The Impact of Complement Activation on Tumor Oxygenation During Photodynamic Therapy†
This invited paper is part of the Symposium-in-Print: Photodynamic Therapy.
Abstract
The response to photodynamic therapy (PDT) mediated by photosensitizer Photofrin was examined with Lewis lung carcinomas growing in either complement-proficient C57BL/6 (B6) or complement-deficient complement C3 knockout (C3KO) mice. The results reveal that Photofrin-PDT was more effective in attaining cures of tumors in C3KO than in B6 hosts. Colony-forming ability of cells from tumors excised immediately after Photofrin-PDT confirmed that the direct cell killing effect was more pronounced in C3KO than in B6 hosts. In contrast, PDT mediated by photosensitizer benzoporphyrin derivative (BPD) produced higher cure rates of tumors in B6 hosts than those in C3KO hosts. Determination of tumor C3 levels by ELISA showed that Photofrin-PDT induced markedly more pronounced complement activation than BPD-PDT. Measurements of tumor oxygen tension immediately after PDT by Eppendorf pO2 histograph showed that Photofrin-PDT induced a marked decline in the oxygenation of tumors growing in B6 mice that was much less pronounced in C3KO hosts. With BPD-PDT the oxygen tensions in tumors in B6 and C3KO hosts decreased to a similar extent. This study indicates that complement activation in PDT-treated tumors that varies with different photosensitizers is an important determinant of tumor oxygen limitation effects directly associated with photodynamic action.
Introduction
Photodynamic therapy (PDT) is a well-established minimally invasive clinical modality for the treatment of malignant and other lesions that exploits drugs (photosensitizers) activated by light focused on the targeted site (1,2). Solid tumors treated with PDT are eradicated by a combination of direct cytotoxic effect of lethal photooxidative lesions, damage induced in tumor vasculature and elicited host reaction (3–5). The host response is initiated as innate immunity-orchestrated tumor antigen-nonspecific protective reaction to tumor-localized injury inflicted by PDT that culminates in the development of adaptive immunity recognizing the treated tumor as its target (6,7). One of the major participants in this response is the complement system.
Functioning as a principal humoral immune effector system, complement integrates over 30 proteins into a cascade of unidirectional enzymatic reactions leading to the formation of anaphylatoxins and the membrane attack complex (MAC) (8,9). The complement system becomes engaged after binding of the recognition component of one of the three activation pathways (classical, lectin and alternative pathways) to an invading organism, other foreign cells or autologous cells altered by sustaining some form of injury. Recent investigations indicate that complement participates in the following important events within the tumor response to PDT: (1) initial recognition of the insult, (2) instigation and propagation of inflammatory response, (3) efferocytosis (dead cell removal) and (4) sustained development of antitumor adaptive immune response (6,10–12).
In the present study, complement-deficient mice and their normal (complement-proficient) counterparts were used as hosts of PDT-treated tumors for investigating the impact of complement activation during the photodynamic illumination on tumor oxygenation.
Materials and methods
Mice and tumor models. Lewis lung carcinoma and MCA205 fibrosarcoma were implanted in syngeneic mice, either C57BL/6 (B6, complement-proficient wild type) or complement-deficient B6.129S4-C3tm1Crr complement C3 knockout (C3KO). The latter have a knockout mutation in the gene encoding C3 component, which prevents the formation of C3 fragments, C5a anaphylatoxin and C5b9 terminal attack complex of the complement system (13). The mice were bred and kept in the Animal Research Centre of British Columbia Cancer Research Centre. They were housed in sterile cages, with food and water provided ad libitum. Subcutaneous tumors were initiated by inoculating 1 × 106 tumor cells into the lower dorsal region of 7- to 9-week-old female mice, as described earlier (14) and were treated 7–8 days later when the largest tumor diameter reached 7–8 mm. Complement depletion in mice prior to tumor therapy was achieved with cobra venom factor (CVF; Quidel Corporation, San Diego, CA) given at 2 U/mouse injected i.p. at 42 and 18 h before PDT light treatment. The animal protocols were approved by the Animal Care Committee of the University of British Columbia.
