hydrophilic character of ALA is a limiting factor leading to a poor permeation through the stratum corneum (11,12). Number derivatives of ALA have been synthesized and tested for PDT (12–15). Lipophilic esters of ALA seem to be more efficient in producing PpIX in cells in vitro (13,16,17). However, the situation is more complicated in vivo in systems. A number of factors influence the selectivity of PpIX production: permeability of the stratum corneum overlaying tumors (18–20), activity of the rate-limiting enzymes ferrochelatase and porphobilinogen deaminase (21,22), iron concentration in tumors (23,24) and tumor temperature compared to normal skin (25–27). Improving the delivery mechanisms and construction of new ALA derivatives are of continuous interest (14,15). Moreover, only few investigations on the penetration depths have been reported (11,28–30). There is an obvious need of such studies, because maximally efficient penetration depths of topically applied ALA and its esters are of interest to achieve high cure rates, particularly for thicker tumors (31). In the present work, we tried to evaluate the depth profile of PpIX production in an amelanotic mouse melanoma after topical application of ALA and two of its esters. For fast and reliable measurements, a simple technique of accurate tumor slicing in situ was developed. The penetration depths of the drugs were assessed by measuring the fluorescence of the generated PpIX at different depths.
Materials and methods
Chemicals. ALA hydrochloride was obtained from Sigma-Aldrich Norway AS (Oslo, Norway). Its methylester (MAL) and hexylester (HAL) hydrochloride were provided by Photocure ASA (Oslo, Norway). All chemicals for cell culture work were purchased from Sigma-Aldrich Norway AS.
Animals. Experiments using mice were approved by the National Animal Research Authority and were performed according to the European Convention for the Protection of Vertebrates Used for Scientific Purposes. Female hairless BALB/c mice were used. The mice were around 8 weeks of age weighing approximately 27 ± 3 g. They were anesthetized with subcutaneous injection of a mixture of Hypnorm (Janssen Pharmaceutica B.V., Tilburg, The Netherlands) and Dormicum (Hoffmann-La Roche AG, Basel, Switzerland) (1:1 vol/vol) for a short period (around 15 min) at the beginning of the experiments in order to facilitate proper application of the creams. The animals were normally active during the rest of the experiment. For ex vivo measurements, the mice were sacrificed by cervical dislocation. In total, five groups were used: control (no drugs), ALA topical, MAL topical, HAL topical and ALA systemic administration, with three animals being in each group.
Tumor model. The human LOX cell line was established as a subcutaneous xenograft in mice from amelanotic axillary lymph node metastasis of a patient with malignant melanoma (32). The cells were grown in RPMI 1640 medium supplemented 2 mm l-glutamine, 10% fetal calf serum, 100 units mL−1 penicillin and 100 μg mL−1 streptomycin and incubated in 25 cm2 cell culture flasks (Nunc AS, Roskilde, Denmark) at 37°C in a humidified 5% CO2 atmosphere in an incubator (Forma Scientific, Inc., Marietta, OH). The cells were subcultured twice a week using 0.01% trypsin in 0.02% EDTA.
For tumor inoculation, cells attached to a plastic substratum were used. The medium was removed using a suction pipette. The flask containing the cells was then washed once with trypsin–EDTA solution. The cells were trypsinized (1 mL trypsin) and placed in the incubator for 5 min. After trypsinization, the flasks were stirred and a pipette (9 mL) of the medium was added to stop the effect of trypsin. The cell suspension was then transferred to a test tube and centrifuged for 5 min at 160 g. The supernatant was removed and the cells were resuspended in fresh medium (1 mL). The number of cells was counted using Glasstic Slides (Hycor Biomedical, Inc., Garden Grove, CA). The suspension was diluted to obtain the desired concentration and was transferred to a sterile 1 mL syringe (Becton Dickinson Ltd., Grogheda, Ireland). Approximately 106 cells (0.1 mL suspension) were injected subcutaneously in each mouse. After about 10 days the tumors were palpable. Fluorescence measurements were performed on tumors with a size large enough to cover the tip of the fiber-optic probe. The average tumor size was 2 cm3.
Topical delivery of the drugs. Creams containing 0.5 mmol g−1 of ALA (around 8.4% w/w), MAL (around 9.1% w/w) and HAL (around 12.9% w/w) were prepared by dissolving the drugs directly in a cream (Unguentum, Merck, Darmstadt, Germany). Approximately 30 mg cm−2 of the freshly prepared cream was applied topically on the tumors and covered with a transparent adhesive dressing (OpSite Flexigrid, Smith & Nephew Medical Ltd., Hull, UK). The adhesive dressings and the creams were removed after approximately 4 h of application. In some experiments, ALA was injected intraperitoneally (i.p.; 0.6 mmol kg−1, around 100 mg kg−1).
