Long-term evaluation of body mass at weaning and postweaning survival rates of Weddell seals in Erebus Bay, Antarctica
Abstract
Variability in juvenile survival rate is expected to be an important component of the dynamics of long-lived animal populations. Across a range of species, individual variation in juvenile body mass has been shown to be an important cause of variation in fates of juveniles. Our goal in this paper was to estimate age-specific apparent survival rates for Weddell seals (Leptonychotes weddellii) in Erebus Bay, Antarctica, and to investigate hypotheses about relationships between body mass at weaning and apparent survival rate for juveniles. Mark–resighting models found average apparent juvenile survival rate (survival from weaning to age 3) was similar between males and females, and revealed positive relationships between body mass at weaning and apparent juvenile survival rate. The effects of body mass on juvenile survival rate differed between the sexes and the relationship between body mass and survival rate was stronger in males. These results indicate that the magnitude of energy transferred from mother to pup during lactation likely has important consequences on offspring survival rate and maternal fitness.
Understanding variation in basic demographic parameters, such as survival rate, is fundamental to understanding population dynamics of long-lived animals. In long-lived vertebrates adult survival rate is expected to be relatively constant, whereas juvenile survival rate is expected to show higher annual variability (Gaillard et al. 1998, Eberhardt 2002, Gaillard and Yoccoz 2003). Juvenile survival rate may be influenced by predation, parasites, or environmental variation, which lead to changes in food supply and body condition (Wickens and York 1997), and juveniles may be more sensitive to environmental variability because of immature immune systems or lack of experience (Gaillard et al. 1998, Gaillard and Yoccoz 2003). Possible mechanisms by which environmental variation may influence juvenile survival rate include direct influences such as food availability and foraging success and indirect influences such as stored maternal reserves and energy transfer during lactation (McMahon and Burton 2005). Among capital breeding species, maternal body mass at parturition is influenced not only by maternal traits such as age but also by environmental variations affecting food availability and maternal foraging success (Proffitt et al. 2007a). Consequently, offspring body mass at weaning shows interannual variation that correlate with environmental conditions (Vergani et al. 2001, Le Boeuf and Crocker 2005, McMahon and Burton 2005, Proffitt et al. 2007b). In addition, recent studies have shown that first-year survival rate is correlated with annual variation in environmental conditions (Beauplet et al. 2005, McMahon and Burton 2005), which suggests that body mass may, through its affects on juvenile survival rate, link environmental conditions, and population dynamics.
Across a range of species, preweaning growth rate, body size, or body mass are positively correlated with juvenile survival rate (Guinness et al. 1978, Murie and Boag 1984, Baker and Fowler 1992, Sedinger et al. 1995, Festa-Bianchet et al. 1997, Beauplet et al. 2005). Among phocid seals, preweaning body condition or body mass and postweaning survival rate may be correlated, with animals in higher body condition or of higher body mass having higher postweaning survival (southern elephant seal (Mirounga leonina), McMahon et al. 2000, McMahon and Burton 2005, gray seal (Halichoerus grypus), Hall et al. 2001). For species such as these that undergo a postweaning fast, the amount of resources accrued prior to weaning may be important in sustaining pups through the transitional period from maternal dependence to nutritional independence and may be important in determining subsequent survival (Hall et al. 2001). Additionally, larger pups may have greater diving capabilities (Burns 1999, Hindell et al. 1999) and lose less heat due to their higher blubber content (Bryden 1964). Thus, larger pups are expected to have an advantage over their smaller counterparts by being able to spend more time searching for food during their first months of nutritional independence (McMahon et al. 2000, Beauplet et al. 2005).