PDT treatment. Tumor-bearing mice received intravenous injection of Photofrin (Axcan Pharma, Inc., Mont-Saint-Hilaire, QC, Canada) at 7.5 or 10 mg kg−1, mTHPC (m-tetrahydoxyphenylchlorin; Biolitec AG, Edinburgh, UK) at 0.1 mg kg−1 or benzoporphyrin derivative (BPD) monoacid ring A, verteporfin formulation (provided by QLT, Inc., Vancouver, BC, Canada) at 2.5 mg kg−1; Photofrin and mTHPC were administered at 24 h before and BPD 3 h before tumor illumination. For Photofrin- and mTHPC-PDT, the light was generated by a FB-QTH-3 high-throughput illuminator (Sciencetech, Inc., London, ON, Canada) based on a 150 W QTH lamp and equipped with interference filters selecting light within 630 ± 10 nm (for Photofrin) or 650 ± 10 nm (for mTHPC). A 250 mW diode laser (SDL7422-H1; Spectra Diode Labs, San Jose, CA) generated 690 ± 1 nm light for BPD-PDT. A liquid light guide (8 mm core diameter; model 77638 by Oriel Instruments, Stratford, CT) was used to deliver monodirectional superficial illumination of the tumors with fluence rate 80–90 mW cm−2; the light doses were 150 J cm−2 for Photofrin-PDT, 100 J cm−2 for BPD-PDT and 20 or 80 J cm−2 for mTHPC-PDT. The mice were immobilized in special holders during tumor light treatment. The treatment area (light spot) encompassed the tumor and 1–1.5 mm of the surrounding skin. No significant increase in tumor temperature was induced by these treatments, as verified by a hypodermic thermocouple measurement. The mice were kept in the dark from the time of photosensitizer injection until 3 days postlight treatment. When tumor cure or regrowth was the endpoint, the mice (eight per treatment group) were monitored for signs of tumor recurrence (palpable growth) every second day and those showing no signs of recurrence at 90 days after PDT were considered cured.
Clonogenic assay. Mice were killed and Lewis lung carcinoma tumors (four per treatment group) were excised immediately following the termination of photodynamic light treatment and enzymatically digested for obtaining single-cell suspensions. Cell viability (based on trypan blue test) and yield per gram of tumor tissue were determined. Known numbers of cells were plated into petri dishes with Alpha Minimal Essential Medium (Sigma Chemical Co., St. Louis, MO) supplemented with 20% fetal bovine serum (HyClone Laboratories, Logan, UT) and left in a CO2 tissue culture incubator for colony-forming growth. Tumor cell colonies were counted 10 days later and the clonogenicity (colony-forming units per gram of tumor) was determined based on untreated control-normalized plating efficiency and cell yield (15).
C3 ELISA assay. Mice (four per treatment group) were killed and tumors excised at 3 h post-PDT light treatment. The levels of complement C3 proteins in Lewis lung carcinoma tissue homogenates were determined with sandwich ELISA assay as previously described (11). Briefly, wells were coated with 1:1000 dilution of goat anti-mouse C3 F(ab′)2 (Cappel Laboratories, Durham, NC), then test samples or C3 standard (protein isolated and purified from DAB/2J mice) were added and finally stained with horseradish peroxide (HRP)-conjugated goat anti-mouse C3 (Cappel) at 1:5000 dilution.
Tumor oxygen tension measurement. Partial pressure of oxygen (pO2) in PDT-treated tumors was measured in situ with polarographic needle electrodes using an Eppendorf histograph Model KIMOC 6650 (Eppendorf-Netherler-Hinz GmbH, Hamburg, Germany), following a procedure described earlier (16). The Eppendorf pO2 histograph is regarded as the “gold standard” for tissue measurements of pO2 (17): its reliability at low pO2 in tissue has been extensively characterized (18,19). Its needle sensors were calibrated before use according to the manufacturer’s instructions. The measurement was initiated immediately following PDT light treatment, with the insertion into the tumor of needle sensors (0.3 mm in diameter) that advanced automatically at 0.5 mm steps. In each tumor, three radial needle tracks with 7–12 advancements per track were performed. After the last step the needle sensor automatically retracked from the tumor tissue. During the measurement, the mice (five or more per treatment group) remained unanesthetized in restraining holders used for PDT treatment. The obtained data for pO2 frequency distributions were plotted on histograms.
