Photochemical Internalization of Bleomycin is Superior to Photodynamic Therapy Due to the Therapeutic Effect in the Tumor Periphery
Abstract
Photochemical internalization (PCI) is under development for clinical use in treatment of soft tissue sarcomas and other solid tumors. PCI may release endocytosed bleomycin (BLM) into the cytosol by photochemical rupture of the endocytic vesicles. In this study, the human fibrosarcoma xenograft HT1080 was transplanted into the leg muscle of athymic mice. The photosensitizer disulfonated aluminum phthalocyanine (AlPcS2a) and BLM were systemically administrated 48 h and 30 min, respectively, prior to light exposure at 670 nm (30 J cm−2). The purposes of this study were to evaluate the treatment response to AlPcS2a-photodynamic therapy (PDT) and AlPcS2a-PDT in combination with BLM (i.e. PCI of BLM) in an orthotopic, invasive and clinically relevant tumor model and to explore the underlying response mechanisms caused by PDT and PCI of BLM. The treatment response was evaluated by measuring tumor growth, contrast-enhanced magnetic resonance imaging (CE-MRI), histology and fluorescence microscopy. The results show that PCI of BLM is superior to PDT in inducing tumor growth retardation and acts synergistically as compared to the individual treatment modalities. The CE-MRI analyses 2 h after AlPcS2a-PDT and PCI of BLM identified a treatment-induced nonperfused central zone of the tumor and a well-perfused peripheral zone. While there were no differences in the vascular response between PDT and PCI, the histological analyses showed that PDT caused necrosis in the tumor center and viable tumor cells were found in the tumor periphery. PCI caused larger necrotic areas and the regrowth in the peripheral zone was almost completely inhibited after PCI. The results indicate that PDT is less efficient in the tumor periphery than in the tumor center and that the treatment effect of PCI is superior to PDT in the tumor periphery.
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of solid tumors with multiple histological subtypes arising in all ages and compromising approximately 1% of all new malignancies. Surgery is the cornerstone of treatment for localized disease and adequate surgical margin is a major prognostic factor for local recurrence (1). Adjuvant radiotherapy improves local control after inadequate resection at the price of early and late complications such as impaired wound healing, infections, edema, fibrosis, necrosis, fractures (2) and secondary malignancies. Technical developments in the last two decades in favor of multidisciplinary diagnostics and surgical therapy have caused a growing number of limb-sparing procedures and the number of recurrences has been reduced. However, advances in nonsurgical and adjuvant therapies have been slow in the last four decades and unfortunately, still 30–50% of newly diagnosed STS patients die within 5 years (1). The development of new treatment approaches to assure tumor eradication at the resection site carries the promise to improve local control and overall survival.
Photochemical internalization (PCI) may be regarded as an enhanced photodynamic therapy (PDT) modality in that PDT and PCI share many fundamental photodynamic mechanisms, but differ in that PCI has the additional function as a drug delivery system by release of endocytosed macromolecules into the cytosol. The PCI technology is based on the use of photosensitizers located in endocytic vesicles. Upon activation by light, the vesicles will release the macromolecules. PCI has been shown to enhance the biological activity of a large variety of molecules that do not readily penetrate the plasma membrane, including type I ribosome-inactivating proteins, immunotoxins, gene-encoding plasmids, adenovirus, oligonucleotides and the chemotherapeutic agents bleomycin and doxorubicin (the latter only in multidrug resistant cells) (3). The results show that PCI can induce efficient light-directed delivery of macromolecules into the cytosol, indicating that PCI may have a variety of useful applications for site-specific drug delivery, e.g. in gene therapy, vaccination and in treatment of solid malignant tumors.
The effect of PCI has previously been well documented on subcutaneous models (4–6). The subcutaneous tumor models are useful for evaluation of treatment effects, but the orthotopic implants better preserve the various aspects of the tumor biology. In case of STS, where tumor infiltration into the normal surrounding tissue often limits the therapeutic outcome of the present treatment regimens, invasive orthotopic models are crucial for assessment of the therapeutic potential of new treatment regimens. The diffusion of photosensitizers in tumor tissue and the response to PDT have been reported to differ between orthotopic and subcutaneous tumor models (7,8). The PCI technology has therefore been evaluated in the HT1080 fibrosarcoma model implanted in the leg and growing invasively into the muscle tissue. The results indicate that PCI is an efficient modality for the treatment of invasive tumors. It has previously been reported that PCI induces therapeutic effects deeper into the target tissue than PDT (6). In the present study it is reported that the treatment effect of PCI is superior to PDT in the tumor periphery.
Materials and Methods
Animals. Female BALB/c (nu/nu) nude mice were bred at the animal department of our institute. The mice were kept under specific pathogen-free conditions. Water and food were given ad libitum. All procedures involving mice were carried out in agreement with protocols approved by the animal care unit at the Norwegian National Hospital—the Norwegian Radium Hospital HF, under control by the National Ethical Committee’s guidelines on animal welfare. The mice were on average 20–25 g (6–8 weeks old) at the start of the experiment. The animals were kept in cages with five animals in each cage and were given a unique number by ear marking.