Tumor slicing in situ. For fast and reliable measurements of the depth profile of the fluorescence distribution, a simple technique was used. Briefly, tumors were accurately sliced in situ ex vivo using a sharp scalpel. The thickness of each tumor slice was accurately measured by means of calipers. Care was taken to avoid squeezing the slices. As it is practically impossible to control the exact thickness of such slicing in situ, and therefore provide constant fluorescence excitation geometry, the fluorescence was always measured on the surface of the remaining tumor and the recorded value was assumed to be a representative for the tumor depth given by the sum of the thickness of the slices. As the excitation wavelength was 407 nm, the fluorescence originates mainly from the upper layers of the remaining tumor, because of the small penetration depth (about 0.5 mm) of 407 nm light (33,34).
Fluorescence measurements. In aqueous solutions, the fluorescence of PpIX is quenched because of aggregation. The fluorescence of PpIX in a lipophilic environment (e.g. in a mixture with serum or triton) is restored and the spectra are similar to those in cells in vitro or tissues in vivo. Based on this spectroscopic experience, we can assume that PpIX in living cells and tissues is in a monomeric form and that the measured fluorescence is linearly related to the concentration. Therefore, the amount of PpIX formed in the tumors was evaluated spectroscopically with a LS50B luminescence spectrometer (Perkin-Elmer, Norwalk, CT). A bifurcated fiber-optic probe (Perkin-Elmer accessory, tip diameter 6 mm) was placed on the surface of the tissue and the fluorescence spectra were measured. The excitation wavelength was 407 nm. A long-pass cut-off 530 nm filter was used on the detection site of the spectrometer. The fluorescence values were notified for the peak at 635 nm. In addition, the fluorescence excitation spectra were recorded at the long-wavelength emission peak (705 nm). A narrow band-pass (710 ± 10 nm) interference filter (Ealing Electro-Optics, Inc., Holliston, MA) was used on the detection site of the spectrometer. There was no significant bleeding of the remaining tumor during the measurements.
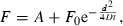 (1)
(1)Results
Porphyrin induction in LOX tumors
Typical PpIX fluorescence spectra with maxima at around 635 and 705 nm were measured after topical application of ALA and its derivatives. The spectra are not shown as they are similar to those obtained in our previous studies (37). After topical application of 4 h, the PpIX fluorescence intensity on the surface of LOX tumors was highest for HAL, followed by ALA and MAL, applied at equimolar concentrations (0.5 mmol g−1) of the drugs (Fig. 1). HAL induced nearly twice as much fluorescence as ALA did and nearly five-fold as much as MAL did.
Fluorescence of PpIX (λexc = 407 nm, λem = 635 nm) measured in situ at different depths of LOX tumor. Inset shows the same data scaled so that low fluorescence at deeper layers is discernible.
For comparison, 0.6 mmol kg−1 (100 mg kg−1) ALA was administered systemically (i.p.). Topical application was superior to i.p. administration for ALA in this tumor model and under the present conditions (Fig. 1).
Depth profile of PpIX fluorescence
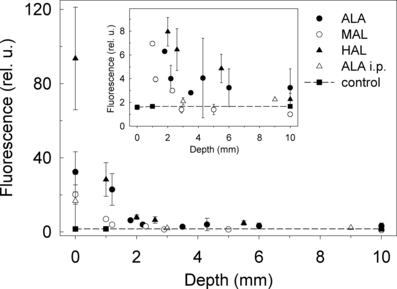
HAL induced the strongest PpIX fluorescence close to the surface of the tumor (Fig. 2). Practically all fluorescence was concentrated within the upper layer (1 mm). The penetration depth was similar for HAL and MAL (Fig. 2). The fluorescence after ALA application was localized within the upper 2 mm (Fig. 2). The fluorescence kinetics after topical application of ALA, MAL and HAL were fitted to Eq. (1) (Fig. 2). According to the data and the model, the diffusion coefficients (D) are 0.16 ± 0.03 mm2 h−1 for ALA, 0.045 ± 0.005 mm2 h−1 for MAL and 0.037 ± 0.003 mm2 h−1 for HAL. It should be noted that the curves presented in Fig. 2 are normalized to the initial fluorescence value at the tissue surface. Therefore, this is a description of the relative drug behavior. The penetration depth of HAL was comparable to that of ALA when considering the fluorescence at deeper layers, at the range 2–10 mm (Fig. 1, inset). The fluorescence after topical application of ALA and HAL was larger than that of the background (control group) down to a depth of around 6 mm, and even at the tumor base (10 mm). The fluorescence after application of MAL was detectable down to a depth of 2 mm. Within the range of 2–6 mm, the fluorescence depth profile after the application of HAL was slightly higher than that after the application of ALA (Fig. 1, inset).
Fitting of the data presented in Fig. 1. The ordinate values are comparable among the different panels.
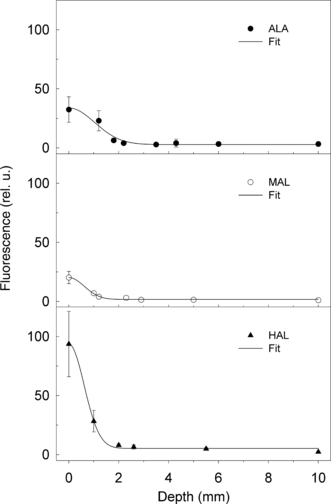
Using the experimentally obtained diffusion coefficients for topically applied ALA, MAL and HAL, the penetration of the drugs after different application times was calculated (Fig. 3). By measuring the PpIX fluorescence at the surface of the tumor, we can now estimate the likely depth of PDT-induced cell inactivation in this tumor model.