Statistical analysis. Tumor response/cure evaluation was based on log-rank test. Nonparametric Wilcoxon matched pairs test was performed for analyzing pO2 measurements and Mann–Whitney test for remaining experiments. A threshold of statistical significance level of 5% was set for determining if the groups were statistically different.
Results
Lewis lung carcinomas were inoculated subcutaneously into syngeneic mice, either immunocompetent wild type (B6) or C3KO, from a common tumor cell suspension. No differences in growth rates or histological characteristics were found with the tumors growing in these two host types. Upon reaching the predetermined size (7–8 mm in largest diameter), the tumors were treated with Photofrin-PDT and then monitored up to 90 days for assessing the curative effects of these therapies. The results reveal that the tumors responded better to this therapy when growing in complement-deficient hosts. All Lewis lung carcinomas in complement-proficient hosts recurred within 2 weeks after Photofrin-PDT while around 30% of those in complement deficient mice were cured (Fig. 1a). The response of tumors growing in wild type mice depleted of complement by CVF pretreatment was very similar to that observed with C3KO mice, confirming that complement deficiency diminishes the resistance of tumors to treatment with PDT mediated by this photosensitizer. The CVF protocol, a classical intervention for achieving a highly effective consumption of C3 and C5b–9 complement components (20), produced no detectable effects on tumor growth when applied in the absence of PDT (not shown).
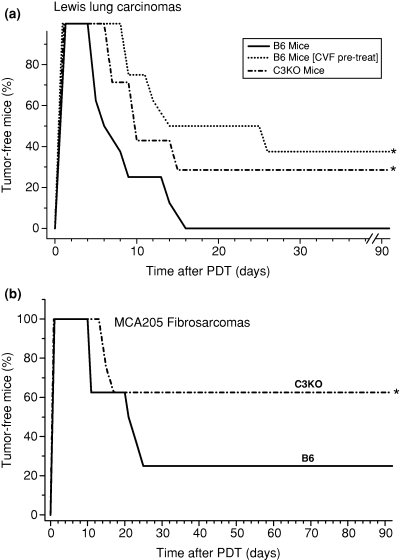
The response of tumors growing in B6 or in C3KO mice to Photofrin-PDT. The light treatment (150 J cm−2) was performed 24 h after Photofrin i.v. injection at 10 mg kg−1 for Lewis lung carcinomas (a) or 7.5 mg kg−1 for MCA205 fibrosarcomas (b). In CVF pretreatment group, mice received CVF (2 U/mouse i.p.) at 42 and 18 h before PDT light treatment. After therapy, the mice (eight per treatment group) were observed for signs of tumor regrowth. *, statistical significance of P < 0.05 for the difference in response compared to the B6 host mice treatment group.
These results with Photofrin-PDT were not specific to chosen tumor model. With MCA205 fibrosarcomas, tumors histologically distinct to Lewis lung carcinomas that are more PDT sensitive, higher levels of cures were reached also in C3KO mice compared to their wild type counterparts (Fig. 1b).
To determine whether the observed effect of Photofrin-PDT reflects the differences in the extent of direct killing of tumor cells, B6 or C3KO mice bearing Lewis lung carcinomas were killed immediately after photodynamic light treatment and the tumors disaggregated into single-cell suspensions that were plated for colony formation assay. The results show that the survival of tumor cells from B6 hosts was about one log greater than that of tumor cells from C3KO hosts (Fig. 2), which is consistent with the interpretation that complement activity renders resistance to tumors from direct killing by Photofrin-PDT. As presented in the following text, untreated Lewis lung carcinomas of the size used in this study are characterized by large hypoxic regions with oxygen levels below the limit requisite for producing PDT-mediated cytotoxic species (21); however, these regions (overlapping at least in part with perinecrotic and necrotic areas) should contain very low numbers of viable tumor cells (22). Hence, there is no contradiction in finding high extents of PDT-mediated tumor cell killing in these tumors as only viable cells are taken into account and others are normalized out with untreated controls while calculating relative colony-forming units.