Xenograft. The human fibrosarcoma cell line HT1080 was purchased from the American Type Culture Collection (ATCC, Rockville, MD). The cell line was propagated by serial transplantations. The tumors were minced to homogeneity by scalpel and approximately 20 μL was injected 3 mm below the patellar tendon into the region of the left lateral gastrocnemius muscle.
Chemicals. Disulfonated aluminum phthalocyanine with the sulfonate groups on adjacent phthalate rings (AlPcS2a) was purchased from Frontier Scientific (Logan, UT). Sixty milligrams of AlPcS2a was dissolved in 1 mL 0.1 m NaOH and diluted with 29 mL phosphate-buffered saline (PBS) to a concentration of 2 mg mL−1. The solution was sonicated for about 1 min, diluted with 18 mL PBS to a final concentration of 1.25 mg mL−1, aliquoted and stored at −20°C. Approximately 200 μL of the stored AlPcS2a solution was injected intraperitoneally (i.p.), to a final concentration of 10 mg kg−1. Bleomycin (BLM; Baxter, Denmark) was provided as powder. 15 000 IU bleomycin was dissolved in 1 mL 0.9% NaCl. Gadolinium-diethylene-triamine-penta-acetic-acid (Gd-DTPA), purchased from Schering (Berlin, Germany), was diluted in 0.9% NaCl to a concentration of 0.06 m and used for intravenous injection. Hypnorm-Dormicum (HD) containing 1 mL of 0.315 mg mL−1 fentanyl citrate and 10 mg mL−1 fluanisone from Vetapharm Ltd. (Sherburn in Elmet, Leeds, UK) and 1 mL of 5 mg mL−1 midozalam from Hoffman-La Roche AG (Basel, Switzerland) was diluted in 6 mL sterile water. The mixture was given subcutaneously for anesthesia related to MRI studies. Bouin’s solution was used as fixative and for staining of metastasis to the lung.
Experimental design and method. For the assessment of therapeutic effect, 80 tumor-bearing animals were allocated randomly into one of the following eight different groups, 10 in each group: (1) no treatment; (2) light only; (3) BLM only; (4) BLM and light; (5) AlPcS2a only; (6) AlPcS2a and BLM; (7) AlPcS2a and light (PDT); and (8) AlPcS2a, BLM and light (PCI).
AlPcS2a (10 mg kg−1) was injected i.p. when the tumors reached a size leading to tumor volumes of 80–150 mm3 at the day of illumination. Forty-eight hours after the injection of AlPcS2a, 1500 IU BLM was injected i.p. Thirty minutes after BLM injection, the animals were fixed in a holder and covered with aluminum foil except for the area above the tumor where a 2 cm2 hole in the foil was made. The tumors treated with AlPcS2a were illuminated using a 670 nm diode laser (CeramOptec GmbH, Bonn, Germany) coupled to a fiber optic microlens applicator (FD1, Medlight, Echlubens, Switzerland). The tumors were exposed to 30 J cm−2 of light at an irradiance of 90 mW cm−2. Animals exposed to AlPcS2a were kept in dark from the time of injection and until day 7 after injection.
The tumor size was measured five times per week and the animals were euthanized by cervical dislocation when the tumor volume reached 1000 mm3. Tumor volume (V) was calculated using the following formula: V = (W × W × L)/2 where W (width) is the shorter and L (length) is the longer of two perpendicular diameters, measured with a caliper. The first 20 animals, in which the tumor reached 1000 mm3, were subjected to analysis for lung metastases with Bouin’s solution and histological examination of hematoxylin–eosin (HE)-stained slides.