Here, we evaluate age-specific apparent survival rate of Weddell seals (Leptonychotes weddellii) in Erebus Bay, Antarctica, and investigate potential relationships between body mass at weaning and apparent juvenile survival rate. Long-term demographic studies on this population have been underway since 1968 (Siniff et al. 1977, Testa and Siniff 1987, Cameron and Siniff 2004), and we used data collected over the past three decades to estimate age-specific apparent survival rate and to evaluate relationships between body mass at weaning and apparent juvenile survival rate (survival from weaning to age 3). We evaluated weaning mass and sex as covariates potentially affecting apparent survival rate in juvenile Weddell seals. Weaning masses are highly variable, showing up to two-fold variations among individuals, and a pup's weaning mass is related to its mother's parturition mass (Wheatley et al. 2006) and annual environmental conditions (Proffitt et al. 2007b). Differences in male and female juvenile survival rate are reported in many mammalian species (Garrott 1991, Hall et al. 2001, Mathisen et al. 2003, Beauplet et al. 2005), including the Weddell seal (Hastings et al. 1999), with juvenile males showing lower survival than females.
We predicted that apparent juvenile survival rate would be positively related to body mass at weaning but that this relationship might be non-linear. Accordingly, we considered two functional forms of the relationship: linear (directional selection hypothesis) and quadratic (stabilizing selection hypothesis). Positive linear relationships between survival rate and body mass reported in previous studies, predict selection would favor evolution of larger body sizes (directional selection hypothesis). Larger females often produce greater numbers of offspring or offspring of better condition than smaller females, and larger males often have greater mating and reproductive success than smaller males (Clutton-Brock et al. 1982, Clutton-Brock 1988, Shine 1989, Honek 1993). The equilibrium view proposes that selection for larger body size is counterbalanced by opposing selective forces (see Blanckenhorn 2000 for review). Costs of being large may include increased predation or parasitism due to reduced agility or increased detectability, decreased mating success of males due to reduced agility or higher energy requirements, or decreased reproductive success of females due to later reproduction. Under the stabilizing-selection hypothesis, pups of average weaning mass are predicted to have higher survival rates than are pups of lower or higher mass. Finally, based on previous studies (Hastings et al. 1999, Cameron and Siniff 2004), we predicted that apparent survival rate would differ by sex, with survival rate being lower in males than in females.
Methods
Study Population
We studied individually marked Weddell seals at breeding colonies in Erebus Bay (western Ross Sea), Antarctica. Weddell seals are intermittent breeders and annually 300–600 females produce a single pup at breeding colonies in Erebus Bay. Pupping occurs on the fast ice surface from late October to early November, and mothers remain in close association with pups throughout a 30–50-d lactation period. Females support the energetic costs of lactation through mobilization of stored energy reserves (Boness and Bowen 1996), and neither mothers nor pups regularly feed during the lactation period. An abrupt weaning follows lactation, after which pups receive no parental care. Maternal energetic investment during lactation and pup weaning masses closely correlate with maternal body mass at parturition (Hill 1987, Wheatley et al. 2006), and both parturition and weaning masses vary annually with variations in environmental conditions that may affect marine food resources (Proffitt et al. 2007a, b).
From 1969 to the present, pups born within the Erebus Bay study area have been marked with four individually numbered livestock tags attached to the interdigital webbing of the rear flippers (Siniff et al. 1977). Tagging primarily occurs from during the peak pupping period (mid-October to November), and since 1982 all pups born within the study area have been marked. Shortly after the peak of the pupping (early November), six to eight systematic surveys of marked and unmarked Weddell seals were conducted in 3- to 5-d intervals. At the time of tagging and during each resighting event, the date, location, tag number, and relative's tag number (if any) were recorded and added to the mark–resighting database. The majority of adults (both pupping and non-pupping females, as well as males) return to traditional breeding colonies within the study area each year, and resighting rates are high (0.92 for females and 0.72 for males in a single survey, Cameron and Siniff 2004; 0.99 for nursing females estimated over multiple surveys, J.R., unpublished data). High fidelity to breeding colonies (Cameron et al. 2007) and high resighting rate allowed comprehensive resighting histories to be recorded for each marked animal.
