The Microenvironment of DNA Switches the Activity of Singlet Oxygen Generation Photosensitized by Berberine and Palmatine
Abstract
The effect of the interaction between DNA and the photosensitizer on photosensitized singlet oxygen (1O2) generation was investigated using DNA-binding alkaloids, berberine and palmatine. These photosensitizers were bound to DNA by electrostatic force. Near-infrared luminescence measurement demonstrated that the photoexcited alkaloids can generate 1O2 only when the photosensitizers are bound to DNA. A fluorescence decay study showed significant enhancement of the lifetime of their photoexcited state with the DNA binding. A calculation study suggested that the electrostatic interaction with DNA inhibits the quenching of the photoexcited state of these alkaloids via intramolecular electron transfer, leading to the prolongation of the lifetime of their excited state. This effect should enhance their intersystem crossing and the yield of energy transfer to molecular oxygen. The results show that the electrostatic interaction with DNA significantly affects the 1O2 generation activity of a photosensitizer. In addition, this interaction may be applied to the control and the design of photosensitizers for medical applications such as photodynamic therapy.
Introduction
Photosensitized generation of reactive oxygen species (ROS) contributes to phototoxicity and photocarcinogenesis (1–7). Furthermore, this process is important in the medical application of photosensitized reactions such as photodynamic therapy (PDT) (8–10). PDT is a relatively new treatment for cancer and other nonmalignant conditions that induces oxidative stress primarily through the light-mediated production of singlet oxygen (1O2). The generation of 1O2 requires oxygen, light and a photosensitizing drug. Light exposure activates the drug and in the presence of O2 produces 1O2 through Type II energy transfer processes (11–13). Critical sites of the generated 1O2 include mitochondria and lipid membranes (8–11,14). Moreover, DNA is also an important target biomolecule of photosensitized reactions (1–5,15–18). As the administered photosensitizers necessarily interact with cellular components, the photosensitized reaction occurs in a microenvironment consisting of biomolecules (12). Therefore, the interaction between biomolecules such as DNA and photosensitizers plays an important role in the photosensitized reaction and may be applied to the control of the activity of PDT photosensitizers (19).
Berberine and palmatine (Fig. 1) are the alkaloid constituents of goldenseal (Hydrastis canadensis L.) (20), and display cytotoxic activities against various human cancer cell lines (21). Their phototoxicity and DNA-photodamaging abilities have been also reported (22–24). These alkaloids bind to the DNA (25–28) and form the fluorescent intermolecular complexes (24,29) with DNA. It has been reported that berberine binds preferentially to the AT-rich minor groove (27), and the binding property of palmatine is consistent with a mixed-mode DNA binding model in which a portion of the ligand molecule intercalates into the duplex, while the nonintercalated portion protrudes into the minor groove (26). The DNA-binding interaction changes the photochemical property and markedly enhances the fluorescence intensity of these alkaloids. The chemical property of alkaloids is useful in designing an experimental system to clarify the environmental effects of DNA, one of the most important biomaterials, on a photosensitized reaction. Moreover, the microenvironmental effect of the DNA strand should be one of the key factors in controlling the activity of photosensitizers, of which the target biomolecule is DNA. In this study, the photosensitized 1O2-generation activity and the photochemical property of these alkaloids were examined.
Structures of berberine and palmatine.
Materials and Methods
Materials. Berberine chloride and sodium azide were from Wako Chemicals Co. (Osaka, Japan). Palmatine chloride was from Aldrich Chemicals Co. (Milwaukee, WI). Calf thymus DNA was from Sigma Chemical Co. (St. Louis, MO). D2O was from Across Organics (Morris Plains, NJ). The spectroscopic grade solvents of acetonitrile (MeCN), dichloromethane (CH2Cl2), dimethyl sulfoxide (DMSO), ethanol (EtOH) and methanol (MeOH) were from Dojin Chemicals Co. (Kumamoto, Japan) and used as received.