Contrast-enhanced magnetic resonance imaging. Contrast-enhanced magnetic resonance imaging (CE-MRI) was performed by using a 1.5-T whole body scanner (Sigma; General Electric, Milwaukee, WI) and a cylindrical slotted tube resonator transceiver coil specially constructed for mice (9). The mice were anesthetized with 40 μL of HD and placed in the scanner with the tumor in the isocenter. The coil was insulated with Styrofoam to prevent excessive heat loss from the mice. The body core temperature of the mice was kept at 37–38°C during imaging by using a thermostatically regulated heating pad. Two calibration tubes, one with 0.5 mm Gd-DTPA in physiological saline and the other with only physiological saline, were placed adjacent to the mice in the coil. The tumors were imaged axially in a single section through the tumor center. Sagital scans were used to localize the tumor and determine the position of the axial scan. A scan thickness of 2 mm was used, and the number of excitations was 1. The image matrix was 256 × 64 with a field of view of 6 × 3 cm2, corresponding to a voxel size of 0.23 × 0.47 × 2.0 mm3. Interpolation algorithms applied by the imaging system resulted in an apparent resolution of 0.23 × 0.23 × 2.0 mm3. Two types of spoiled-gradient recalled sequences were used: proton density images were recorded by using a repetition time TR = 900 ms, echo time TE = 3.2 ms, and flip angle α = 20° and T1-weighted images were recorded by using a TR = 200 ms, TE = 3.2 ms and α = 80°. One proton density image and one T1-weighted image were recorded before administration of the contrast agent, and one T1-weighted image was recorded 60 s after administration of the contrast agent. The contrast agent (Gd-DTPA) was administered in the tail vein of the mice in a bolus dose of 5.0 mL kg−1 body weight during a period of 5 s. The administration was performed after the mice had been placed in the MR scanner and by using a 24-G neoflon connected to a syringe by polyethylene tubing. CE-MRI was performed before and approximately 2 h after PDT or PCI treatment. Tumor images were stored in the DICOM format and analyzed on a voxel-by-voxel basis by using software developed in IDL (Interactive Data Language, Boulder, CO). Gd-DTPA concentrations were calculated from signal intensities by using the method of Hittmair et al. (10).
Histology. The tumors were harvested 7 days after PDT or PCI. The specimens were fixed in 4% buffered formalin, embedded in paraffin, sectioned and stained with HE.
In vivo localization of AlPcS2a. AlPcS2a (10 mg kg−1) was injected i.p., and 48 h later the mice were killed. Both tumor-bearing and nontumor-bearing hind limbs were removed, bone elements dissected out and the soft tissue components directly frozen in liquid nitrogen. The tissue blocks were mounted in Tissue Tek II embedding compound (BDH, Poole, UK), and a series of tissue sections were then cut with a cryostat microtome to a thickness of 8 μm and mounted on clean glass slides. Fluorescence microscopy was carried out using an Olympus AX70 microscope with a 100 W mercury lamp. Fluorescence images were made with a thermoelectrically cooled CCD camera (Retiga 1300; QImaging, Surrey, Canada) with 1280 × 1024 pixel resolution and 12 bit dynamic range. The filter combination used was composed of a 590–650 nm excitation filter, a 660 nm beam splitter and a 660–735 nm emission filter. The same lens (10×, NA = 0.30) and integrated exposure time of 2 s that causes less than 1% photobleaching of the dye fluorescence were used in the whole study. The sections were then stained with HE and transmission images of the sections were made with a color CCD camera (SyncroCOOL 435; Syncroscopy, Cambridge, UK), on the same microscope, so as to determine the exact histological localization of the dye in the tissues. Multiple adjacent images were joined to cover the whole tumor and the surrounding normal muscle tissue. Image analysis was then used to measure the average intensity in the fluorescence image as a function of the distance to the tumor border as determined from the HE images.
Statistical analysis of the data. The tumor growth data are subjected to survival analysis, using the day when the tumor volume supersedes the volume Vcrit = 1000 mm3 as the failure time, and the duration of the experiment as the censoring time, if the tumor does not obtain this volume. Statistical differences in treatment response were evaluated by pairwise log-rank analyses in spss 15.0.
Because both the PDT and the BLM groups induced a delay in tumor growth, documentation of synergism requires more detailed analyses, as described in Berg et al. (4). The documentation of synergy is based on a log-logistic survival function. Synergy is regarded as statistically significant when the additional interaction variable, β3, is significantly different (P < 0.05) from the sum of the individual survival variables, β12 = β1 + β2.
Results
Tumor model
At the time of light delivery, 6–7 days after tumor cell injection, an intramuscular nodule was present at the injection site on all the animals. Histological examination of the tumor at this time showed irregular tumor cells with infiltrative behavior within the deep muscle compartment. No surrounding fibrous capsule was present in the transitional zone on the border between tumor and muscle tissue, giving the HT1080 tumor a subjective impression of being highly malignant (Fig. 1). Lung metastases were not observed in tumor-bearing mice, regardless of the treatment regime. This may however be due to rapid growth of the tumor, requiring euthanization before lung metastasis can be observed. HT1080 cells injected intravenously were found to cause lung metastases.
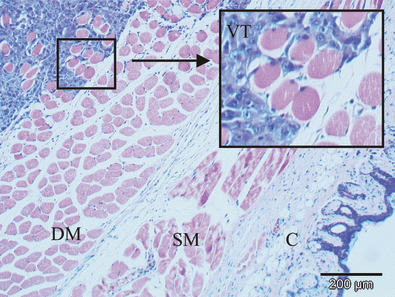
The HT1080 human fibrosarcoma xenograft located intramuscularly in the leg examined with HE staining. A part of the transitional zone between viable tumor (VT) and the muscle tissue is magnified and inserted in the figure. Deep muscle layer (DM), superficial muscle layer (SM) and cutis (C) are shown in the figure.