Pup Weaning Mass Measurements
Weaning masses were collected periodically over a 33-yr period from 1974 to 2006. Weaning masses were measured approximately 40 d postparturition (range 35–45). The exact date individual animals were weaned could not be determined in the field; however, we strived to standardize the age at which pups were sampled. Therefore, our body mass measurements represent the body mass of pups at approximately 40 d of age and the true weaning mass may be underestimated for pups weaned either earlier than day 40 or later than day 40. From day 35 to day 45, unweaned pups are gaining body mass slowly (1.4 kg/d, n= 15, this study unpublished data). Therefore, variability due to the timing of body mass measurement is small relative to the overall variability of weaning masses. While this variability may make the effects of body mass on juvenile survival more difficult to detect, errors introduced from variability of weaning dates are not expected to bias results. Mass was measured by rolling the pup into a sling and weighing using a spring scale (estimated precision, 1.5 kg).
Mark–Resighting Models
We estimated apparent survival and sighting probabilities and evaluated relationships between covariates and these parameters using extensions of the Cormack–Jolly–Seber (CJS) models (Pollock et al. 1990, Lebreton et al. 1992) in Program MARK (White and Burnham 1999). All animals included in the analysis were initially marked as pups and their body masses were measured at weaning. We used resighting data collected during the 1974–2006 breeding seasons (1 October to 15 December) for pups marked from 1974 to 2004.
To evaluate our predictions, we developed a set of a priori models that included two types of parameters: apparent survival rate (φ) and resighting rate (p), where φi was defined as the probability that a seal alive in year i remains available for resighting until year i+ 1 (i.e., survives and does not permanently emigrate from the study area), and pi was defined as the probability that a seal alive in year i that has not permanently emigrated is observed in year i. Our primary interest was in the effects of body mass at weaning on φ; however, it was important to also adequately model variation in φ and p.
Before evaluating models that considered individual covariates (sex and body mass at weaning), we first determined which of two age structures was most appropriate to use for modeling possible age-related variation in φi. Based on results of previous studies (Hastings et al. 1999, Cameron and Siniff 2004, Hadley et al. 2006), we compared models in which age structures for φ contained either three age classes (a3: 1-, 2-, and ≥3-yr olds), or six age classes (a6: 1-, 2-, 3–6-, 7–10-, 11–14-, and ≥15-yr olds). However, because few yearlings were resighted during the study (n= 6), we constrained φi to be equal for 1- and 2-yr olds in all models. This resulted in age structure for φ containing two and five age classes (a2: 1–2-, and ≥3-yr olds, a5: 1–2-, 3–6-, 7–10-, 11–14-, and ≥15-yr olds). In all models, estimates of pi were allowed to vary among four age classes (a4: 1-, 2-, 3–6, and ≥7-yr olds) (Hastings et al. 1999, Cameron and Siniff 2004, Hadley et al. 2007). We evaluated competing models to determine whether it was most appropriate to constrain age-specific estimates of pi to follow linear or quadratic trends across age classes or to allow them to vary independently across age classes.
After determining the most appropriate age structure to use, we then evaluated the amount of support in the data for models that included effects of body mass at weaning (Mass) and sex of the animal (Sex). In our a priori models, Mass, in both linear and quadratic forms, was related to φ for 1- and 2-yr-old animals only, which expressed the prediction that high weaning mass would have the greatest benefits in the years immediately following weaning. We hypothesized that both φ and p might vary by animal sex because of strong sex-specific differences in behavior. Further, we predicted that p for males would be lower than that for females and that the difference would increase with age because males guard underwater territories and are more likely to do so as they mature (Siniff et al. 1977, Hill 1987). Therefore, we evaluated models that allowed p to vary as a function of either additive or interactive affects of Sex and Age (Age–SexAdd and Age–SexInt, respectively). Although φ and p likely vary to some degree among cohorts and years (Cameron and Siniff 2004, Hadley et al. 2006), the annual sample sizes available for the analyses presented here prevented us from estimating cohort- or year-specific estimates.