Tentative depth profile of PpIX fluorescence calculated using experimentally obtained diffusion coefficients.
Discussion
It has been suggested that the hydrophilic character of ALA limits its transport through the stratum corneum and reduces its penetration depth (11,12). To overcome this problem, a number of derivatives of ALA have been synthesized and tested (14,15). Our study intended to compare three derivatives of different lipophilicities with respect to the depth of PpIX formation in a tumor model. The relevance of the used tumor model is questioned as skin tumors in humans are located within the skin, not underneath the skin. However, as mouse skin is few times thinner in comparison with human skin (38), a more relevant transplanted tumor model will be difficult to find.
Ester derivatives of ALA are more lipophilic than ALA and are expected to penetrate biological barriers better (17). The stratum corneum is considered to be the major penetration barrier for topically applied drugs (19,20). Esters of ALA are considerably more efficient to synthesized PpIX in cells in vitro than ALA itself (13,16,17). However, this is not the case in tissues in vivo. While some biopharmacokinetic differences exist, such as the time lag between drug application and the onset of PpIX formation (19,37), no surprising differences were found when comparing ALA with its derivatives in tissues in vivo with respect to the amount of generated PpIX (37,39). The methylester, ethylester and the propylester of ALA were found to give more PpIX in vivo than ALA (14,40). The penetration depths of topically applied ALA and its derivatives in vivo are not drastically different (41,42). The fluorescence is mainly localized in the stratum corneum and in the epidermis of normal skin (39,42) and is found down to 1–2 mm in mouse tumors (11,29,42,43). In some studies, the penetration depths of different drugs were directly compared in the same tumor model. Data have been reported for pentylester in UVB-induced skin tumors (42), and for butylester and hexylester in human skin (41). The penetration depths were reported to be similar for these drugs. Some studies on comparison of the penetration depths in skin chamber models in vitro have been published. Van den Akker et al. (44) reported that the penetration through skin was the highest for ALA and lowest for HAL, with MAL being in between. De Rosa et al. (12) found an increased permeation of HAL compared to other esters (butyl, methyl, octyl) and ALA. The transport of the octylester of ALA is negligible compared to that of the hexylester and of ALA (12). In our amelanotic mouse melanoma model, we find that ALA has the highest penetration depth as estimated by the depth profile of PpIX fluorescence compared to MAL and HAL. This is most likely due to the higher ability of ALA to penetrate normal skin and enter systemic circulation, thereby generating fluorescence “outside” the application area (20,37,40). The methylester of ALA is more selectively accumulated in skin disorders than ALA (18,29). This may also be linked to the lack of systemic uptake of ester derivatives of ALA.
Earlier we performed a study on the possibility to estimate penetration depths of topically applied drugs by taking advantage of the wavelength dependence of light penetration into tissues and that of the excitation light (33). For deeply penetrating drugs, the contribution of the long wavelength peak (around 633 nm) should be larger than that for drugs with shallow penetration. The fluorescence excitation spectra showed no significant differences for ALA, MAL and HAL (data not shown). This may be due to the low fluorescence (almost on detection limit) excited via the 633 nm PpIX absorption peak.
The finding that HAL induces around twice as much PpIX as ALA does at the tumor surface (Fig. 1) may be due to either a higher degree of penetration of HAL through the mouse skin, or a higher ability of HAL (per mole of substance) to generate PpIX within the tumor tissue.
Intraperitoneal injection is often applied in animal experiments when testing systemic behavior of drugs due to the simplicity of the procedure. Our earlier work (45) shows that the generation profiles of PpIX in mouse skin over time after i.p. administration of ALA and MAL is similar to that after oral administrations. Therefore, in our experiments i.p. was used to represent systemic application of 0.6 mmol kg−1 (100 mg kg−1) ALA. Topical application was superior to i.p. administration for ALA in this tumor model (Fig. 1).
Our study concerns the application of MAL and HAL on a nodular tumor model in mice. The reason for the relatively low penetration depth of MAL and HAL may be binding to the upper tissue layers or lack of penetration through normal skin. Such a binding or retention can be expected for lipophilic drugs and will reduce the penetration depth.
In conclusion, using a simple in situ technique the depth profile of PpIX fluorescence can be measured. The deepest localization was found for ALA (2 mm), and the lowest for MAL and HAL (1 mm). Slightly elevated PpIX fluorescence above the background level was found down to a depth of 2–10 mm for ALA and HAL. The penetration behavior of the drugs for different application times was tentatively described using experimentally obtained diffusion coefficients. By measuring the relative PpIX fluorescence at the surface of the tumor, it will be possible to estimate the largest depth of PDT-induced necrosis in LOX tumors for a certain drug and application time.
Acknowledgements— The present work was financed by the Norwegian Cancer Society (DNK) and by the Norwegian Radium Hospital Research Foundation (RF).