Treatment response to PDT and PCI
The therapeutic potential of AlPcS2a-PDT and AlPcS2a-PDT in combination with BLM (i.e. PCI of BLM) on HT1080 xenograft tumors, compared with relevant control groups, was assessed over time. The tumor response to the various treatments is described in the Kaplan–Meier plot in Fig. 2. The mean time for the tumors to reach the endpoint volume of 1000 mm3 (Table 1) and the statistical analysis (Table 2) indicate that BLM delays tumor growth by approximately 1 day (P < 0.05). There was no effect of light or AlPcS2a alone on tumor growth in BLM-treated mice. The PDT treatment, delaying the tumor growth by approximately 5 days, was significantly more effective than all other treatments except for the PCI treatment. The treatment with PCI of BLM was significantly more effective than all other treatments, inducing a mean growth delay of 21 days compared with the controls and 15 days compared with PDT (P < 0.001). The PCI of BLM treatment was found to act in a synergistic manner (P < 0.001) as compared with the expected sum of the individual treatments BLM and PDT.
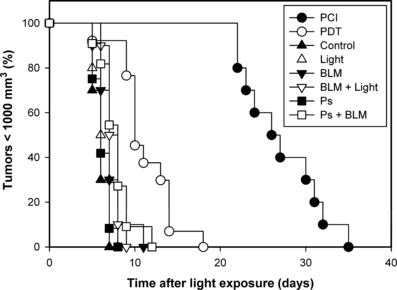
Kaplan–Meier plots of responses to treatment of HT1080 tumors with 1500 IU bleomycin (BLM) with and without combination with photochemical treatment. The various treatments are indicated in the figure. The endpoint is the time after light exposure when the individual tumors have reached a tumor volume of 1000 mm3. The results are based on interpolations of the measured tumor volumes. For clarity, all data points are not shown.
| Group | No. of animals | Mean time to reach 1000 m3 | SE |
|---|---|---|---|
| Control | 10 | 6.0 | 0.26 |
| AlPcS2a | 12 | 6.3 | 0.28 |
| Light only | 10 | 6.6 | 0.37 |
| BLM only | 10 | 7.2 | 0.51 |
| BLM + light | 10 | 7.5 | 0.27 |
| AlPcS2a + BLM | 11 | 7.8 | 0.55 |
| PDT | 13 | 11.3 | 0.90 |
| PCI | 10 | 26.6 | 1.35 |
- SE, standard error; BLM, bleomycin; PDT, photodynamic therapy; PCI, photochemical internalization.
| Treatment | Treatment groups | ||||||
|---|---|---|---|---|---|---|---|
| Control | Light only | BLM only | BLM + light | AlPcS2a | AlPcS2a + BLM | PDT | |
| Control | |||||||
| Light only | 0.135 | ||||||
| AlPcS2a | 0.460 | 0.346 | 0.102 | 0.008 | |||
| BLM only | 0.031 | 0.481 | |||||
| BLM + light | 0.001 | 0.111 | 0.608 | ||||
| AlPcS2a + BLM | 0.003 | 0.071 | 0.291 | 0.509 | 0.009 | ||
| PDT | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | |
| PCI | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
- BLM, bleomycin; PDT, photodynamic therapy; PCI, photochemical internalization.
- The values are based on paired log-rank analyses of the time for the HT1080 tumors to reach 1000 mm3.
MRI evaluation of treatment response
The effect of PCI and PDT on the tumor perfusion was evaluated by subjecting the animals to CE-MRI with Gd-DTPA as contrast agent. Prior to treatment and contrast administration, the T1-weighted images revealed a rim of slightly higher intensity in the tumor periphery, indicating possible hemorrhagic areas in the transitional zone between tumor and muscle compared with the central zone (Fig. 3a). The rim was not dramatically altered after treatment (Fig. 3b). After contrast administration but before treatment, the tumors including the surrounding transitional zone were clearly demarked by the high uptake of Gd-DTPA (Fig. 3c). Two hours after PDT or PCI, the uptake of Gd-DTPA was completely blocked in the tumor center (Fig. 3d). However, in the peripheral zone, the Gd-DTPA uptake was still high. The T1-weighted images recorded prior to contrast administration were subtracted from the T1-weighted images recorded after contrast administration to eliminate background intensity (Fig. 3e,f). The subtraction images computed from CE-MRI after PDT clearly verify a peripheral zone with well-perfused tissue and a central nonperfused zone (Fig. 3f). The color-coded concentration images, superimposed on the T1-weighted CE-MRI images, indicate that the peripheral zone is unequally affected by the light exposure. After treatment, the peripheral zone located profound for tumor has higher Gd-DTPA uptake than the peripheral zone superficially to the tumor (i.e. closer to the light source) (Fig. 3h). These two areas had equally high uptake of Gd-DTPA before treatment (Fig. 3g). These results indicate a light fluence-dependent vascular shutdown in the peripheral zone but a higher fluence is required to inhibit peripheral perfusion than in the center of the tumor. The MRI study did not reveal any differences between AlPcS2a-PDT and AlPcS2a-PDT in combination with BLM (i.e. PCI of BLM) with respect to inhibition of vascular perfusion (3, 4).
