Red Porgy, Pagrus pagrus, Larvae Performance and Nutritional Condition in Response to Different Weaning Regimes
Abstract
Red porgy, Pagrus pagrus, is a candidate species for aquaculture diversification. The aim of this work was to assess whether an early supply of enriched Artemia (D1) or a direct step to dry diets (D3) would be advantageous weaning strategies for red porgy larvae, compared to a later supply of Artemia followed by dry diets (D2). Direct weaning to dry diet resulted in significantly lower growth, survival, pancreatic (trypsin and lipase), and intestinal (alkaline phosphatase) enzyme-specific activity, with the exception of leucine-alanine peptidase. The direct weaning strategy presented severe nutritional restrictions from early weaning stages with an associated delay of the maturation of digestive system. The two-step strategy presented in D1 and D2 resulted in comparable results in most parameters, including survival. Weaning using enriched Artemia as an intermediate step is confirmed as the most adequate strategy for red porgy larvae. Digestive enzymes and selected fatty acids correlated well with performance responses to dietary regimes, thereby supporting the use of these parameters as sensitive and reliable indicators of red porgy nutritional or physiological status during larval stages.
Most marine fish larvae depend on live feed for the initial life stages. In marine hatcheries, the production of live prey is considered a somewhat unpredictable, expensive task with results of variable nutritional quality (Person-Le Ruyet 1989; Callan et al. 2003; Rao 2003; Carvalho et al. 2006). Therefore, weaning fish larvae, as soon as possible, to artificial diets is a major goal in commercial operations.
Weaning should ensure that mortality due to cannibalism, delayed development of active feeding behavior, and starvation are kept to a minimum, while maintaining good growth rates (Fletcher et al. 2007). Knowledge of the nutritional condition or physiological status of the fish larvae is also critical to interpret the adequacy of the diet regimes (Cunha et al. 2003; Gisbert et al. 2004). The choice of methods to evaluate larval nutritional condition vary according to the duration of experiments, with morphometric parameters and lipid analysis oriented for trials on a weekly basis, whereas nucleic acid ratios and enzyme activity are utilized when the time scale is in days or hours, respectively (Suthers 2000).
Red porgy, Pagrus pagrus, is a sparid fish regarded as a strong candidate species for the diversification of the fish farming industry in southern Europe (Basurco and Abellan 1999; Divanach 2002). A major constraint on the farming success of red porgy has been the low survival rate during larval culture due to the scant knowledge of the biology of the species and inadequate technology for the mass production of larvae (Papandroulakis et al. 2004).
Rearing methods used for red porgy larvae were adapted from sea bream culture (Divanach 2002). Recent advances have promoted improvements of red porgy larvae performance under production conditions. Rod cells in the eye were found to develop at about 7.0 mm total length (TL) by 20 days after hatching (d.a.h.), improving retina sensitivity (Roo et al. 1999) and instigated a reduction to a 12-h photoperiod (Roo et al. 2010). Larval live feed demand rises sharply from 14 d.a.h. at about 5.6 mm TL and their needs can be met at an exponential rate of 0.159 ± 0.007 mg feed/larvae (Papandroulakis et al. 2004). Poor larvae growth performance with rotifers enriched at a docosahexaenoic acid (DHA) to eicosapentaenoic acid (EPA) ratio lower than 1 (Hernández-Cruz et al. 1999) and associated skeletal deformities (Roo et al. 2009) have prompted the need to produce new and more efficient enrichment protocols and/or diets high in DHA (Roo et al. 2009, 2010). Nevertheless, red porgy culture still has its challenges during the transformation period from larval to juvenile stage and in the weaning to an inert diet, with high size heterogeneity from 20 d.a.h., TL range 6–8 mm (Mihelakakis et al. 2001) and consequent high mortalities due to cannibalism by 30 d.a.h. at 8–8.5 mm TL (Roo et al. 2010).
Weaning protocols for large-scale production of red porgy larvae fall into two main categories in regard to the feed schedule: a) an early supply of enriched Artemia and/or inert diets, starting 15–20 d.a.h. at about 5–6.5 mm TL (Kentouri et al. 1995; Kolios et al. 1997; Papandroulakis et al. 2004; Roo et al. 2010), and b) a delayed introduction of enriched Artemia and inert diets, after 25 d.a.h. at about 7–12.0 mm TL (Mihelakakis et al. 2001; Büke et al. 2005; Aristizábal and Suárez 2006).
The significant development of gastric glands of the digestive tract from 26 d.a.h. at about 7.5 mm TL (Darias et al2005, 2007) and pepsin-specific activity detected at 28 d.a.h. at approximately 13 mm TL (Suzer et al. 2007) is evidence of late maturation of the red porgy digestive system compared to that of other sparids, which is a strong argument for using a delayed weaning strategy. This also concurs with observations by Roo et al. (2010) of undigested Artemia evacuated by larvae at 20 d.a.h. at 6–7 mm TL.
Conversely, weaning protocols for red porgy larvae were partially evaluated in terms of growth and survival by Aristizábal and Suárez (2006) showing better results for an early co-feeding regime with a commercial dry diet. However, this study did not present data on the physiological condition of larvae, nor gave any indication of energy allocation and nutrients in larval tissues.
This study aimed to assess P. pagrus larvae performance and nutritional condition over different feeding schedules, with early or delayed introduction of Artemia and commercial starting diet. Growth parameters and survival, complemented by enzyme activity, major fatty acids (FAs) composition, protein content, and nucleic acid ratios, were used a) to evaluate effects of diet shifts, b) to compare results from different feeding schedules, and finally, c) to determine the most sensitive variables for future works.