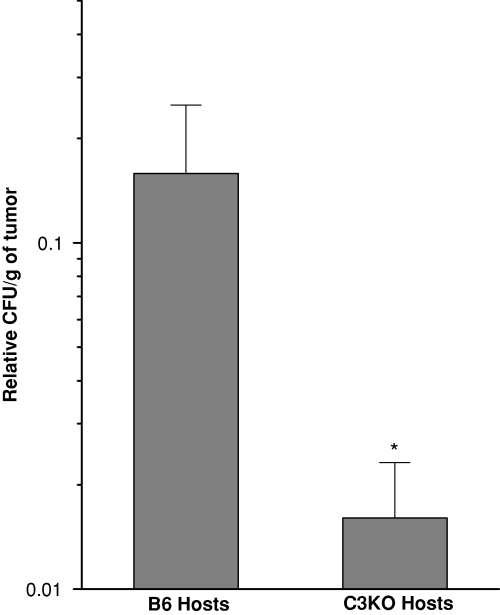
Clonogenicity of Lewis lung carcinoma cells isolated from tumors growing in either B6 or C3KO mice immediately after Photofrin-PDT. Lewis lung carcinomas were treated by Photofrin-PDT as described for Fig. 1b and excised immediately after tumor illumination. Cell suspensions were prepared by enzymatic disaggregation of PDT-treated and control untreated tumors, and known numbers of cells plated for clonogenic assay. Colony-forming units per gram of tumor relative to values from untreated controls (clonogenicity) were derived from plating efficiency and cell yield. n = 4, bars are SD. *, statistical significance of P < 0.05 for the difference in clonogenicity compared to the B6 host mice treatment group.
The PDT response of Lewis lung carcinomas growing in either complement-proficient or complement-deficient mice was also tested with two other photosensitizers, mTHPC and BPD. The response to PDT mediated by mTHPC, a photosensitizer with many characteristics resembling those of Photofrin more than BPD (23–25), followed the same pattern as with Photofrin-PDT exhibiting greater sensitivity with C3KO hosts than in the wild type mice (Fig. 3a). In fact, almost half of the complement deficient hosts were cured with the chosen dose (0.1 mg kg−1 of mTHPC plus 20 J cm−2), while no cures were reached with the complement proficient hosts even if the light dose was increased to 80 J cm−2. The CVF-mediated complement depletion before mTHPC-PDT treatment of another tumor type, FsaR fibrosarcoma growing in a different mouse strain (C3H/HeN, complement-proficient), increased the cure rates in a similar fashion as seen in Fig. 1a (not shown). In contrast to these results, the response of Lewis lung carcinomas treated with PDT mediated by photosensitizer BPD revealed an opposing pattern. The tumors growing in complement-proficient hosts showed a greater sensitivity to BPD-PDT than those in their complement-deficient counterparts, as their recurrence rate following initial complete ablation was significantly greater in C3KO mice (Fig. 3b).
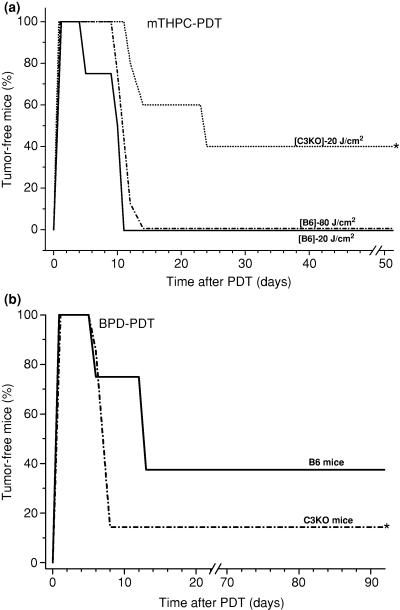
The response of Lewis lung carcinomas to mTHPC-PDT and BPD comparing B6 and C3KO hosts. Tumor-bearing mice received mTHPC (0.1 mg kg−1 i.v.) followed 24 h later by either 20 or 80 J cm−2 (a) or BPD 2.5 (mg kg−1) followed 3 h later by 100 J cm−2 (b). Other details were as described for Fig. 1. *, statistical significance of P < 0.05 for the difference in response compared to the B6 host mice treatment group.