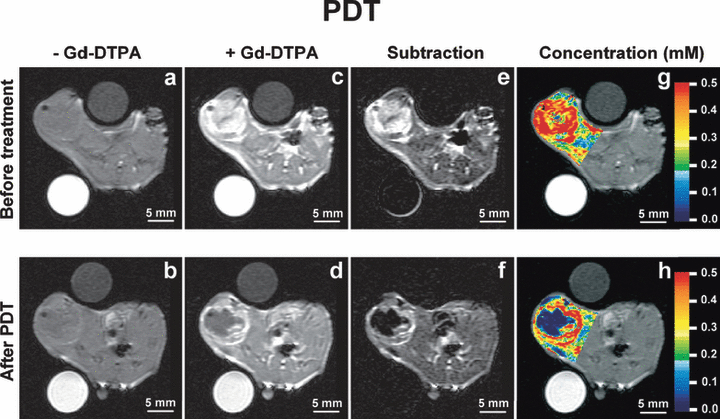
Contrast-enhanced magnetic resonance imaging (CE-MRI) before and 2 h after photodynamic therapy (PDT). (a) (b): T1-weighted images before contrast administration; (c) (d): T1-weighted images after contrast administration; (e) (f): background subtracted T1-weighted images; (g) (h): color-coded concentration images superimposed on the T1-weighted images. The concentration scale (mm) is given by the color bar. The upper panels (a,c,e,g) refer to the CE-MRI session prior to treatment, whereas the lower panels (b,d,f,h) refer to the CE-MRI session 2 h after PDT.
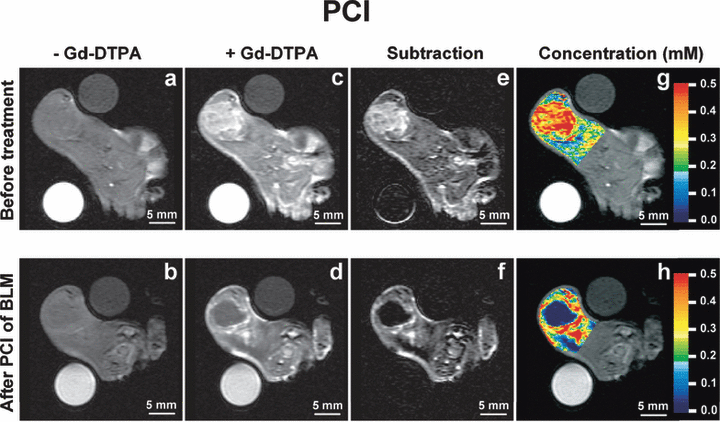
Contrast-enhanced magnetic resonance imaging (CE-MRI) before and 2 h after photochemical internalization (PCI). (a) (b): T1-weighted images before contrast administration; (c) (d): T1-weighted images after contrast administration; (e) (f): background subtracted T1-weighted images; (g) (h): color-coded concentration images superimposed on the T1-weighted images. The concentration scale (mm) is given by the color bar. The upper panels (a,c,e,g) refer to the CE-MRI session prior to treatment, whereas the lower panels (b,d,f,h) refer to the CE-MRI session 2 h after PCI.
Histological evaluation of treatment response
HT1080 is well perfused and appears with small areas of focal necrotic tissue when increasing in size (Fig. 5a). Seven days after the PDT, large areas of viable tissue were observed in the tumor periphery together with central necrosis (Fig. 5c,d). After the PCI of BLM, the central necrosis was dominating with only a thin rim of viable tumor tissue in the deep layer of the periphery (Fig. 5b).

Hematoxylin–eosin (HE)-stained histological sections after photodynamic therapy (PDT) and photochemical internalization (PCI) of bleomycin (BLM). The specimens are oriented with the superficial part, closest to the light source, heading up. The tumors were isolated 7 days after treatment. (a) Nontreated control tumor with dominating viable tumor cells (V) and small areas of necrotic tissue (N) nontreated tumor was isolated after 5 days. (b) After PCI of BLM, only a thin rim of viable tumor tissue (white arrow) was found adjacent to the deep muscle layer. The necrotic tumor tissue (N) is dominating. (c) (d) After PDT, large areas of viable tumor tissue (V) is found in the tumor periphery both superficial (white arrow in d) and deep to the necrotic central tumor tissue (N). Scale bar 1 mm.