We evaluated goodness of fit for the most general model that did not include individual covariates (included only age structure) using a parametric bootstrap procedure in Program MARK (White and Burnham 1999), and estimated a variance inflation factor,  , from 100 simulations. We adjusted AICc scores for overdispersion, and used QAICc scores to compare models. Akaike model weights (wk) were computed and used to address model-selection uncertainty and to produce model-averaged estimates (Burnham and Anderson 2002). Model-averaged coefficient estimates for body mass were computed across all models containing mass in the same functional form. Tag loss is known to occur at a low rate in our study population: the probability that a seal will retain at least one tag from 1 yr to the next was estimated to range from 0.963 to 0.998 by Cameron (2001) and Cameron and Siniff (2004). As described by Nichols et al. (1992), if some animals lost both tags, then estimates of φ represented the product of the underlying apparent survival rate and the tag-retention rate
, from 100 simulations. We adjusted AICc scores for overdispersion, and used QAICc scores to compare models. Akaike model weights (wk) were computed and used to address model-selection uncertainty and to produce model-averaged estimates (Burnham and Anderson 2002). Model-averaged coefficient estimates for body mass were computed across all models containing mass in the same functional form. Tag loss is known to occur at a low rate in our study population: the probability that a seal will retain at least one tag from 1 yr to the next was estimated to range from 0.963 to 0.998 by Cameron (2001) and Cameron and Siniff (2004). As described by Nichols et al. (1992), if some animals lost both tags, then estimates of φ represented the product of the underlying apparent survival rate and the tag-retention rate 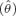 . Thus, we adjusted all estimates of φ using the equation
. Thus, we adjusted all estimates of φ using the equation  (Arnason and Mills 1981), where
(Arnason and Mills 1981), where  was 1 minus the age-, sex-, and cohort-specific estimates of tag loss provided by Cameron (2001) and weighted according to the sample sizes used here.
was 1 minus the age-, sex-, and cohort-specific estimates of tag loss provided by Cameron (2001) and weighted according to the sample sizes used here.
Results
Data were available for 486 animals marked as pups (238 males and 248 females), of which 109 (56 males and 53 females) were resighted in subsequent years. At least one pup was weighed at weaning in each of 17 yr (average n/yr = 29.8 pups, SE = 7.2). Overall, weaning mass averaged 105.8 kg (SD = 20.5). For the 13 yr in which at least 10 pups were weighed, average annual weaning mass ranged from a low of 81.9 kg (SD = 16.9) to a high of 122.1 kg (SD = 20.7). Weaning mass was similar for males (average = 106.2 kg, SD = 21.4) and females (average = 105.5 kg, SD = 19.6 kg).
A total of 24 a priori CJS models were evaluated, and  was 1.092 for the most general model that did not include either Mass or Sex, which indicated that for the most general model there was little overdispersion present and that reasonable goodness of fit was achieved. The data provided more support for models that estimated φ for five age classes and p as a quadratic function of four age classes (Table 1). Therefore, that age structuring of φ and p was included in all models used to evaluate effects of individual covariates on φ and p. For the top model that only considered age class, annual estimates of φ adjusted for tag loss were 0.52 (SE = 0.03) for 1- to 2-yr olds, 0.92 (SE = 0.02) for 3- to 6-yr olds, 0.93 (SE = 0.02) for 7- to 10-yr olds, 0.95 (SE = 0.03) for 11- to 14-yr olds, and 0.73 (SE = 0.07) for animals ≥15 yr old; and estimates of p were 0.03 (SE = 0.01) for 1-yr olds, 0.15 (SE = 0.02) for 2-yr olds, 0.43 (SE = 0.03) for 3- to 6-yr olds, and 0.69 (SE = 0.03) for animals ≥7 yr old.