A possible reason for these contrasts could be differences in the activity of complement system. The activation of the complement by Photofrin-PDT has been well documented (10–12), but the extent to which this can vary among different photosensitizers was not addressed. To examine this, B6 mice bearing Lewis lung carcinomas were treated with either Photofrin-PDT or BPD-PDT and killed at 3 h post therapy with tumor tissues collected for the quantification of key complement component (C3). The ELISA-based analysis revealed that the C3 content was significantly elevated after both types of PDT, but the extent of the increase was much higher with Photofrin-PDT than with BPD-PDT (Fig. 4). After the same PDT protocols, the genes encoding C3, C5 and C9 complement proteins are upregulated in Photofrin-PDT treated (26) but not in BPD-PDT treated Lewis lung carcinoma tumors (B. Stott and M. Korbelik, unpublished results).
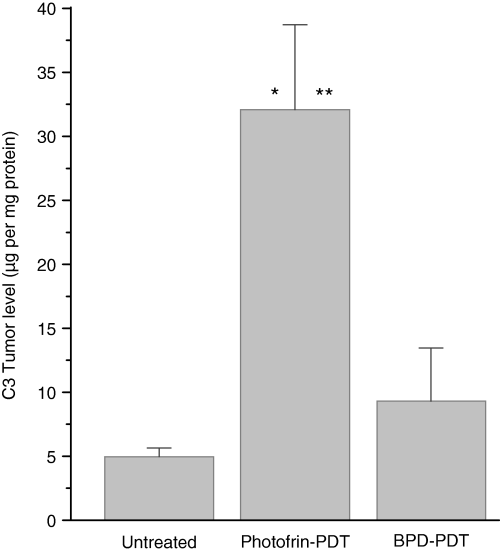
Complement C3 protein levels in Lewis lung carcinomas treated by Photofrin-PDT or BPD-PDT. The tumors were PDT treated as described for Fig. 1 and excised at 3 h after therapy. The levels of C3 proteins were determined from tumor homogenates using ELISA technique. n = 4; bars are SD. *, statistical significance of P < 0.05 for the difference in C3 levels compared to the untreated tumors group; **, statistical significance of P < 0.05 for the difference in C3 levels compared to the BPD-PDT treatment group.
The difference in the extent of Photofrin-PDT mediated direct tumor cell killing between complement-proficient and complement-deficient hosts (Fig. 2) raises the possibility that complement activity impacts the tumor oxygenation during photodynamic light treatment. To verify this, Eppendorf pO2 needle sensor was employed to determine oxygen levels in tumors immediately after PDT. The partial pressures of oxygen showed no significant differences between untreated Lewis lung tumors growing either in B6 or C3KO mice (median mmHg 3.75 and 3.90, respectively). Histograms presenting the distribution of pO2 values in these tumors show that tumor oxygen status markedly deteriorated by the end of Photofrin-PDT in B6 hosts (as evidenced by markedly more recorded events in the 0–2.5 mmHg range), but was less affected in C3KO hosts (Fig. 5). With BPD-PDT, the data with B6 hosts show that the fraction with the lowest oxygen tensions (0–2.5 mmHg) decreased (compared to untreated tumors) but the next 2.5 mmHg fractions increased; in C3KO hosts, there were only minor changes in the distribution of oxygen tension fractions. Altogether, the pO2 measurements were performed with 20 untreated tumors (770 pO2 values generated in total), while 25 mice were distributed in PDT treatment groups (not including other controls) that generated 141–305 pO2 values per group. Following Photofrin-PDT the mean tumor oxygen tension dropped to 33.9 ± 12.0 (SD) percent of the pretreatment level in B6 hosts, while in C3KO hosts this drop was only to 71.8 ± 24.0 (SD) percent; statistical analysis showed that this induced decrease in tumor oxygen tension was significantly greater in B6 than in C3KO hosts (P < 0.05). The drops in the mean oxygen tension following BPD-PDT in B6 and C3KO hosts were to 53.2 ± 19.1 and 62.3 ± 18.7 (SD) percent of the pretreatment level, respectively. Statistically, BPD-PDT had not induced significantly different declines in tumor oxygenation between B6 and C3KO hosts. Tumor oxygen parameters were not significantly affected with mice administered Photofrin or BPD in the absence of light exposure or by tumor light treatment alone (not shown).