Materials and Methods
Source of Larvae
Red porgy eggs were obtained from spontaneous spawning of wild broodstock. The eggs were incubated and the larvae were reared in a 40 m3 fiberglass cylinder culture tank according to standard methods in use at Centro de Maricultura da Calheta hatchery, Madeira Island adapted from Divanach and Kentouri (2000). The seawater was filtered (10 µm) before entering the tank and provided with gentle aeration. Increasing daily water changes of 10–150% were maintained starting on 3 d.a.h. A light regime of 12 h of light at 2000 lx at the water surface was provided by fluorescent lamps. Nannochloropsis sp. was added daily to keep a density of 250 × 103 cell/mL. The first feeding of larvae was initiated 3 d.a.h. with enriched rotifers supplied twice a day and maintained at a density of 5 per mL.
Experimental Procedures
Two days before the larvae reached 20 d.a.h., they were transferred to 12 trial tanks (100-L volume cylinder tanks), at a stocking density of 20 per L. The tanks were supplied with filtered water (10 µm) of 20.5 ± 0.5 C and flow rate of about 3 L/min. All tanks had a 24-h light regime and light intensity at the water surface, measured with a photometer (Model HD8366; Delta OHM SRL, Padova, Italy), was maintained at 400 lx. Rotifers were supplied as previously mentioned. Oxygen saturation was measured daily by probe (Model Handy Polaris; OxyGuard International A/S, Birkerød, Denmark) and registered a minimum of 91%. Salinity registered 36 ± 1 ppt. The tank bottoms were siphoned every day (10–15% water volume renewal) before the first feeding and dead larvae were counted. Total ammonia nitrogen (TAN) was measured daily by using the indophenol method (Koroleff 1983) and it was consistently below 0.42 mg/L. Following Person-Le Ruyet et al. (1997), this corresponded to 0.008–0.017 mg NH3/L, thus below the critical level of 0.024 mg NH3/L for seabream larval culture recommended by Parra and Yúfera (1999).
The trial consisted of three treatments or dietary regimes for the larvae (Fig. 1), with three replicates each: D1, the control group, which consist of rotifers and Artemia from 20 d.a.h., followed by inert diets from 30 to 35 d.a.h., the end of trial; D2, a delayed feeding regime starting with rotifers from 20 d.a.h., followed by Artemia from 25 d.a.h. and inert diets from 30 d.a.h.; and D3, a direct shift strategy with inert diets and enriched rotifers from 20 d.a.h. Changes of diet allowed for a phasing-out period of 3 d to allow for larvae adaptation to a new diet. Feeding included rotifers added twice a day in order to maintain a density of 5/mL as recommended for P. pagrus (Hernández-Cruz et al. 1999; Roo et al. 2010). Artemia was added at increasing densities 1–5 metanauplii/mL/d. The artificial diet on the first day was supplied according to the daily ration of rotifers dry weight (DW) at 20 d.a.h., that is, 0.28 mg/larva (Papandroulakis et al. 2004) and at 15% larvae biomass/d the following days (Roo et al. 2010).
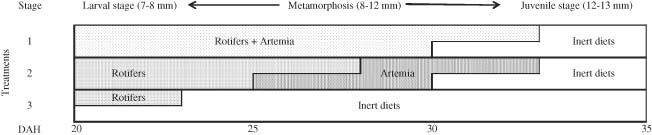
Schematic representation of the feeding regimes used in red porgy weaning trial. Developmental stages and body sizes (mm total length) according to Mihelakakis et al. (2001).
Rotifers, Brachionus rotundiformis T., and Artemia metanauplii (EG Artemia cysts; INVE Aquaculture NV, Dendermonde, Belgium) were enriched with DHA-Protein Selco (INVE Aquaculture NV) at 0.55 g/L seawater for 8 h and Protein Selco (INVE Aquaculture NV) at 0.6 g/L seawater for 24 h, respectively, according to the manufacturers' instructions. Inert diets provided were 100–200 µm initially and 200–400 µm from 30 d.a.h. (Proton; INVE Aquaculture).
Sampling and Measurements
Three pools of 20 larvae each were sampled at random in each tank at days 20, 25, 30, and 35 after hatching, before diet transition. One pool of samples was used to photograph the left side of each larva with a Sound Vision SV Micro camera (Sound Vision, Inc., Newton, MA, USA) mounted on a stereoscopic microscope (Model Stemi SV11; Carl Zeiss Microimaging GmbH, Göttingen, Germany). Total body length (TL) was measured to the nearest 0.01 mm from the photographs using Zeiss Ks 300 v. 3.0 software package (Carl Zeiss Vision, GmbH, Eching bei München, Germany). After being measured, the larvae were frozen at −20 C, freeze-dried, and the DW was measured, with a ±0.001 mg precision. Three samples of rotifers and Artemia were collected for routine quality assessment at 20 and 25 d.a.h. (N = 6).
Lipids and Essential FAs
The live diets and the larvae were analyzed for lipid content and essential FAs. Total lipids were extracted with a chloroform–methanol mixture (1/2, volume basis) containing 0.01% butylated hydroxytoluene and quantified by gravimetric method, according to Bligh and Dyer (1959). FA methyl esters (FAMEs) were prepared according to the Lapage and Roy (1986) method. The FAMEs quantitative analyses were performed by Gas Chromatography (Model 8700; Perkin Elmer Ltd., Beaconsfield, UK).