was 1.092 for the most general model that did not include either Mass or Sex, which indicated that for the most general model there was little overdispersion present and that reasonable goodness of fit was achieved. The data provided more support for models that estimated φ for five age classes and p as a quadratic function of four age classes (Table 1). Therefore, that age structuring of φ and p was included in all models used to evaluate effects of individual covariates on φ and p. For the top model that only considered age class, annual estimates of φ adjusted for tag loss were 0.52 (SE = 0.03) for 1- to 2-yr olds, 0.92 (SE = 0.02) for 3- to 6-yr olds, 0.93 (SE = 0.02) for 7- to 10-yr olds, 0.95 (SE = 0.03) for 11- to 14-yr olds, and 0.73 (SE = 0.07) for animals ≥15 yr old; and estimates of p were 0.03 (SE = 0.01) for 1-yr olds, 0.15 (SE = 0.02) for 2-yr olds, 0.43 (SE = 0.03) for 3- to 6-yr olds, and 0.69 (SE = 0.03) for animals ≥7 yr old.
 calculated from model φa5, pa4 was 1.092.
calculated from model φa5, pa4 was 1.092.| Model | ΔQAICc | wk | K |
|---|---|---|---|
| Suite evaluating potential age structure | |||
| φa5, pa4.QUAD | 0.00 | 0.41 | 8 |
| φa5, pa4 | 0.49 | 0.32 | 9 |
| φa5, pa4.LIN | 1.60 | 0.18 | 7 |
| φa2, pa4.QUAD | 4.80 | 0.04 | 5 |
| φa2, pa4 | 5.35 | 0.03 | 6 |
| φa2, pa4.LIN | 6.08 | 0.02 | 4 |
| Suite evaluating potential effects of weaning mass and sexa | |||
 |
0.00 | 0.21 | 14 |
 |
1.01 | 0.13 | 13 |
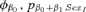 |
1.24 | 0.12 | 12 |
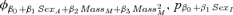 |
2.05 | 0.08 | 15 |
 |
2.14 | 0.07 | 13 |
 |
2.16 | 0.07 | 13 |
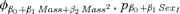 |
2.45 | 0.06 | 14 |
 |
2.89 | 0.05 | 14 |
 |
3.05 | 0.05 | 14 |
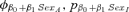 |
3.28 | 0.04 | 13 |
 |
3.63 | 0.03 | 14 |
 |
4.23 | 0.03 | 15 |
 |
4.27 | 0.03 | 14 |
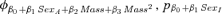 |
4.49 | 0.02 | 15 |
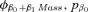 |
49.24 | 0.00 | 10 |
 |
49.37 | 0.00 | 9 |
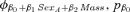 |
50.76 | 0.00 | 11 |
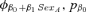 |
50.91 | 0.00 | 10 |
- aAll models in the effects of weaning mass and sex suite had an underlying φa5, pa4.QUAD structure. The covariate Mass was estimated for the first two age classes (ages 1 and 2) of each sex unless otherwise denoted by the subscript “M” for male only and “F” for female only.
The data provided support for our prediction that body mass at weaning is positively related to apparent survival rate for the first two years of life (φjuv), particularly for male animals. The two top-ranked models both included weaning mass as a covariate related to φjuv and together received 47% of the Akaike model weight (Table 1). In the top model, weaning mass was positively and linearly related to φjuv (directional selection) for males (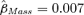 , SE = 0.003, 95% CI = 0.001–0.014), but not to φjuv for females (Fig. 1). For males,
, SE = 0.003, 95% CI = 0.001–0.014), but not to φjuv for females (Fig. 1). For males, 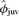 after being adjusted for tag loss ranged from 0.44 (95% CI = 0.35–0.53) for a male that was 39 kg (minimum weaning mass in data set) at weaning to 0.68 (95% CI = 0.55–0.82) for one that was 177 kg (maximum weaning mass in the data set). Based on parameter estimates from the top model, the estimated odds of a male pup surviving from weaning to age 3 increased 16.2% (CI = 2.0%–34.9%) for every 21.4 kg increase in body mass accrued by weaning, where 21.4 kg was one standard deviation in the sample used in analysis. In the second-best model, weaning mass was positively and linearly related to
after being adjusted for tag loss ranged from 0.44 (95% CI = 0.35–0.53) for a male that was 39 kg (minimum weaning mass in data set) at weaning to 0.68 (95% CI = 0.55–0.82) for one that was 177 kg (maximum weaning mass in the data set). Based on parameter estimates from the top model, the estimated odds of a male pup surviving from weaning to age 3 increased 16.2% (CI = 2.0%–34.9%) for every 21.4 kg increase in body mass accrued by weaning, where 21.4 kg was one standard deviation in the sample used in analysis. In the second-best model, weaning mass was positively and linearly related to 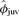 for both males and females (