In vivo localization of AlPcS2a
To reveal the causes of vascular shutdown and the low responsiveness of the transitional zone to PDT, the spatial distribution of AlPcS2a was evaluated by analyzing the fluorescence in frozen sections at the time of illumination. The fluorescence from a photosensitizer correlates well with its treatment effect. Fluorescent measurements is therefore a relevant measure of photochemically active photosensitizer (11,12). In the center of the tumor, AlPcS2a was relatively homogeneously distributed. However, in the tumor periphery AlPcS2a fluorescence slightly declined towards the tumor border and continued to decline in the muscle proportional with distance to the tumor tissue (Fig. 6). In the transitional zone, a peak of average AlPcS2a accumulation was detected. This area was of high cellular density (Fig. 7, square) with different morphology than the tumor (Fig. 7, asterisk) and the muscle (Fig. 7, arrow) cells. Furthermore, AlPcS2a was localized in the extracellular matrix surrounding the muscle rather than specifically in the muscle cells. The tumor cells in the transitional zone seemed to contain AlPcS2a levels comparable to those in the central tumor. Hence, the peak seen in Fig. 6 does represent not only the high cellular density area but also the tumor cells containing AlPcS2a.
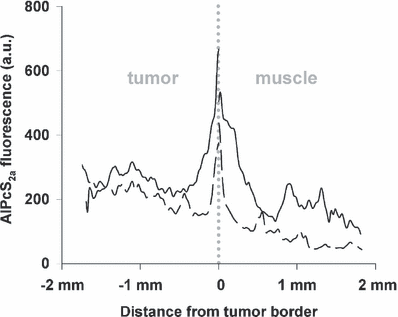
Semiquantitative analysis of AlPcS2a fluorescence distribution at various points related to the tumor-muscle transitional zone. Biodistribution of AlPcS2a as a function of distance from the tumor border. Each line represents one animal, and is the average fluorescence intensity of a section through a tumor and its surrounding muscle tissue.
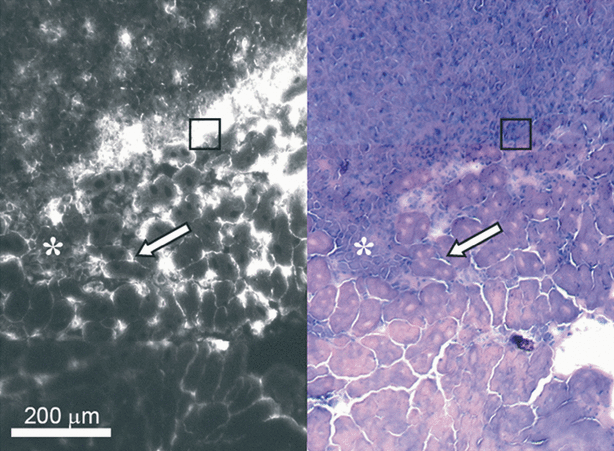
Distribution of the AlPcS2a in tumor and adjacent muscle 48 h after i.p injection of 10mg kg−1. AlPcS2a fluorescence from a frozen section is shown on the left side and the corresponding HE staining of the same section on the right side. The asterisk indicates area of tumor infiltration. The arrow points to muscle fibers adjacent to the tumor. Note that AlPcS2a is not particularly enriched inside the myocytes but located in the interstitium, see text. The square is located in an area of very intense fluorescence in the transitional zone. In the corresponding HE-stained section, one can see cells apparently morphologically different from muscle and tumor cells. See text.
In HT1080 tumors, the larger vessels are sparse and difficult to detect on standard HE-stained sections (13). To detect endothelial localization of AlPcS2a, sections of muscle tissue were evaluated. As shown in Fig. 8, AlPcS2a was found located to the endothelium of larger vessels in the contralateral leg 48 h after administration. There was no sign of photosensitizer in the vascular lumen at this time.
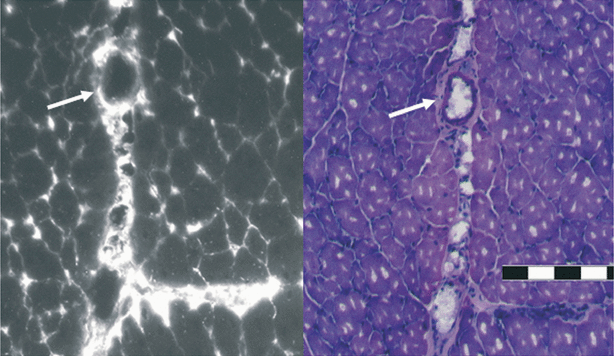
Intramuscular distribution of AlPcS2a. The distribution of AlPcS2a in the right gastrocnemius muscle 48 h after i.p injection of 10 mg kg−1 AlPcS2a. AlPcS2a fluorescence from a frozen section is shown on the left side and the corresponding HE staining of the same section on the right side. The pictures indicate AlPcS2a in the vascular endothelium and not in the lumen. A white arrow points to the endothelium. Scale bar 200 μm.