Digestive Enzyme Assays and RNA, DNA Determinations
Red porgy larvae were dissected on ice to isolate the abdominal segments of the digestive tract for enzymatic assays. Abdominal segments were homogenized in five volumes (1 g/5 mL) of ice-cold distilled water. Trypsin, amylase, alkaline phosphatase, and leucine-alanine peptidase activities were measured using the following substrates: Nα-benzoyl-dl-arginine-p-nitroanilide, Bapna (Holm et al. 1988), starch (Métais and Bieth 1968), p-nitrophenylphosphate, pNPP (Bessey et al. 1946), and leucine-alanine (Nicholson and Kim 1975). Lipase activity was assayed according to a spectrophotometric method (Hitachi U-2000 Spectrophotometer; Hitachi High Technologies Corporation, Tokyo, Japan) slightly modified from Iijima et al. (1998), using p-nitrophenyl myristate (Sigma, St. Louis, MO, USA) as substrate dissolved in 65.8 mM of dimethylsulfoxide (Merck-Schuchardt, Hohenbrunn, Germany). Enzyme-specific activities were expressed as micromoles of substrate hydrolyzed per min per mg of protein (i.e., U mg/protein) at 37 C for alkaline phosphatase and leucine-alanine peptidase, and 25 C for trypsin. Amylase-specific activity was expressed as the equivalent enzyme activity that was required to hydrolyze 1 mg of starch in 30 min at 37 C per mg of protein. Protein was determined by the Bradford method (Bradford 1976).
RNA and DNA contents were determined in the whole larval body using the dual wavelength absorbance method (Ashford and Pain 1985).
Data Analysis
Results were given as mean ± SD. Relative growth rate (RGR, %/d) was calculated between sampling points as (eg− 1) × 100, with g = (ln final weight −ln initial weight)/time, following Ricker (1958). Coefficient of variance (CV) was calculated for both TL and DW as percentage for each treatment. As red porgy larvae at 35 d.a.h. have isometric growth (Mihelakakis et al. 2001; Socorro et al. 2001), the condition factor (K) was determined at the end of the experiment as (weight/TL3) × 100. Percent Survival in each tank (S%) was calculated as (number of larvae at sampling/[number of larvae at start − number of larvae sampled from previous count]) × 100, where number of larvae at sampling = number of larvae at start − cumulative mortality over the sampling period. Data from each treatment (morphometric variables, growth, survival, larval chemical composition, and enzymatic activity) were compared by one-way analysis of variance (ANOVA). The effects of independent variables “age,”“diet,” or the interaction of variables were tested by two-way ANOVA. The assumptions of normality adjustment and homogeneity of variance were checked using Kolmogomorov–Smirnov's and Levene's tests, respectively, and a significance level of 0.05 (Zar 1996). When significant differences were found, Tukey's HSD multiple test was performed. All statistical analyses were carried out using SPSS for Windows, v12.0.1 (SPSS, Chicago, IL, USA).
Results
Biochemical Composition of Diets
Among the different tested feeds, enriched Artemia presented the highest content of protein and lipids (Table 1). The FA composition of enriched rotifers and Artemia was very similar, with a high content of unsaturated FA which may be the result of the same type of enrichment products and protocols (Table 2).
| Diets | Total protein (%DW) | Total lipid (%DW) |
|---|---|---|
| Enriched rotifers | 51.31 ± 4.46 | 21.38 ± 2.83 |
| Enriched Artemia | 55.62 ± 3.78 | 23.98 ± 2.04 |
| Dry diet1 | 54 | 15 |
- 1Dry feeds: Proton; INVE Aquaculture NV.
| Fatty acid | Enriched rotifers2 | Enriched Artemia3 |
|---|---|---|
| 14:0 | 0.80 ± 0.22 | 0.48 ± 0.31 |
| 16:0 | 13.81 ± 1.23 | 12.58 ± 1.01 |
| 16:1 | 3.51 ± 0.31 | 3.21 ± 0.25 |
| 18:00 | 7.64 ± 1.30 | 6.34 ± 1.19 |
| 18:01 | 32.92 ± 2.96 | 26.96 ± 3.28 |
| 18:2n-6 | 6.20 ± 0.11 | 6.09 ± 0.56 |
| 18:3n-3 | 17.09 ± 1.77a | 25.86 ± 2.42b |
| 18:4n-3 | 1.63 ± 0.06a | 5.48 ± 0.44b |
| 20:01 | 1.17 ± 0.09 | 1.25 ± 0.11 |
| 20:4n-6 (ARA) | 0.70 ± 0.19 | 0.63 ± 0.12 |
| 20:5n-3 (EPA) | 5.78 ± 1.93 | 6.29 ± 1.43 |
| 22:5n-3 | 0.17 ± 0.01 | 0.14 ± 0.02 |
| 22:6n-3 (DHA) | 4.18 ± 0.35 | 3.48 ± 0.26 |
| SFA | 24.85 ± 2.23 | 22.60 ± 1.99 |
| MUFA | 37.60 ± 3.19a | 30.95 ± 3.54b |
| PUFA | 36.10 ± 2.84 | 44.95 ± 4.98 |
| HUFA | 27.05 ± 2.49a | 36.21 ± 3.34b |
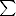 n-3 n-3 |
29.20 ± 3.54 | 36.80 ± 5.95 |
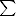 n-6 n-6 |
6.89 ± 0.70 | 6.16 ± 0.24 |
| n-3/n-6 | 4.24 ± 0.72 | 5.92 ± 1.15 |
| DHA/EPA | 0.72 ± 0.08 | 0.54 ± 0.09 |
| EPA/ARA | 8.32 ± 0.36a | 10.01 ± 1.07b |
- ARA = Arachidonic acid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; HUFA = highly unsaturated fatty acids.
- 1Values are means (N = 3) ±SD; if followed by different superscript letters within a row were significantly different (P < 0.05).
- 2Enrichment product: DHA-Protein Selco; INVE Aquaculture NV.
- 3Enrichment product: Protein Selco; INVE Aquaculture NV.