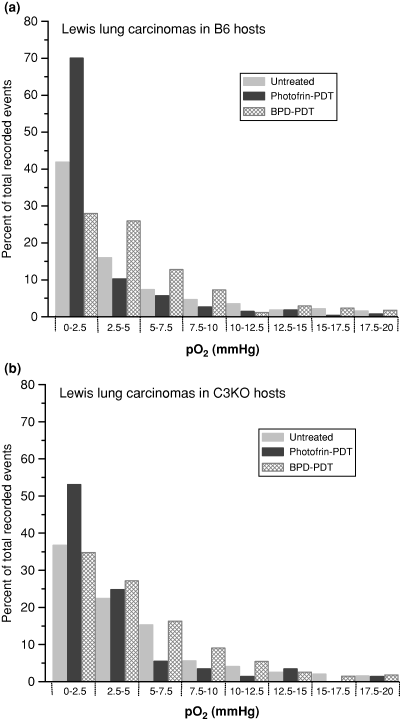
Changes in the distribution of pO2 fractions in Lewis lung carcinomas growing in either B6 or C3KO mice following treatment with either Photofrin-PDT or BPD-PDT. The tumors were PDT treated as described for Fig. 1a and immediately thereafter subjected to oxygen tension measurement using the Eppendorf pO2 needle sensor. This measurement was also performed with untreated tumors of the same size. The data is presented as the percent of total events recorded and distributed into increments of 2.5 mmHg using the pooled values from all tumors within same treatment groups; n ranged from 5 to 14.
Discussion
Inferior therapeutic outcome with Photofrin-PDT in complement-proficient compared to complement-deficient hosts was demonstrated in this study with two different mouse tumor models (Lewis lung carcinoma and MCA205 fibrosarcoma). The same conclusion was derived from complement knockouts and from normal mice depleted of complement before PDT using CVF. The finding of a much more pronounced direct tumor cell killing with complement-deficient than with complement-proficient hosts (determined with tumors excised immediately after photodynamic light exposure, Fig. 2) indicates that this effect of complement is exerted during the illumination for Photofrin-PDT. Similar complement-dependent impact on tumor cures was found with PDT mediated by the photosensitizer mTHPC, but the opposite was the case with photosensitizer BPD.
The pharmacokinetics of Photofrin and mTHPC differ greatly from those of BPD. At the time of tumor illumination (24 h postinjection or up to 72 h with patients), Photofrin is well distributed within tumor parenchyma and much lower levels remain in the circulation (27,28). At 3 h post-BPD injection (time of tumor illumination in this study), the levels of this photosensitizer are approximately twice as high in the blood than the tumor (29). While direct tumor cell killing is induced by Photofrin-PDT, this is not the case with BPD-PDT (30). A factor emerging as critical for the contrasting PDT outcomes with complement-proficient vs complement-deficient hosts is the impact on tumor oxygenation. It is well established that with Photofrin-PDT, especially with clinically relevant fluence rates similar to that used in the present study, there is a significant depletion of tumor oxygen (23,31). This was proposed to be caused by at least two mechanisms, photochemical oxygen depletion and vessel constriction occurring rapidly during PDT light treatment (31). In the latter, complement system is bound to have a major role. The key vasoconstricting agent involved is thromboxane (32), and the release of this arachidonic acid metabolite is caused by complement anaphylatoxins (33,34). Complement-mediated secretion of thromboxane-like eicosanoids also acts on circulatory platelets causing thromboembolic effects (35). In addition to triggering and/or accelerating the release of vasoactive mediators through anaphylatoxins, activated complement can participate in vascular effects in Photofrin-PDT treated tumors by forming MAC, the cell-lysing terminal complex of complement cascade, which was shown to assemble on the vascular epithelium in tumors treated by this therapy (36). Deposition of activated complement components on Photofrin-PDT treated human endothelial cells was also demonstrated in vitro (10). Owing to the essential role of oxygen in the production of cytotoxic lesions by PDT (23), the above described effects of complement impair the therapeutic potential of some photosensitizers.