for both males and females ( , SE = 0.005, 95% CI =−0.002–0.016). Thus, the point estimate for the mass coefficient remained the same, but the precision of the estimate decreased, and the confidence interval slightly overlapped zero. The model-averaged coefficient for body mass (in the linear form) was similar to estimates in the top models (
, SE = 0.005, 95% CI =−0.002–0.016). Thus, the point estimate for the mass coefficient remained the same, but the precision of the estimate decreased, and the confidence interval slightly overlapped zero. The model-averaged coefficient for body mass (in the linear form) was similar to estimates in the top models (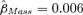 , SE = 0.004, 95% CI =−0.002–0.015). The third-ranked model, which was structured the same as the top model, but with the Mass covariate removed, also received some support from the data (ΔQAICc= 1.24).
, SE = 0.004, 95% CI =−0.002–0.015). The third-ranked model, which was structured the same as the top model, but with the Mass covariate removed, also received some support from the data (ΔQAICc= 1.24).

The estimated φjuv of male and female Weddell seals in Erebus Bay, Antarctica as a function of their body mass at weaning. Estimates and 95% confidence intervals are corrected for tag loss and generated from the top-ranking model in the suite evaluating potential effects of weaning mass and sex ( Table 1).
Table 1).
We found little support for our prediction that the relationship between body mass and survival rate was nonlinear. When the top model was modified such that Mass2, as well as Mass, was related to φjuv for males, the 95% confidence interval for 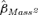 was almost perfectly centered about zero (
was almost perfectly centered about zero (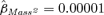 , 95% CI =−0.0001–0.0001) and the QAICc score for the resulting model was 2.05 units worse than the value for the top model.
, 95% CI =−0.0001–0.0001) and the QAICc score for the resulting model was 2.05 units worse than the value for the top model.
We did not find support for our prediction that φjuv would be lower in males than in females. In fact, the top model provided evidence that all but the lightest males survive at a higher rate than do females (Fig. 1). Based on estimates from our top model,  was 0.56 (SE = 0.04, 95% CI = 0.48–0.63) for males of average mass (106.2 kg) and 0.49 (SE = 0.03, 95% CI = 0.43–0.55) for females regardless of their mass. Point estimates of φjuv were >0.49 for males >69 kg, but when uncertainty of estimates was incorporated, confidence intervals of φjuv were broad enough to preclude strong inferences regarding sex-specific differences in juvenile survival rate (Fig. 1). When estimates were averaged across all models (and adjusted for tag loss), there was evidence of an increase in φjuv for both males and females as a function of increasing mass, but the trend was imprecisely estimated when all models were considered. For pups that were 81 kg at weaning (10% quantile in data set),
was 0.56 (SE = 0.04, 95% CI = 0.48–0.63) for males of average mass (106.2 kg) and 0.49 (SE = 0.03, 95% CI = 0.43–0.55) for females regardless of their mass. Point estimates of φjuv were >0.49 for males >69 kg, but when uncertainty of estimates was incorporated, confidence intervals of φjuv were broad enough to preclude strong inferences regarding sex-specific differences in juvenile survival rate (Fig. 1). When estimates were averaged across all models (and adjusted for tag loss), there was evidence of an increase in φjuv for both males and females as a function of increasing mass, but the trend was imprecisely estimated when all models were considered. For pups that were 81 kg at weaning (10% quantile in data set), 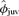 was 0.50 (SE = 0.06) for males and 0.48 (SE = 0.06) for females. If a pup was weaned at 107 kg (50% quantile),
was 0.50 (SE = 0.06) for males and 0.48 (SE = 0.06) for females. If a pup was weaned at 107 kg (50% quantile), 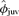 increased to 0.54 (SE = 0.04) in males and 0.51 (SE = 0.03) in females. For pups that weaned at 132.5 kg (90% quantile),
increased to 0.54 (SE = 0.04) in males and 0.51 (SE = 0.03) in females. For pups that weaned at 132.5 kg (90% quantile),  was 0.58 (SE = 0.07) for males and 0.53 (SE = 0.07) for females.