Larvae Performance
The larvae fed actively in the three treatments since the beginning of the experiment. As different items of the diet were differently colored, it was possible to confirm their presence in the stomachs. Larvae under feeding regimes D1 and D2 presented better growth performance (P < 0.05; one-way ANOVA) compared to D3 (Table 3), with no significant differences between the first two diet regimes throughout the trial. Early supplementation of Artemia in D2 did not significantly affect the RGR when compared with regime D1, but it had a significant effect on larval survival 25 d.a.h. (P < 0.05; one-way ANOVA). The CV of DW and TL was also similar in both treatments at 25 d.a.h, but significant differences were detected in the subsequent sampling days (30 and 35 d.a.h.) (P < 0.05; one-way ANOVA). Larvae under feeding regimes D1 and D2 also presented comparable condition factors (K). Inversely, a direct shift to inert diet and enriched rotifers (D3) decreased RGR, leading to size heterogeneity (significantly higher CVDW) at 25 d.a.h. and lower survival than the other diet regimes (P < 0.05; one-way ANOVA). Rotifers persisted in D3 tanks for 3 d after their use were discontinued. Final survival was over 40% for all treatments.
| Treatments | |||
|---|---|---|---|
| D1 | D2 | D3 | |
| 20 d.a.h. | |||
| DW (mg) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| TL (mm) | 6.48 ± 0.21 | 6.48 ± 0.21 | 6.48 ± 0.21 |
| CV (weight) | 12.86 | 12.86 | 12.86 |
| CV (length) | 3.47 | 3.47 | 3.47 |
| 25 d.a.h. | |||
| DW (mg) | 0.19 ± 0.02a | 0.16 ± 0.01a | 0.12 ± 0.02b |
| TL (mm) | 8.69 ± 0.18a | 8.62 ± 0.33a | 7.61 ± 0.12b |
| RGR (%/d) | 28.08 ± 2.29a | 23.78 ± 1.55b | 16.04 ± 4.05c |
| CV (weight) | 9.12 | 6.25 | 17.84 |
| CV (length) | 2.02 | 3.80 | 1.55 |
| Survival (%) | 77.00 ± 2.90a | 85.77 ± 4.83a | 62.72 ± 2.57b |
| 30 d.a.h. | |||
| DW (mg) | 0.24 ± 0.01a | 0.21 ± 0.03ab | 0.15 ± 0.02b |
| TL (mm) | 10.91 ± 0.5a | 10.41 ± 0.52ab | 8.18 ± 0.16b |
| RGR (%/d) | 4.55 ± 2.51a | 5.11 ± 2.24 | 5.84 ± 6.55 |
| CV (weight) | 4.88 | 15.55 | 13.58 |
| CV (length) | 4.59 | 4.98 | 1.91 |
| Survival (%) | 92.38 ± 0.71 | 92.32 ± 0.56 | 93.11 ± 1.93 |
| 35 d.a.h. | |||
| DW (mg) | 0.30 ± 0.02a | 0.28 ± 0.05a | 0.17 ± 0.04b |
| TL (mm) | 13.14 ± 0.57a | 12.62 ± 0.82a | 8.98 ± 0.62b |
| RGR (%/d)2 | 4.86 ± 2.33 | 6.49 ± 0.53 | 5.89 ± 0.54 |
| CV (weight)3 | 6.67 | 16.30 | 24.25 |
| CV (length)3 | 4.32 | 6.51 | 6.85 |
| Survival (%)4 | 97.67 ± 0.52a | 96.15 ± 0.97a | 75.51 ± 0.79b |
| Cumulative survival (%) | 69.47 ± 2.36a | 76.18 ± 5.54a | 44.12 ± 2.76b |
| Condition factor (K)5 | 0.01 | 0.01 | 0.02 |
- 1Values are means (N = 3) ±SD; if followed by different superscript letters within a row were significantly different (P < 0.05).
- 2RGR (%) = (eg− 1) × 100, with g = (ln final weight −ln initial weight)/time.
- 3CV = (treatment SD/treatment mean) × 100.
- 4Survival (%) = (number of larvae at sampling/[number of larvae at start − number of larvae sampled from previous count]) × 100.
- 5Condition factor (K) = (larva weight/TL3) × 100.
Biochemical Composition of Larvae
Total protein was not significantly different for the three treatments, apart from 35 d.a.h., when D3 larvae had a higher content, followed by D2 and D1 larvae, respectively (Table 4). Two-way ANOVA detected significant effects of age rather than diet regime on protein contents.
| Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 d.a.h. | 25 d.a.h. | 30 d.a.h. | 35 d.a.h. | |||||||||
| D1 | D2 | D3 | D1 | D2 | D3 | D1 | D2 | D3 | D1 | D2 | D3 | |
| Protein | 61.98 ± 8.52 | 61.94 ± 8.95 | 63.35 ± 3.24 | 47.74 ± 10.10 | 54.40 ± 6.91 | 69.06 ± 4.51 | 41.95 ± 7.05 | 48.28 ± 7.48 | 39.49 ± 13.44 | 53.00 ± 5.19 | 38.62 ± 3.57 | 30.78 ± 4.79 |
| FA | ||||||||||||
| 16:0 | 10.04 ± 0.45a | 7.19 ± 0.32b | 9.08 ± 0.4a | 7.67 ± 1.08 | 7.62 ± 0.47 | 7.66 ± 0.40 | 6.99 ± 0.32 | 6.30 ± 0.77 | 6.80 ± 0.73 | 5.84 ± 1.26 | 5.34 ± 0.35 | 5.36 ± 0.63 |