As BPD is known as a photosensitizer that acts primarily by inducing vascular damage in the treated tumors (37), it may seem counterintuitive that complement-associated effects compromising tumor oxygenation were found to affect Photofrin-PDT but not BPD-PDT. However, vascular effects of Photofrin-PDT are dominated by a rapid yet transient constriction (23). With BPD-PDT, when a 3 h interval is used between drug injection and light treatment as in the present study, no constriction and acute vascular damage is observed and only a gradual reduction of tumor tissue perfusion develops after PDT (37). It can be speculated that this is due to a slow apoptotic death of endothelial cells and the absence of significant complement action in the vascular wall. Based on electron paramagnetic resonance oxymetry, Pogue and coworkers have registered an increase in tumor oxygen levels during and immediately after BPD-PDT treatment with the 3 h drug-light interval using a BPD dose lower than that used in this work (1 mg kg−1) and a fairly hypoxic mouse RIF-1 fibrosarcoma model (38). They suggested that such rise in tumor pO2 may have originated from either reduced metabolic consumption of oxygen due to direct phototoxicity or vasodilatation-induced changes in blood flow. Interestingly, even with the 15 min drug-light interval (that maximizes vascular targeting by BPD-PDT) these authors have not detected significant decreases in tumor oxygen tensions.
Tumor oxygen tension measurements made in this study immediately after PDT treatment reveal that a marked decline in the oxygenation of tumors growing in complement-proficient hosts induced by Photofrin-PDT was not matched in complement-deficient hosts, while after BPD-PDT the decrease was similar in both host types. The most plausible explanation for this finding is that the degree of complement activation in treated tumors differs between BPD- and Photofrin-PDT, and this was confirmed by our measurement (Fig. 4). Such a result may originate from different cellular localization patterns of the photosensitizers. A significant fraction of Photofrin remains localized in the outer membrane resulting in the abundance of phototoxic lesions formed on the cell surface (39), where they can be detected by complement recognition elements (10). In contrast, BPD predominantly localizes in perinuclear membranes/mitochondria (40) producing much less lesions on the cell surface.
It is becoming clear that complement activity can have both positive and negative impact on the therapeutic outcome of tumor PDT. Rapid complement activation during PDT light delivery associated with an enhanced decline in tumor oxygen supply with a photosensitizer such as Photofrin impairs the generation of phototoxic species and prevents PDT to exert its full therapeutic potential. Such negative impact of complement is not exhibited with PDT mediated by BPD and probably also other photosensitizers characterized by similar vascular effects. The positive impact unfolds after PDT treatment when activated complement system instigates and promotes the elicited host response including its tumor-destructive activity (6,11); if vascular effects of complement negatively influencing tumor oxygenation persist after PDT they would also be beneficial (hamper tumor growth). These therapeutically favorable effects of complement system can be further amplified by adjuvant treatment with complement-activating agents applied after PDT (41,42).
Negative effects of activated complement system occurring during photodynamic tumor illumination with photosensitizers such as Photofrin result in the overall negative impact on the outcome of therapy, implying that their consequences cannot be fully compensated by positive (antitumor host response promoting) effects of complement system (6,10–12,43) expressed after PDT treatment. The awareness of this negative aspect of complement activation has two important implications. First, it inspires an approach of attempting to improve the outcome of tumor PDT by agents temporarily blocking complement activity (during illumination). Second, as complement-activating agents are emerging as effective adjuvants to tumor PDT (41,42), it becomes clear that their application must be restricted to post-PDT light treatment.
Acknowledgments
Acknowledgements— This study was supported by the National Cancer Institute of Canada, with funds from the Canadian Cancer Society and by the Doctoral Trainee Award of the Michael Smith Foundation for Health Research to I. C.