Measurement of absorption and fluorescence spectra of berberine and palmatine. Absorption spectra of berberine and palmatine were measured with a UV–Vis spectrophotometer (UV-1650PC; Shimadzu, Kyoto, Japan). Fluorescence spectra of berberine and palmatine were measured with a RF-1500PC spectrophotometer (Shimadzu) on 365 nm excitation. The samples contained 50 μm berberine or palmatine with or without calf thymus DNA in 10 mm sodium phosphate buffer (pH 7.6). The binding constant (K) between DNA and the alkaloids was estimated according to a previous report (24). Free energy change (ΔG) was calculated from the binding constants using the equation: ΔG = −RT ln K. The enthalpy change (ΔH) and the entropy change (ΔS) were calculated using the equations: d(ln K)/dT = ΔH/RT2 and ΔS = (ΔH − ΔG)/T, respectively.
Detection of near-infrared luminescence from 1O2. 1O2 generation was directly measured by near-infrared luminescence around 1270 nm from deactivated 1O2, which corresponds to the 1O2(1Δg)−3O2(3Σg−) transition. The sample solutions contained 50 μm berberine or palmatine, with or without calf thymus DNA in D2O. A direct detection system which consists of a Nd:YAG laser (THG/355 nm, 30 Hz; Tempest-30, New Wave Research) as an excitation light source (355 nm, intensity: 280 mW cm−2), a quartz cuvette as an irradiation cell, a spectroscope and a near-infrared gated multi-channel detector ICCD camera (NIR-II; Hamamatsu Photonics, Shizuoka, Japan) has been built (30,31). Gate time and accumulation time are 5–50 μs after laser pulse and 128 s (total: 36 J cm−2), respectively.
Time-correlated single photon counting spectrofluorimetry. Fluorescence decays of the alkaloids were measured by a time-correlated single photon counting technique using FluoTime 100 (PicoQuant GmbH, Berlin, Germany) (excitation wavelength: 360 nm, detection wavelength >550 nm). The sample solution contained 50 μm berberine or palmatine with or without 100 bp-μm calf thymus DNA.
Measurement of fluorescence quantum yields. Fluorescence quantum yields of berberine and palmatine in solvents were measured with an absolute PL quantum yield measurement system C9920-02 (Hamamatsu Photonics).
Calculations of intermolecular complex between DNA and the alkaloids. The equilibrium geometry of an intermolecular complex between double-stranded DNA and berberine or palmatine was obtained by molecular mechanics calculation utilizing Spartan 04’ (Wavefunction, Inc., CA). The geometry of 20-mer of double-stranded DNA was constructed using Spartan 04’. The absorption transitions of these alkaloids binding to DNA were calculated by the semi-empirical Zerner’s intermediate neglect of differential overlap (ZINDO) procedure utilizing the CAChe WorkSystem Pro 6.0 (Fujitsu Ltd. 2003, Tokyo, Japan). The energy of their orbitals was estimated by DFT (B3LYP/6-31G*) calculation utilizing Spartan 04’.
Results
Binding interaction between the alkaloids and calf thymus DNA
Berberine and palmatine formed binding complexes with DNA, resulting in fluorescence enhancement (24,29). Fluorescence intensity of these DNA–alkaloid complexes was decreased by the addition of sodium chloride (Fig. 2), suggesting that the interaction between alkaloids and DNA was caused by electrostatic force (32,33). The binding constant (K) of berberine and palmatine to DNA was estimated from an analysis of their fluorescence enhancement according to a previous report (24). The values of K decreased along with a rise in temperature. The resulting thermodynamic parameters are listed in Table 1. These parameters, ΔG, ΔH and ΔS of berberine are almost similar to those of palmatine. The negative values of ΔH show that these photosensitizers are stabilized through electrostatic interaction in the minor groove. The positive values of ΔS suggest that the binding of photosensitizers causes the removal of water from DNA.
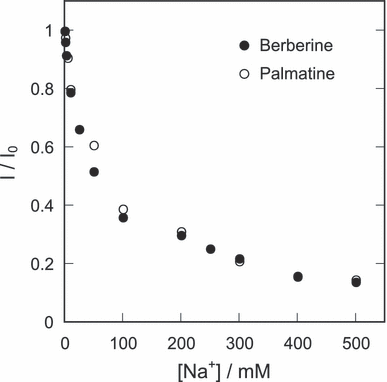
Dependence of the fluorescence intensity of the DNA-binding alkaloids on the concentration of sodium chloride. The sample solution contains 50 μm berberine or palmatine, 100 bp-μm calf thymus DNA and indicated concentration of sodium chloride in distilled water.