| 16:1n-7 | 4.10 ± 0.75 | 2.51 ± 0.57 | 3.94 ± 0.77 | 3.22 ± 0.51a | 2.60 ± 0.37a | 1.75 ± 0.13b | 2.77 ± 0.17 | 2.27 ± 0.40 | 1.46 ± 0.73 | 1.64 ± 0.34 | 1.38 ± 0.13 | 1.47 ± 0.13 |
| 18:0 | 6.03 ± 0.12 | 4.99 ± 0.47 | 5.76 ± 0.54 | 5.21 ± 0.41 | 4.87 ± 0.30 | 5.17 ± 0.06 | 4.9 ± 0.39 | 4.65 ± 0.38 | 5.10 ± 0.15 | 4.33 ± 0.72 | 3.92 ± 0.38 | 4.28 ± 0.27 |
| 18:1n-9 | 8.79 ± 0.97 | 6.36 ± 0.47 | 8.16 ± 1.11 | 8.50 ± 0.35a | 6.82 ± 0.67b | 5.45 ± 0.02c | 9.43 ± 0.84 | 7.97 ± 1.07 | 6.40 ± 2.75 | 6.80 ± 1.22 | 5.41 ± 0.66 | 6.17 ± 0.26 |
| 18:1n-7 | 2.63 ± 0.39 | 3.09 ± 0.28 | 2.22 ± 0.20 | 4.75 ± 0.52a | 4.33 ± 0.53a | 1.55 ± 0.07b | 5.2 ± 0.28a | 4.48 ± 0.56a | 2.59 ± 2.20b | 3.49 ± 0.45 | 3.00 ± 0.24 | 3.25 ± 0.17 |
| 18:2n-6 | 5.65 ± 0.52 | 3.59 ± 0.26 | 5.62 ± 0.47 | 3.41 ± 0.34 | 2.77 ± 0.19 | 4.09 ± 0.23 | 3.49 ± 0.22a | 2.90 ± 0.41b | 3.44 ± 0.22a | 2.90 ± 0.55 | 2.32 ± 0.08 | 2.36 ± 0.13 |
| 18:3n-3 | 2.64 ± 0.50 | 1.43 ± 0.36 | 2.58 ± 0.39 | 0.93 ± 0.09 | 0.64 ± 0.09 | 0.89 ± 0.07 | 6.17 ± 0.76 | 4.83 ± 0.91 | 2.33 ± 3.08 | 3.34 ± 0.42 | 2.62 ± 0.12 | 2.99 ± 0.16 |
| 20:1n-9 | 1.10 ± 0.13 | 0.73 ± 0.06 | 1.11 ± 0.18 | 0.67 ± 0.00 | 0.55 ± 0.02 | 0.69 ± 0.05 | 0.55 ± 0.02 | 0.50 ± 0.03 | 0.59 ± 0.02 | 0.47 ± 0.11 | 0.36 ± 0.04 | 0.40 ± 0.01 |
| 20:1n-11 | 0.16 ± 0.04 | 0.11 ± 0.01 | 0.16 ± 0.03 | 0.10 ± 0.00 | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.00 |
| 20:4n-6 (ARA) | 2.09 ± 0.04 | 2.77 ± 0.01 | 1.96 ± 0.23 | 3.57 ± 0.19 | 3.95 ± 0.23 | 1.75 ± 0.02 | 3.08 ± 0.14 | 3.03 ± 0.30 | 2.15 ± 0.93 | 2.54 ± 0.05 | 2.58 ± 0.11 | 2.49 ± 0.11 |
| 20:5n-3 (EPA) | 5.70 ± 0.67a | 7.08 ± 0.38b | 4.89 ± 0.38a | 12.55 ± 0.82a | 11.78 ± 1.55a | 4.18 ± 0.18b | 8.95 ± 0.24a | 8.02 ± 0.87a | 5.55 ± 3.04b | 6.46 ± 0.57 | 5.72 ± 0.31 | 6.04 ± 0.38 |
| 22:6n-3 (DHA) | 10.67 ± 0.42 | 8.00 ± 1.99 | 10.41 ± 1.45 | 7.19 ± 1.02a | 7.40 ± 0.46a | 12.61 ± 0.74b | 5.60 ± 0.51a | 5.67 ± 0.51a | 11.68 ± 4.57b | 7.60 ± 1.68 | 6.38 ± 0.41 | 6.07 ± 0.57 |
| Total FA | 74.22 ± 5.06a | 60.65 ± 0.74b | 68.86 ± 4.08a | 74.17 ± 4.33a | 68.81 ± 4.79a | 56.67 ± 1.73b | 69.16 ± 3.64a | 62.00 ± 6.28a | 58.38 ± 8.92b | 55.45 ± 8.50 | 48.58 ± 3.27 | 50.58 ± 2.63 |
| PUFA | 31.56 ± 1.25a | 26.34 ± 1.32b | 30.18 ± 2.57a | 30.91 ± 1.48 | 29.61 ± 1.79 | 27.51 ± 1.01 | 29.93 ± 1.79 | 26.96 ± 3.02 | 28.42 ± 2.05 | 25.17 ± 3.72 | 21.61 ± 1.19 | 22.21 ± 1.32 |
| SFA | 18.18 ± 0.87a | 13.89 ± 0.55b | 16.44 ± 0.75a | 15.70 ± 1.56 | 15.13 ± 0.91 | 13.72 ± 0.52 | 13.44 ± 0.74 | 12.42 ± 1.29 | 12.93 ± 0.27b | 11.31 ± 2.19 | 10.25 ± 0.76 | 10.58 ± 0.97 |
| MUFA | 19.72 ± 3.10 | 16.10 ± 1.79 | 17.73 ± 2.55 | 23.17 ± 1.93a | 20.17 ± 2.40a | 11.03 ± 0.48b | 21.36 ± 1.33a | 18.48 ± 2.54a | 12.75 ± 6.97 | 14.58 ± 2.55 | 12.17 ± 1.39 | 13.31 ± 0.70 |
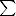 n-3 n-3 |
22.95 ± 0.84 | 19.32 ± 1.42 | 21.78 ± 2.06 | 23.18 ± 1.03 | 22.13 ± 0.82 | 20.76 ± 0.80 | 22.80 ± 1.41 | 20.46 ± 2.26 | 22.03 ± 1.38 | 19.14 ± 3.07 | 16.17 ± 0.95 | 16.78 ± 1.10 |
 n-6 n-6 |
7.75 ± 0.49 | 6.36 ± 0.26 | 7.58 ± 0.51 | 6.98 ± 0.53a | 6.73 ± 0.24a | 5.85 ± 0.22b | 6.56 ± 0.35 | 5.93 ± 0.70 | 5.59 ± 0.89 | 5.44 ± 0.60 | 4.89 ± 0.18 | 4.85 ± 0.23 |
| n-3/n-6 | 2.97 ± 0.09 | 3.05 ± 0.35 | 2.87 ± 0.17 | 3.32 ± 0.20 | 3.29 ± 0.66 | 3.55 ± 0.05 | 3.47 ± 0.03 | 3.45 ± 0.03 | 3.98 ± 0.39 | 3.51 ± 0.98 | 3.3 ± 0.13 | 3.45 ± 0.10 |
| DHA/EPA | 1.9 ± 0.31 | 1.14 ± 0.35 | 2.13 ± 0.18 | 0.58 ± 0.11a | 0.63 ± 0.17a | 3.02 ± 0.14b | 0.63 ± 0.04a | 0.71 ± 0.04a | 2.76 ± 1.80b | 1.18 ± 0.06 | 1.12 ± 0.04 | 1.00 ± 0.04 |
| EPA/ARA | 2.72 ± 0.32 | 2.56 ± 0.15 | 2.49 ± 0.17 | 3.51 ± 0.16a | 2.98 ± 0.78ab | 2.39 ± 0.11b | 2.91 ± 0.06 | 2.65 ± 0.05 | 2.58 ± 0.26 | 2.54 ± 0.90 | 2.22 ± 0.05 | 2.43 ± 0.05 |
- ARA = Arachidonic acid; DHA = docosahexaenoic acid; DW = dry weight; EPA = eicosapentaenoic acid; FA = fatty acid; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; HUFA = highly unsaturated fatty acids.