was 0.58 (SE = 0.07) for males and 0.53 (SE = 0.07) for females.
Exploratory analyses did not provide support for a relationship between body mass at weaning and survival rates in animals ≥3 yr old. Finally, no exploratory models were found to fit the data better than a priori models (ΔQAICc≥ 8.5).
Discussion
Body mass at weaning was found to be an important determinant of postweaning survival rate, suggesting that factors affecting the magnitude of energy delivered to offspring during lactation likely have important consequences on offspring survival rate and individual fitness. Mark–resight models revealed positive relationships between body mass at weaning and apparent survival rate of 1- and 2-yr-old Weddell seals, and models containing body mass covariates received strong support from the data. The most supported model contained body mass as a covariate on male juvenile survival but not female juvenile survival. However, models containing body mass as a covariate on both male and female or female only juvenile survival also received strong support from the data. The effects of body mass on apparent survival rate were more precisely estimated for male pups than female pups; however, overall model results provide evidence that body mass at weaning affects both male and female juvenile survival. These results indicate that energy transferred from mother to pup during lactation is important in determining offspring survival rate, and links energetic investment during lactation with a female's fitness.
Several studies, including an earlier study on Weddell seals, found sexual differences in juvenile survival rate, with males having a lower rate of survival than do females (Garrott 1991, Hastings et al. 1999, Hall et al. 2001, Beauplet et al. 2005). Although we found little evidence of differences in juvenile survival rate for males and females, we did find that male juvenile survival rate was more strongly influenced by body mass at weaning than female juvenile survival rate, which is consistent with findings from two previous seals studies. Hastings et al. (1999) found a positive relationship between reproductive rate (representing annual body condition of reproductive females) and male (but not female) first-year survival rate, suggesting that survival of male pups is more dependent upon maternal or environmental conditions than survival of females. A gray seal (Halichoerus grypus) study also found that the effects of body condition at weaning on survival rate were stronger in males than females (Hall et al. 2001). The effects of body condition at weaning may be more pronounced in male pups due to differences in metabolic activities, with males relying more heavily on lipid metabolism than females (Biuw 2003, Wheately et al. 2006) or to behavioral differences. The result that male juvenile survival rate is more sensitive to body condition at weaning predicts that high-quality females should invest more heavily in their male pups because the return, in terms of maternal fitness, from additional expenditure on male pups is greater than that for an investment in female pups (Hall et al. 2001).
Our finding that higher body mass at weaning resulted in higher postweaning survival rate is consistent with findings from a variety of taxa (Guinness et al. 1978, Baker and Fowler 1992, Sedinger et al. 1995, Festa-Bianchet et al. 1997, McMahon et al. 2000). We found a positive linear relationship between body size at weaning and juvenile survival rate, and this relationship was stronger in males than females. These results support the directional selection hypothesis; however, in order for selection for larger body sizes to occur, traits coding for either larger body size or increased capacity for maternal energetic expenditure during lactation must be heritable and there must be no countervailing selection at another life stage. Although several marine mammal studies have found positive relationships between body mass or condition at weaning and postweaning survival (Baker and Fowler 1992, McMahon et al. 2000, Hall et al. 2001), the effects of body mass or condition at weaning and long-term survival and reproductive success are unknown. For Weddell seals, body mass at weaning was an important factor in juvenile survival (survival from weaning to age 3) but appeared to have little affect on survival rate later in life.