| Alkaloids | ΔG (kJ mol−1) | ΔH (kJ mol−1) | −TΔS (kJ mol−1 K−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|---|
| Berberine | −21.5 | −18.8 | −2.7 | 9.0 |
| Palmatine | −21.9 | −18.5 | −3.4 | 11.5 |
Photosensitized 1O2 generation by berberine and palmatine under interaction with DNA
Emission at ca 1270 nm was observed during photo-irradiation of the DNA–alkaloid complexes (Fig. 3). This emission was quenched by sodium azide, a scavenger of 1O2. More than 0.5 mm sodium azide completely quenched the emission (data not shown). In the absence of DNA, berberine or palmatine did not show any emission. These findings demonstrate that photoexcited alkaloids can generate 1O2 only when the DNA–alkaloid complex is formed. The 1O2 emission by photoexcited berberine and palmatine was not observed in ethanol (data not shown), in which the polarity of the surroundings is similar to that in the DNA minor groove (34). This study showed that the environment of the DNA strand activates 1O2 generation from the photosensitizer. The apparent quantum yields of 1O2 generation (ΦΔ) were estimated from the comparison of the 1O2 emission intensities by these alkaloids and methylene blue (ΦΔ = 0.52 in D2O) (35). The estimated value of ΦΔ increased in a dose-dependent manner up to 100 bp-μm of DNA addition (Fig. 4), indicating that the 1O2 generation activity depends on the DNA–alkaloid complex formation. ΦΔ decreased at concentrations over 100 bp-μm possibly due to the quenching of 1O2 by DNA.
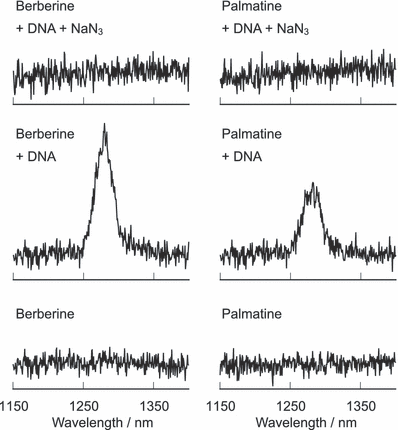
Emission spectra of 1O2 generated through photosensitized reaction of berberine and palmatine. The sample mixture contains 50 μm berberine or palmatine in D2O. Where indicated, the sample solution contains 100 μm/base calf thymus DNA and 50 mm sodium azide.
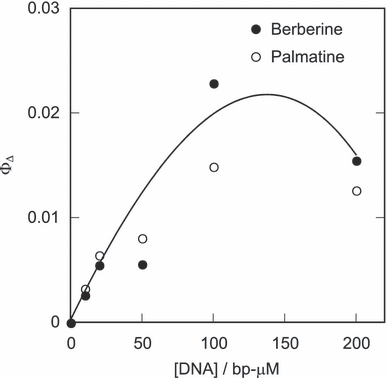
The apparent quantum yield of 1O2 generation. The sample mixture contains 50 μm berberine or palmatine and indicated concentrations of calf thymus DNA in D2O.
Lifetimes of photoexcited states of the alkaloids and their complexes with DNA
Fluorescence decay of the photoexcited alkaloids is shown in Fig. 5. Fluorescence intensities of berberine and palmatine rapidly decreased, and the curve was fitted by single-exponential decay and their photoexcited state lifetimes (τ) were 90 and 150 ps for berberine and palmatine, respectively. Their lifetimes significantly increased by the addition of DNA. Therefore, their fluorescence enhancement by DNA is due to the elongation of their lifetimes of the photoexcited singlet states (S1). The fluorescence decay curves in the presence of DNA were multi-exponential decay curves, suggesting the various conformations of the complexes and/or binding modes. Indeed, the various binding modes between DNA and these alkaloids other than the AT-rich minor groove binding should be possible (26–28). The S1 lifetimes of DNA–alkaloid complexes were roughly estimated from an assumption of three conformations (berberine: 90 ps [56%], 2.52 ns [36%], 8.67 ns [8%], palmatine: 150 ps [64%], 2.24 ns [31%], 7.07 ns [5%]). These results show that the lifetimes of the photoexcited states are prolonged about 20–80-fold by their complex formation.