- 1Values are means (N = 3) ±SD; if followed by different superscript letters within a row were significantly different (P < 0.05).
On the other hand, total FA and several FA classes varied and were significantly influenced by the three diet regimes. D3 presented significantly lower total FA, MUFA, total n-6, EPA, and ARA than the other two diet regimes (P < 0.05; ANOVA). These results are in accordance with the higher contents of these FA in live prey. The exception to this lower FA pattern is at 35 d.a.h., when differences between treatments were not significant. An opposite trend occurred with DHA, as D3 had significantly higher contents than D1 and D2 (P < 0.05; ANOVA), but no significant differences were detected at 35 d.a.h. Other FA classes such as total SFA and total PUFA presented no significant differences between treatments or sampling ages.
Larval Enzymatic Activity
No significant differences were found in trypsin-specific activity for D1, D2, and D3 larvae at 25 d.a.h. (Fig. 2). In later sampling points, D1 and D2 larvae remained stable and showed significantly higher trypsin-specific activity than D3. Segmental activity increased during the experiment for D1 and D2 larvae, but it was relatively constant for D3. Again for lipase, the specific and segmental activity as well showed an increasing pattern for D1 and D2 treatments with slightly higher values D1 at 30 d.a.h. and about the same levels for D3 treatment. In contrast, amylase-specific and segmental activity decreased in all treatments, showing a significantly higher activity for D1 treatment at 30 d.a.h. (P < 0.05; ANOVA). Alkaline phosphatase-specific and segmental activity presented no significant differences between treatments at 25 d.a.h., but increased onward for D1 and D2 treatments, while in D3 it was lower at 35 d.a.h. Finally, the activity of leucine-alanine peptidase for D1 and D2 treatments presented a decreasing pattern at the specific level, but increasing for segmental activity. D3 treatment had increasing patterns for specific and segmental activity and was significantly higher at 35 d.a.h. (P < 0.05; ANOVA).
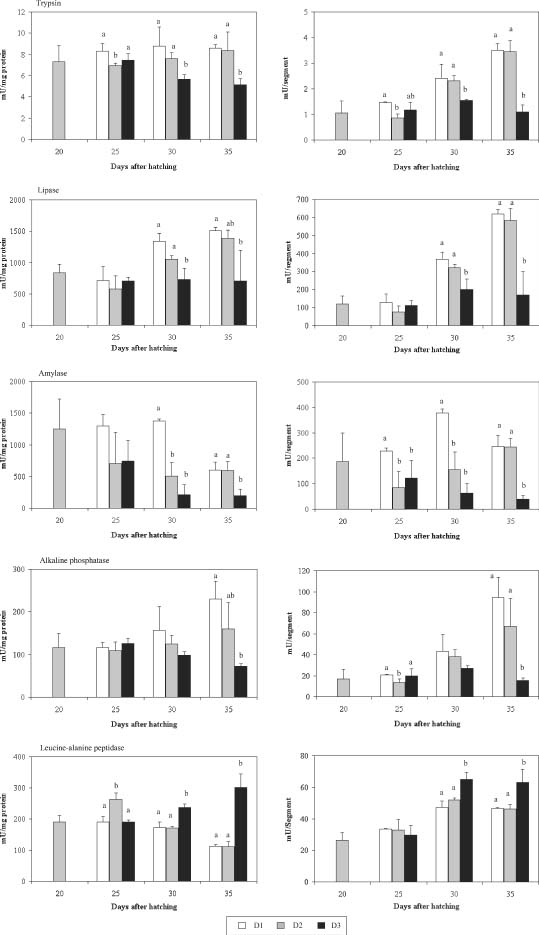
Enzymatic specific (mU/mg protein) and segmental (mU/segment) activity of the enzymes trypsin, lipase, amylase, alkaline phosphatase, and leucine-alanine peptidase in red porgy larvae for the three diet treatments (D1, D2, and D3) at 20, 25, 30, and 35 days after hatching. Values are means (N = 3)±SD; columns with different superscript letters were significantly different (P < 0.05).