These findings also support the hypothesis that body mass may be a mechanism linking maternal traits such as age with patterns of reproductive performance (Hadley et al. 2007). Recent studies on this population have found that middle-aged and older mothers produce offspring with higher survival rates than those of their younger counterparts (Hadley et al. 2007). Maternal body mass at parturition, the primary determinant of offspring body mass at weaning (Hill 1987, Arnbom et al. 1997, Mellish et al. 1999, Crocker et al. 2001, Wheatley et al. 2006), is positively correlated with maternal age (Proffitt et al. 2007a), which yields the prediction that older, larger females produce larger offspring. Our findings that offspring with higher body mass at weaning (predicted to have older, larger mothers) had higher rate of survival than their smaller counterparts (predicted to have younger, smaller mothers) are consistent with predictions from previous studies that older, larger mothers produce offspring with higher survival rate, and suggest that body mass may be an important mechanism linking maternal age and reproductive performance. These results improve our understanding of the reproductive strategy of female Weddell seals and suggest that the costs of weaning larger, successful offspring may be higher for younger, smaller mothers.
Environmental variations influencing prey distribution and abundance may affect animals' body condition and be important factors to consider in the analysis of population dynamics. The diet composition, reproductive performance, and population dynamics of Antarctic top-trophic species may be affected by variations in sea-ice extent and the Southern Oscillation (Testa et al. 1991; Ainley et al. 1998; Barbraud and Weimerskirch 2001a, b; Vergani et al. 2001; Proffitt et al. 2007b). Based on our findings regarding the effects of body mass on survival rate, environmental variations that produce interannual variations in maternal parturition mass and offspring weaning mass will potentially affect offspring survival rate. Previous studies on this population have found annual variations in maternal body mass and offspring weaning mass correlate with variations in sea-ice extent and the Southern Oscillation (Proffitt et al. 2007a, b). The findings of this study predict that environmental conditions resulting in reduced Weddell seal body mass (high sea-ice extent and a negative southern oscillation state) will reduce juvenile survival rate and negatively impact population growth. Understanding these linkages between large-scale oceanographic–environmental variations and biological responses is increasingly important because the environment of the Ross Sea region of Antarctica is changing (Vaughn et al. 2001, Doran et al. 2002), and significantly altered biological dynamics, are expected should such changes continue (Smith et al. 2007).
We expect our estimates of apparent survival to be quite close to actual survival because both male and female Weddell seals are strongly philopatric (Stirling 1969, Cameron and Siniff 2004, Cameron et al. 2007), and extensive surveys outside of the study areas have indicated that permanent emigration is rare (Cameron 2001). We were unable to directly estimate the effects of environmental variations or cohort effects on apparent survival rate because of modest annual sample sizes. However, annual effects are likely important in explaining additional variation in survival rate (Hastings et al. 1999, Cameron and Siniff 2004, Beauplet et al. 2005). Future studies exploring linkages between annual variations in vital rates and environmental conditions are needed to clarify relationships between environmental variability and Weddell seal population dynamics. Additionally, future studies investigating the dispersion patterns of weaned seals may clarify the observed inter-sexual variations in relationships between body mass at weaning and juvenile survival rate.
Acknowledgments
Funding for this project was provided by a National Science Foundation grant, OPP-0225110, to R. Garrott, J. Rotella, and D. Siniff. The data were collected under various National Science Foundation grants to R. Garrott and J. Rotella at Montana State University, D. Siniff at the University of Minnesota, M. Castellini at the University of Alaska, Fairbanks, and J. W. Testa at the University of Alaska, Fairbanks. We thank the past leaders of this project, D. Siniff and J. W. Testa, for their dedication in maintaining the long-term Weddell seal studies and we thank all the personnel who have participated in the long-term Weddell seal demography study. This manuscript was greatly improved by insightful comments from T. Williams and two anonymous reviewers.