Discussion
Disease-free survival after treatment of STS in humans is associated with resection margin, tumor size, malignancy grade, relation to deep fascia and certain histological findings like necrotic areas and infiltrative growth (1,14). In the model used in the present study, the tumor is localized deep in the gastrocnemius muscle, showing an infiltrative growth pattern in the transitional zone (Fig. 1), resulting in a fast expanding aggressive tumor similar to a STS with high malignancy grade in human. Intramuscular HT1080 tumors in mice share many fundamental characteristics with STS tumors in humans and are therefore of high clinical relevance.
In previous in vivo PCI studies, subcutaneous tumors have been utilized. This is the first PCI study on an invasive and more demanding tumor model. Exploring the effect of PCI on an orthotopic, deep and invasive sarcoma is essential for evaluating the clinical potential of the PCI technology.
The PCI of BLM was found to retard growth of the HT1080 tumors in a synergistic manner in accordance with our previous report on three subcutaneously growing tumors (4). The synergistic response to the combination treatment is strong in all the four tumor models. In contrast to the response in the WiDr colon adenocarcinoma and the TAX-1 fibrosarcoma models, the HT1080 model used in this study and the previously reported subcutaneously growing CT26 colon carcinoma induced only retardation of tumor growth and not any complete responses. The treatment doses used in these studies have been selected to evaluate synergisms and to explore the mechanisms of action and have not been optimized with respect to treatment outcome. Thus, curative response may be obtained by raising the PDT and/or BLM doses. Indeed, almost complete response (>80%) has been obtained after PCI of BLM in another CT26 model with a significantly lower cure rate after PDT (O.-J. Norum and K. Berg, unpublished data).
The growth kinetics of the HT1080 tumors is comparable to that of the CT26 tumors, i.e. time to reach a 10-fold increase in tumor volume in untreated tumors is 6 and 7 days, respectively. The response to BLM and PDT appeared similar, i.e. 25% and 80–90% increase in time to reach 1000 mm3 after BLM and PDT, respectively. The delay in tumor growth after PCI of BLM appeared to be larger in the HT1080 tumors as compared with the CT26 tumors (340%vs 180% increased time to reach 1000 mm3). Thus, the invasive properties of the HT1080 tumor do not appear to influence negatively on the synergism. PCI of BLM seems therefore to be a treatment modality adequate for treatment of invasive cancers.
CE-MRI with Gd-DTPA is reported being a valid method of evaluating the initial vascular response to PDT (15). The MRI-based evaluation of the treatment response to AlPcS2a-PDT and AlPcS2a-PDT in combination with BLM (i.e. PCI of BLM) 2 h after light exposure revealed a complete blockage of Gd-DTPA uptake in the central zone of the HT1080 tumors. This indicates an inhibition of tumor perfusion. As the BLM treatment through PCI of BLM did not inhibit the perfusion level in tumors beyond that resulting from PDT alone, it suggests that the inhibition of tumor perfusion is mainly due to the PDT element in the PCI-treated tumors (Fig. 4).
The irreversibility of the inhibited perfusion after PDT and PCI is reflected in the central necrosis seen in the histological sections (Fig. 5). Assuming that the AlPcS2a-PDT induced blockage of the perfusion in the central zone of the tumor leads to irreversible ischemic cell death, the remaining peripheral zone is playing an important role in explaining the observed differences in growth inhibition on Kaplan–Meier plots (Fig. 2) seen between AlPcS2a-PDT in combination with BLM (i.e. PCI of BLM) compared to AlPcS2a-PDT. Previously we have reported that the increased effect of PCI was due to increased therapeutic effect deeper into the tumor tissue (6). This study indicates the additional role of the peripheral zone, both superficial and deep in the tissue, in explaining the difference in treatment effect. The histology shows only a rim of viable tumor cells in the tumor border that was clearly thinner after PCI than after PDT (Fig. 5b–d). It should be noted that viable tumor cells were observed regularly after PDT in the tumor periphery closest to the skin. As the light penetration through tissue decreases exponentially, the sensitivity of the tumor periphery to PDT must be dramatically lower than that of the central part of the tumor. Based on interstitial fluence rate measurements in subcutaneous sarcoma xenografts (E. Angell-Petersen and K. Berg, unpublished data), the fluence 3–4 mm into the tumors were about three times lower than in the superficial layers of the tumor. Altogether, these results indicate that better treatment effect conducted by PCI of BLM than by PDT is due to its effect in the peripheral zone of the tumor, close to the normal surrounding tissue and not only due to better efficacy in the deeper tissue layers as previously reported. It may also be deduced from the present study that PCI exerts a stronger cytotoxic effect than PDT irrespective of the fluence received by the tissue. This conclusion may be drawn by evaluating the regrowth of tumor tissue located superficial and deep. The superficial half of the tumor (located near the skin/surface) may be regarded as a model receiving high fluences and the lower and deeper located part of the tumor may be regarded as a model receiving low fluences. As the therapeutic effect evaluated on histological sections in Fig. 5d is creating a “mirror image” with a central necrotic part and a peripheral viable part after PDT both in high fluence regions and low fluence regions, this indicates that the phenomenon of reduced sensitivity to PDT in the peripheral part is true for different fluences. The cytotoxic effect in the periphery after PCI may be induced by direct BLM effect on the tumor parenchyma cells or be due to BLM targeting of the vascular endothelial cells resulting in a more slowly induced vascular shutdown.