RNA/DNA and RNA/Protein Indices
RNA/DNA index was not affected by different diet regimes (Table 5). This ratio had a pronounced and significant similar peak for the three treatments at 30 d.a.h. (P < 0.05; ANOVA). At 35 d.a.h., all diet treatments presented no significant differences. (P < 0.05; ANOVA). A similar trend was found for RNA/protein index.
| d.a.h. | Treatments | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | ||||
| RNA/DNA | RNA/protein | RNA/DNA | RNA/protein | RNA/DNA | RNA/protein | |
| 20 | 2.365 ± 0.253 | 0.002 ± 0.000 | 2.475 ± 0.201 | 0.002 ± 0.001 | 0.293 ± 0.153 | 0.002 ± 0.001 |
| 25 | 4.354 ± 0.920 | 0.033 ± 0.007a | 4.310 ± 0.303 | 0.022 ± 0.003a | 0.481 ± 0.465a | 0.012 ± 0.003b |
| 30 | 22.215 ± 6.076 | 0.104 ± 0.039 | 21.741 ± 3.757 | 0.104 ± 0.040 | 19.794 ± 10.800 | 0.144 ± 0.110 |
| 35 | 4.838 ± 1.689 | 0.021 ± 0.004 | 3.619 ± 0.559 | 0.030 ± 0.009 | 3.614 ± 1.671 | 0.033 ± 0.001 |
- 1Values are means (N = 3) ±SD; if followed by different superscript letters within a row were significantly different (P < 0.05).
Discussion
It is generally accepted that the adaptation of fish larvae to dry diets demands protocols to adapt to a period of drastic morphological, physiological, and behavioral changes and to their nutritional and environmental requirements (Rao 2003).
The evaluation of three weaning regimes for P. pagrus made in this study revealed that age or development stage is the most important independent variable affecting growth performance and nutritional condition of larvae. This emphasizes the difficulties in dealing with diet assays during fish larval stages.
Red porgy larvae showed a distinct response to diet feeds and schedules, whether considering a two-step weaning regime with enriched Artemia as intermediate live feed or direct step weaning from live rotifers to dry diet.
Two-Step Weaning Treatments
The early introduction of Artemia did not significantly influence growth rates, as previously reported for cod larvae weaning by Baskerville-Bridges and Kling (2000). Larvae from diet regimes supplied with Artemia had higher TL growth rates than those reported from laboratory conditions (Mihelakakis et al. 2001; Socorro et al. 2001; Darias et al. 2007; Suzer et al. 2007) and from semi-intensive or intensive production methods, although of smaller size (Kentouri et al. 1995; Roo et al. 2010). Survival was considerably high at 69.74% for D1 and 76.18% for D2, as in large-scale production conditions, it may vary from about 60 to 70% for mesocosm and intensive culture, respectively, according to estimates by Roo et al. (2010). Significant differences in mortality between D1 and D2 were only observed at 25 d.a.h., possibly due to difficulties faced by larvae in digesting Artemia at that early stage in the former diet treatment, as previously mentioned by Roo et al. (2010).
In both treatments, the live feeds rich in protein and FA modulated the pancreatic enzymes involved in their digestion by maintaining trypsin-specific activity at high levels and increasing lipase-specific activity, respectively. Ribeiro et al. (2008), studying blackspot seabream larvae, observed a similar trend of constant values for trypsin-specific activity with the sequence of three diets used. On the other hand, amylase decreased activity even when inert diet, usually a high carbohydrate source, was added. This is a common occurrence with other marine species, denoting an apparent importance, although poorly understood, of this enzyme at earlier larval stages (Moyano et al. 1996; Ribeiro et al. 1999, 2008; Zambonino Infante and Cahu 2001).
D1 and D2 treatments did not evidence an abrupt decrease of cytosolic enzymes, such as leucine-alanine peptidase, with a simultaneous sharp increase in alkaline phosphatase of the brush border membrane, the general pattern of variation of enzymes during fish larvae development (Cahu and Zambonino Infante 2001), and previously described for P. pagrus (Suzer et al. 2007). Whether the more subtle trend was an adaptive response to the diet regimes needs to be further investigated. However, this may suggest that there is an overlap period of adaptation with both pancreatic and intestinal enzymes and associated forms of digestion, before the shift to dominant luminal digestion characteristic of adult fish. A combined mechanism for intra- and extracellular digestion was previously suggested for red porgy based on histological evidence (Darias et al. 2007). Peak responses of the enzymes were later than reported by Suzer et al. (2007). However, there were differences in the diet regimes, including the first Artemia supply 5 d later than reported by those authors. In addition, larvae from our study were smaller in size at the same age, thus less developed (Rosenlund et al. 1997), and consequently were expected to have a later maturation of the digestive enzymes.
One-Step Direct Weaning Treatment
Early weaning of larvae with a co-feeding regime of inert diet (D3) depressed growth, caused a sudden high size heterogeneity (expressed as CVweight at 25 d.a.h.) and higher mortalities over the several weaning stages. Furthermore, the size heterogeneity of larvae may increase agonistic interactions and cannibalism later than 35 d.a.h. D3 is not an appropriate feeding regime for red porgy larvae. The growth performance results partly agree with Aristizábal and Suárez (2006), but denote relative higher mortalities with direct supply of inert diet compared to the other diet regimes. Taking into account the different artificial diet and more scattered data from those authors, comparisons of results should be treated with caution.
Several constraints have been reported using dry compound diets in weaning, such as the difficulties of larvae in learning and catching feed (Appelbaum 1985), a low diet acceptance by larvae (Yúfera et al. 2000; Cuvier-Péres et al. 2001), or dry diets considered as a second choice in a co-feeding regime with live prey (Fernández-Diaz et al. 1994). However, these limitations can hardly explain the growth performance results in this study. In fact, D3 larvae were observed actively feeding and dry diet was observed in their gut. It is more likely that the compound diet ingested contributed to the reduction of rotifer consumption and together with the limited capacity of fish larvae to digest artificial diet at 20 d.a.h. may have left larvae with less energy and nutrients available for growth.
