SUCKLING BEHAVIOR IN SPERM WHALE CALVES: OBSERVATIONS AND HYPOTHESES
Abstract
This study is the first to describe quantitatively the apparent suckling behavior of sperm whale, Physeter macrocephalus (Linnaeus, 1758), calves using observations from both above and below the surface. Peduncle dives are short (mean 14 s) dives made by sperm whale calves beside the peduncle of an adult female, which were previously assumed to be indicative of suckling. Photo-identification and focal calf-follows were used to collect data during 177 peduncle dive bouts from 22 different calves (11 calves from the Caribbean Sea, 11 from the Sargasso Sea), one of which was followed on forty different days. We found that peduncle diving in sperm whale calves is laterally asymmetrical with a bias to the left side of the escorting adult (69.8% of peduncle dives) and that calves generally do not switch sides during a bout of peduncle dives (switches occurred in only 10.8% of bouts). Further subsurface observations gave insight into potential alternative functions of peduncle diving. These alternative hypotheses, including nasal suckling, and the existing supporting evidence for each are discussed. It is likely that peduncle diving is related to suckling but that the exact function of the dives and manner in which sperm whale calves ingest milk remains unclear.
The production and care of offspring are central to the social interactions and associations among members of complex societies. Among mammals, the social organization of many species has at its base the mother–offspring relationship, as mothers provide the bulk of parental care by virtue of the nursing relationship (Kleiman and Malcolm 1981, Klopfer 1981, Clutton-Brock 1991, Reeve and Shellman-Reeve 1997). Although some mammals wean their offspring abruptly after birth, others nurse their young for years and maintain contact among generations over a lifetime (Klopfer 1981).
Sperm whales, Physeter macrocephalus (Linnaeus, 1758), live in partially matrilineal social units consisting of females and their descendant offspring. The principal function of these units seems to be to provide protection for calves while mothers make lengthy foraging dives at depth (Best et al. 1984, Gordon 1987, Arnbom and Whitehead 1989, Whitehead 1996). Much of our knowledge about social units of sperm whales has been derived from studies that focus on mothers and their adult associates. Detailed studies of the other half of the mother–offspring unit, the calf, are lacking (Whitehead 2003).
Three important behaviors must be performed on a daily basis by sperm whale calves: breathing, swimming, and suckling. As Whitehead (2003) pointed out, calves do not have difficulty breathing even immediately after birth but are unable to make prolonged dives with older individuals in the social unit. Quite soon after birth, sperm whale calves are proficient swimmers (Weilgart and Whitehead 1986) and manage to keep up with the foraging adults below presumably by following their echolocation clicks (Gordon 1987). Suckling is more of an enigma. The predominantly accepted theory, which is supported by both nineteenth-century whalers and brief modern observations by snorkellers, suggests that the nipple is held in the gape of the calf's jaw when the calf dives subsurface and rolls under the adult female's underbelly (Beale 1839, Best et al. 1984, Gordon 1991a). However, Best et al. (1984) cite an observation by Bennett (1840) who describes an adult female rolling onto her side with her pectoral fin above water and a calf suckling from the exposed nipple with its blowhole above water.
Previous work (Gordon 1987, Gordon et al. 1998) assumed that suckling was occurring based on above-water observations of repeated short dives underneath the peduncle of an escort, referred to here as peduncle diving. Gordon (1987) makes a point of stating that such behavioral observations must be treated with caution as one is unable to determine if milk is being transferred while the calf is below the surface. Cameron et al. (1999) showed that there was no relationship between behavioral observations of suckling and milk or energy intake in horses (Equus caballus). As such, we support precaution when interpreting the functional relationship between observable surface behaviors and those occurring subsurface and do not assume that suckling is occurring when peduncle diving is observed. Given the lack of clear descriptions of calf behavior and, in particular, behaviors relating to suckling, this study was undertaken to describe calf behavior in detail and to act as a basis from which to develop a more complete ethogram of infant sperm whale behavior.
Methods
Field Methods
Groups of female and immature sperm whales were located and followed both acoustically, using a directional and towed hydrophone, and visually, by observers on a dedicated 13-m auxiliary sailing vessel (Whitehead and Gordon 1986). Fieldwork was split between two sites. The first (5 May–20 June 2004; 38 d effort) was located in the Sargasso Sea. The 2005 fieldwork (14 January–13 April 2005; 58 d effort) was completed in the Caribbean Sea off the leeward shore of Dominica in an area that covered approximately 2,000 km2 along the entire west (leeward) coast of the island, in waters sheltered from the trade winds.