The vascular response to PDT has been extensively described and is regarded as one of the main mechanisms causing therapeutic response to PDT. Vascular leakage, platelet aggregation, vasoconstriction or dilatation leading to hemorrhage and/or complete stasis are well-known observations in experimental tumor systems after PDT (16). The impact of vascular targeting depends however on the photosensitizer and on the drug–light interval. In comparison to many other photosensitizers, e.g. AlPcS4 and Photofrin, AlPcS2a seems to target the tumor parenchyma cells to a higher extent than the vasculature. However, the in vitro tumor cell survival decline with time for which the tumors remained in situ following in vivo AlPcS2a-PDT, indicating a vascular component in the treatment effect of AlPcS2a-PDT (16). Chan et al. also reported a reduction of about 50% 24 h in blood flow after AlPcS2a, as revealed by tumor retention of 99mTc-MIBI (technetium-99m-hexakis-2-methoxy-isobutyl isonitrile) (17). The present MRI analyses are in accordance with previous reports, but indicate that the vascular response is spatially dependent in that the vascular shutdown is predominantly in the center of the tumor. The previously reported incomplete inhibition of tumor perfusion after AlPcS2a-PDT, which reflects the mean perfusion of the whole tumor volume, may therefore reflect the sum of areas with complete inhibition of perfusion and unaffected vasculature.
We have previously reported that AlPcS2a is mainly intracellular and only to a minor extent localized in the vascular lumen 48 h after administration (18). These results, together with the indication of AlPcS2a localization in the endothelial cells (Fig. 8), point towards endothelial cell targeting as the cause of the reduced perfusion in the HT1080 tumors after AlPcS2a-PDT, though it is difficult to trace the vessels in the HT1080 tumors as previously reported (13).
The low response to PDT in the tumor periphery correlates with the unchanged perfusion rate in this tissue area after PDT. However, AlPcS2a has been reported to accumulate in the tumor cells in vivo and the tumor cells in the tumor periphery could therefore be expected to be sensitized to photochemical inactivation. AlPcS2a was found to be relatively homogeneously distributed in the tumor and the tumor cells in the transitional zone seemed to contain AlPcS2a at a level comparable to that deeper into the tumor (Fig. 7). Thus, the low response to PDT in the tumor periphery cannot be explained by a lower uptake of AlPcS2a into the tumor cells.
The AlPcS2a was relatively homogeneously distributed in the central area of the tumor declining towards the lower concentration in the muscle tissue. This is in accordance with the previous reports (8,19) showing a higher content of photosensitizers in normal tissue adjacent to the tumor tissue. In the transitional zone there was however an area with high content of AlPcS2a fluorescence located between the muscle bundles which appears not to be enriched in the tumor cells. In the transitional zone (Fig. 7), the neovascularization is particularly active and typically the endothelial lining is less well organized than in the more mature parts of the tumor (20,21). In this area, extravasation in general is exaggerated and may cause increased extravasation of AlPcS2a. In the transitional zone, there may be many inflammatory cells, like macrophages, known to have especially high endocytotic rate and thereby retaining high amounts of AlPcS2a (22,23). Further studies are required to evaluate the impact of this difference in the vasculature on the lower sensitivity to AlPcS2a-PDT and to reveal the cause of the highly fluorescent cells in the transitional zone. The cause of the striking differences in PDT-induced vascular shutdown in the peripheral zone and the central part of the tumor is beyond the focus of this study. However, it cannot be explained in a simple manner from fluorescence studies or attenuation of light due to absorption in the tissue. The explanation might be increased resistance to peripheral vasculature or increased resistance to peripheral tumor cells.
In this study, we have demonstrated that the PCI technology is effective in an invasive tumor model, showing synergistic effect in combination with BLM. This is an important prerequisite for future clinical application to invasive human cancers. Further, we have demonstrated that central vascular shutdown is a significant element in the initial response to both PDT and PCI. Previous reports have indicated that the PCI technology can induce necrosis in deeper tissue layers than PDT and that this is the main cause of the superior therapeutic effect of PCI as compared to PDT (6). The present results indicate that the observed difference in growth retardation between PDT and PCI is in addition originating from the peripheral zone.
Acknowledgments
Acknowledgement— Thanks to Marie-Therese Roppestad Strand for skilful technical assistance. This study has been financially supported by the Norwegian Radium Hospital Research Foundation and the Norwegian Cancer Society.