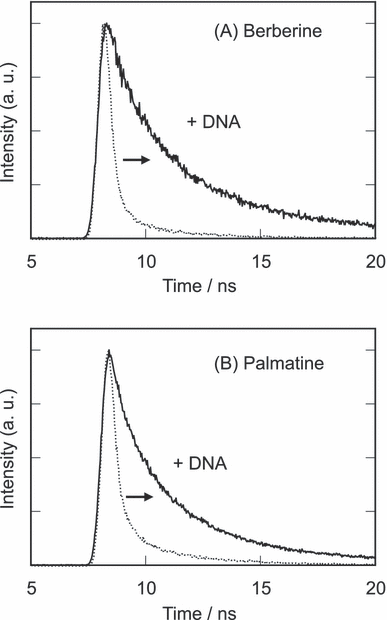
Fluorescence decay of berberine and palmatine. The sample mixture contains 50 μm berberine or palmatine with or without 100 bp-μm calf thymus DNA in 10 mm sodium phosphate buffer (pH 7.6). Excitation and detection wavelengths are 360 and >550 nm, respectively.
Absorption and fluorescence maximum of berberine and palmatine
The absorption maximum of these photosensitizers showed a blueshift depending on the solvent polarity (Fig. 6A), showing that this absorption is due to the n–π* transition. The absorption spectra of DNA-binding photosensitizers were estimated by subtracting the absorption spectra of free photosensitizers from those of DNA-containing photosensitizers (Fig. 7). The binding ratio was calculated from the binding constants and the DNA concentration. The obtained spectra of these alkaloids showed a large redshift through the complex formation with DNA in an aqueous solution. The long wavelength absorption maxima (berberine: 449 nm; palmatine: 445 nm) were similar to those in dichloromethane. This large redshift could not be explained by the reduced polarity effect of the DNA microenvironment, in which the polarity of surroundings is almost similar to that of ethanol (34). ZINDO calculation of absorption spectra of the alkaloids interacting with phosphate anion of DNA showed a large redshift (data not shown), suggesting that the spectral shifts by the DNA binding was due to the electrostatic interaction. Moreover, the ZINDO calculation showed that the structure change of these photosensitizers by the binding to DNA scarcely affected their spectra. Fluorescence maximum redshifted depending on the solvent polarity (Fig. 6B). The fluorescence maximum (berberine and palmatine: 527 nm) was shorter than that predicted from the polarity effect of DNA. These findings suggest that the photochemical property of these photosensitizers mainly depends on the electrostatic interaction with DNA rather than on other effects.
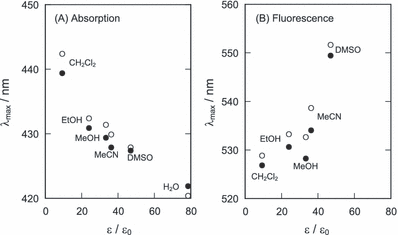
Dependence of the absorption (A) and the fluorescence (B) maxima of berberine (closed circle) and palmatine (open circle) on the relative dielectric constant of solvent. The values of the ε/ε0 of solvents are as follows: 9.1 (CH2Cl2), 23.8 (EtOH), 33.1 (MeOH), 36.0 (MeCN), 46.7 (DMSO) and 78.3 (H2O).
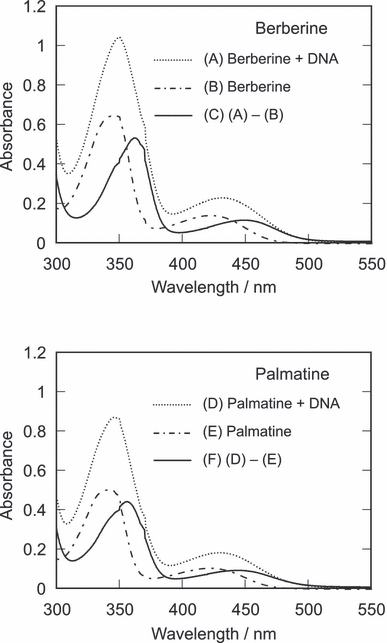
Absorption spectra of berberine and palmatine and their complexes with DNA. The sample contains 50 μm berberine (A) or palmatine (D) in the presence of 200 bp-μm calf thymus DNA in sodium phosphate buffer (pH 7.6). The absorption spectra of free berberine (B) and palmatine (E) in the above samples were calculated from their binding constants and concentrations of alkaloids and DNA. The absorption spectra of the DNA-alkaloids complexes were estimated by subtracting the spectra of free alkaloids from those of DNA-containing samples.