According to Ryan et al. (2007), Murray cod juveniles of 1 g during weaning and under such nutritional limitations first utilize accumulated fat resources to support metabolic activity to survive and then, once the feeding situation is improved, use again dietary lipid source for growth. Our results seem to evidence selected FA mobilization and priority use for catabolism by larvae as a response to the nutritional constraints imposed by D3 treatment in these two phases.
In the first phase, until 25 d.a.h., there was a DW reduction simultaneous with the decrease of total FA, priority mobilization of (n-6) in relation to (n-3) PUFA and conservation of total PUFA, which are signs of larvae starvation previously detected in red seabream by Tandler et al. (1989) and in gilthead seabream by Rainuzzo et al. (1994). DHA was conserved in line with what was reported by those authors, and the percentage of total EFA was maintained. DHA is a component of cell membrane structure and essential for the development of neural and visual systems (Sargent 1995; Izquierdo 1996). The lack of available essential FA, EPA, and ARA, in particular, was insufficient to sustain good growth and survival as previously suggested by several authors (Izquierdo et al. 1989; Reitan et al. 1994; Bell et al. 1995; Rainuzzo et al. 1997). The proportions of different essential FA and their ratios were closer to those of 35 d.a.h. larvae reared in an intensive system than to those of larvae reared in a mesocosm as reported by Roo et al. (2010). These balances within PUFA contributed to the absence of significant differences in this FA class during the trial. Conversely, MUFAs were mobilized from 25 d.a.h., as these FAs are more easily transported and energetically more profitable than long chain PUFA according to Rueda et al. (1997). In contrast to major FA, protein was conserved by larvae and protein synthesis, as indicated by RNA/DNA and RNA/protein ratios, which did not vary significantly with diet treatments and seemed more dependent on age or development stage. Taken together, these data suggest that in the first weaning period, lipid store mobilization contributed to main protein metabolism homeostasis.
In the second phase, 25–30 d.a.h., the biology of the species had a main role in larvae response to D3 treatment. The maturation of the digestive system takes place during this period (Darias et al. 2007) and larvae would then be able to digest dry diet. FA increased to levels not significantly different from those of other treatments at the end of trial. Consequently, growth was promoted and survival was not significantly different from other treatments already at 30 d.a.h.
Suboptimal maintenance rations are likely to originate size heterogeneity in cultured fish larvae, which may cause and/or be an effect of cannibalism and size-dependent mortality (Kestemont et al. 2003). D3 larvae population went from the highest CVweight at 25 d.a.h. to the lowest among treatments at 30 d.a.h., a period of high cannibalistic behavior according to Roo et al. (2010). Cannibalism is likely to have removed the smaller and less fit larvae. This generates size selective mortality and consequently, an overestimation of the population growth rate (Folkvord 1997). In our experiment, we only observed partial cannibalism (fin nipping) independently of the treatment. As we did not measure dead larvae, we were unable to verify the occurrence and potential effects of size selective mortality caused by cannibalism. However, the putative effects of cannibalism on D3 larvae, re-establishing FA composition, and condition at 35 d.a.h. might not be negligible.
Direct feeding with dry diet had a detrimental effect in the larvae physiological condition. There was a general trend of significant deterioration of enzymatic activity, decreasing (trypsin) or maintaining (lipase) the early pancreatic enzyme activity at specific level, and preventing the rise of sequent intestinal enzyme-specific activity (alkaline phosphatase), all indicative of inadequate diet (Cahu and Zambonino Infante 2001) or even larvae starvation (Bolasina et al. 2006). Concurrently, the exception to this situation was the increase of leucine-alanine peptidase, a brush border enzyme previously suggested as being indicative of delayed maturation (Ribeiro et al. 2002). Higher RNA ratios at 35 d.a.h. for D3 could eventually be considered another indication of a delayed development relative to the other diet treatments.
Concluding Remarks
Age or developmental stage is determinant in growth, survival, and physiological parameters of red porgy larvae. Diet regime through diet item and schedule may affect the performance and physiological status of larvae by modulating the digestive enzyme activity and FA composition.
Artemia supplied at 20 d.a.h. to red porgy larvae promoted better growth and nutritional condition, but these parameters were not significantly different from those of rotifer-fed larvae. Given the significantly higher mortality at this stage, Artemia should be supplied at low feeding levels. This strategy is likely to attenuate problems associated with the more unpredictable rotifer production, but costs need to be evaluated. The supply of inert diet should be adapted from 30 d.a.h. onward, when larvae are physiologically prepared to digest more complex feed items. However, the relatively high survival in treatment D3 encourages the development of optimized diets for early weaning of red porgy. These should take into account: a) adequate particle size (100–200 and 200–400 µm) used for the larval size range 6.5–11.0 mm, b) water stability to avoid water quality problems, c) nutritionally well-balanced ingredients providing high protein, high energy, and high HUFA levels including DHA, and d) high digestibility assured by ingredient selection and adequate diet production technology.
Enzymatic activity and FA were sensitive parameters for the detection of the influence of the diets in red porgy larvae at different physiological levels over the duration of the experiment and should be considered for future works. The different results and complementary information obtained from the several methods used corroborate previous suggestions by Cunha et al. (2003) and Rosenlund et al. (1997) to combine approaches in the evaluation of the nutritional condition, in order to take into account the species-specific biological and ecological response to diet changes.
Acknowledgments
This study was partially funded by the projects Pargogen (Interreg III B, FEDER, European Union) and Mais Peixe (Interreg III B, FEDER, European Union). C. A. P. A. acknowledges his gratitude for the PhD grant from Centro de Ciência e Tecnologia da Madeira (CITMA). We thank two anonymous referees and the editor for their constructive review of the manuscript, and E. Porter for the English revision.