Solvent polarity dependence of fluorescence quantum yields of berberine and palmatine
Berberine and palmatine demonstrated relatively strong fluorescence in less polar solvent. The fluorescence quantum yields (Φf) were plotted against the solvent dielectric constant (Fig. 8). The values of Φf of these alkaloids decreased with an increase in solvent polarity. As electron transfer is enhanced in polar solvent (36), these results suggest that the quenching of their photoexcited states is through intramolecular electron transfer.
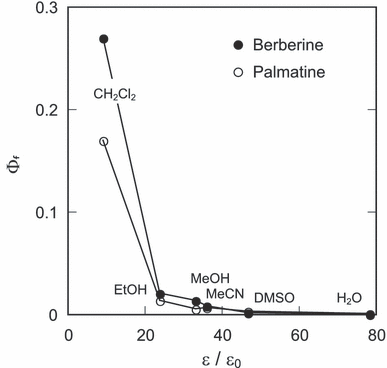
Dependence of fluorescence quantum yields of berberine and palmatine on the relative dielectric constant of solvent. Values of ε/ε0 are presented in the caption of Fig. 6.
Calculation study of berberine and palmatine
Berberine and palmatine consist of isoquinoline moiety and dimethoxy benzene moiety. The absorption spectrum of the isoquinoline moiety was calculated from the ZINDO method (Fig. 9). The comparison between the observed and calculated absorption spectra showed that the S1 state of berberine and palmatine is excitation of the isoquinoline moiety. The DFT calculation showed that the highest occupied molecular orbital (HOMO) of these alkaloids is localized on the dimethoxy benzene moiety (data not shown). These calculation results suggest that S1 of berberine and palmatine can be quenched through intramolecular electron transfer from their dimethoxybenzene moiety and the short photoexcited lifetimes of these alkaloids are due to this effect.
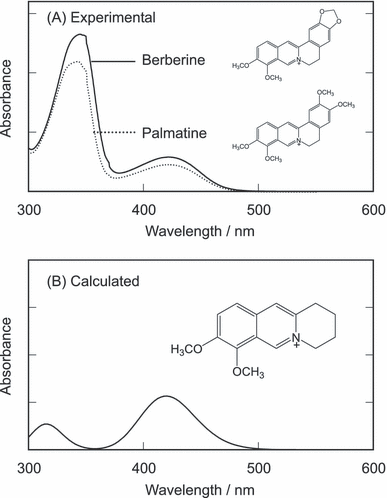
Observed absorption spectra of berberine and palmatine and the calculated absorption spectrum of the isoquinoline moiety.
Discussion
There is wide agreement that ROS play a key role in both constructive and destructive fundamental biological processes. The photosensitized ROS generation is the initial event of PDT. DNA is one of the most important target biomolecules of ROS. Berberine and palmatine bind to DNA by an electrostatic interaction, leading to the formation of a fluorescent inter-molecular complex (24,29). The thermodynamic study showed that DNA binding is enthalpy driven. The activity of photosensitizers in generating 1O2 is sensitive to their surroundings. The photosensitized 1O2 generation from berberine and palmatine was markedly enhanced by DNA binding. The typical emission from 1O2 at ca 1270 nm was observed during photo-irradiation of the DNA–alkaloid complexes. In the absence of DNA, the photo-irradiated alkaloids showed no emission around 1270 nm in aqueous solution. These findings demonstrate that the photoexcited alkaloids can generate 1O2 only when the DNA–alkaloid complex is formed. 1O2 emission by photoexcited berberine and palmatine was not observed in ethanol, in which the polarity of the surroundings is similar to that in the DNA minor groove (34). This study showed that the microenvironment of the DNA strand activates 1O2 generation of the photosensitizer.
The S1 state of these alkaloids immediately returns to the ground state via a nonradiative mechanism (22,23). This rapid deactivation should be the reason for the decreased 1O2 generation by photosensitized berberine and palmatine in an aqueous solution (Fig. 10). The measurement of the fluorescence decay demonstrated that the DNA-binding interaction stabilizes the photoexcited states of berberine and palmatine, resulting in the enhancement of their S1 lifetimes. The ZINDO calculation showed that the S1 state is produced by an excitation of their isoquinoline moieties. The DFT calculation showed that their HOMOs are localized on their dimethoxybenzene moieties. Therefore, the S1 of berberine and palmatine can be quenched through intramolecular electron transfer from their dimethoxybenzene moieties. Their fluorescence quantum yields were very small in polar solvents and increased in a less polar solvent. As electron transfer is promoted depending on the polarity of the surroundings (36), this solvent effect supports the quenching via intramolecular electron transfer. An inhibition of the intramolecular electron transfer can increase the lifetime of the photoexcited states of these alkaloids. The spectral measurements suggest that the photochemical property of these alkaloids is mainly affected by the electrostatic interaction with anionic polymer DNA. Indeed, the interaction between the alkaloids and an anionic polymer, poly(sodium 4-styreneslfonate), also demonstrated similar effects—enhancement of fluorescence emission and 1O2 generation in aqueous solution (data not shown). The electrostatic interaction between the positive charge of the alkaloids and the negative charge of the phosphate group of DNA should increase the energy levels of the isoquinoline moieties of berberine and palmatine. The large redshift of their spectra of these alkaloids by DNA binding is possibly due to the electrostatic interaction. Therefore, the levels of the charge transfer (CT) states are raised through this interaction, leading to the inhibition of the intramolecular electron transfer and a prolongation of their photoexcited states. Consequently, the intersystem crossing yields of these alkaloids should be increased by DNA binding, resulting in the enhancement of energy transfer to 3O2.
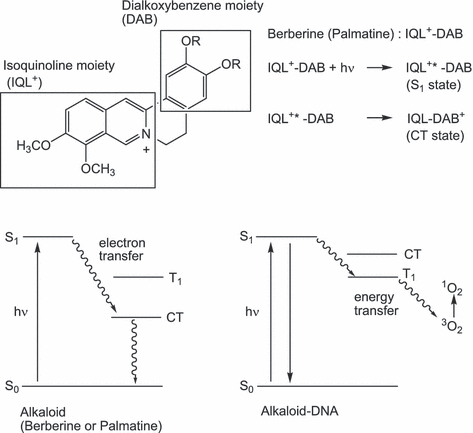
Energy diagram of berberine, palmatine and their complexes with DNA.
Photoexcited states of these photosensitizers are prolonged in the DNA microenvironment, leading to the enhancement of photochemical reactions. During irradiation of these photosensitizers, superoxide was not detected in the experiment using cytochrome c and superoxide dismutase as reported previously (37) (data not shown). Visible-light photosensitizers can hardly reduce molecular oxygen into superoxide because of the large reorganization energy of an oxygen molecule (38,39). In the mechanism of DNA damage by photoexcited berberine and palmatine, contributions of other types of ROS or photo-induced electron transfer were not observed (24). These results show that berberine and palmatine can induce biomolecular damage only through photosensitized 1O2 generation. 1O2 is the major oxidative and damaging species formed during the Type II process of photosensitization (11–13,17) and plays an important role in the process of PDT (8–10). 1O2 is able to induce the oxidation of cellular DNA (3,4). 1O2 can diffuse in a very short distance during its lifetime, which is much shorter in the cell (0.01–0.2 μs) than in simple aqueous solutions (2–4 μs) (11,13). Therefore, the contact of a photosensitizer with biomacromolecules, such as DNA, is very important. In addition, control of key therapeutic parameters, including 1O2 generation level, is also important and optimized synthetic procedures of PDT photosensitizers have been developed (19,40). The switch of 1O2 generation activity of photosensitizers through contact with biomolecules is advantageous for the development of functional PDT photosensitizers.
In summary, this study demonstrated that berberine and palmatine bind to DNA, and their activity in the photosensitized 1O2 generation is markedly enhanced. This study showed that the electrostatic interaction with a DNA strand can change the 1O2 generation activity of photosensitizers. This mechanism through the interaction with the DNA microenvironment may be applicable to the activity control of the PDT photosensitizers.
Acknowledgments
Acknowledgements— This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (417) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of the Japanese Government and NST Co., Ltd.