During daylight hours, individuals resting at the surface were approached and photographs of calf dorsal fins were taken from alongside the animals for individual identification purposes. During the 2004 field season, photographs were taken using a Canon EOS Elan II SLR camera with a Canon EF fixed 300-mm lens and Ilford HP5 400 black and white film. For the 2005 field season, a Canon D10 digital SLR base was used. Digital pictures were taken in full color at a resolution of 3,072 × 2,048 pixels and were saved in JPEG format. Slough skin samples, for genetic determination of sex, were collected in the slicks of individuals after identification (Whitehead et al. 1990, Bérubé and Palsbøll 1996).
Focal-Calf Follows
Focal-animal follows (Altmann 1974, Mann 1999) conducted on calves were completed within the larger group-follow of the entire sperm whale group (Whitehead 2004). Behavioral data were collected using continuous sampling for all calf behaviors, including peduncle dives (Altmann 1974, Mann 1999). As in Cowie et al. (1951), we use “suckling” to refer to the behavior of a calf whose aim is to ingest milk from an adult female, whatever those actions may be. A “bout” of peduncle dives is defined as a series of peduncle dives that began at the first observed peduncle dive when the dorsal fin disappeared beneath the surface and that ended when the calf stopped making peduncle dives, swam away from the escort at the surface, or if the escort started a fluke-up foraging dive. Bouts of peduncle dives were timed using a digital stopwatch. Both the duration of each dive within the bout and the surface interval between dives were recorded. The side of the escort on which the calf was located while at the surface between peduncle dives (referred to as “dive side”) was also recorded, as well as any switches in the dive side during a bout. When possible, peduncle dives were observed underwater by a snorkeller swimming alongside of the whales within 10 m of the calf.
Analyses
Identifications—As young calves rarely lift their flukes, these animals were individually identified using the shape of their dorsal fin and distinct markings on their dorsal fin and body. A quality rating (Q) between 1 and 5 was designated to each photograph, where 1 indicated a very poor photograph, and 5 indicated a very high quality photograph (Arnbom 1987, Dufault and Whitehead 1993). We used similar criteria (focus, exposure, angle of dorsal fin relative to the negative plane, percent of the dorsal fin not submerged, and the proportion of the frame filled by the dorsal fin) to assign a quality rating to the calf dorsal fin photographs as those used to assign quality ratings to fluke pictures used to identify adult sperm whales in previous studies (Arnbom 1987, Dufault and Whitehead 1993). Only pictures with a Q≥ 3 were used for the analyses. The best picture for each individual within each encounter was assigned an identification number then matched among encounters by eye.
Dive side—The records for a bout were classified into one of two categories based on whether or not all observed dives within a bout were completed on one side of the adult escort (“consistent”) or if the calf switched sides during the bout (“switch”). Dive side was compared within and between bouts for a given individual, as was the frequency of switches. Only calves in which dive side for three or more bouts was recorded were included in these analyses (n= 6 for each study area). Finally, a t-test was used to compare the mean duration of peduncle dives on left and right sides. Dives from all individuals for which dive times and side were recorded were included in the t-test.
Results
Data were collected from 177 bouts from 22 different calves (11 from the Caribbean Sea, 11 from the Sargasso Sea). Of those, only 23 were considered complete bouts, such that we observed them from when the calf surfaced, began to peduncle dive, and either the calf stopped diving or, as in most cases, the adult began a fluke-up dive. On average, complete bouts were 24.4 dives long (range 19–31 SD = 3.44) and had a mean duration of 5.7 min (range 3.5–8.2 SD = 1.14).
Encounters in the Caribbean Sea were dominated by the continuous presence of one particular social unit, “The Group of Seven” (GOS), which consisted of five adult females, one juvenile male (about 8.8 m long from acoustic measurement, Gordon 1991b), and one male calf (4.5 m, ∼3 mo old; length estimated using photographs with a fixed focal length from the crow's nest on the mast of the sailing vessel, age based on a growth curve from fig. 10 of Best et al. 1984). Sexes of these individuals were determined by an ongoing genetic analysis.1 The GOS was encountered on 40 different days over a 54-d period.
Underwater Observations
Underwater observations of peduncle diving by the GOS male calf were made for a total of twelve bouts (totaling 173 dives), all with the same adult escort, its mother (confirmed by genetic relatedness).1 The same behavior was observed during all dives observed underwater. The calf arched down without rolling onto its side; remaining somewhat parallel to its mother and in an upright position, the calf moved its head under the belly of its mother and pressed its blowhole to the escort's genital area (Fig. 1). The calf remained in this position for a few seconds and then turned away and surfaced. This behavior would be repeated every peduncle dive until the end of the bout or observations. If the mother fluked-up at the end of the bout, the calf generally dove in parallel with the mother, and no attempts to suckle were made while descending. The entire bout of peduncle dives was accomplished while slowly moving forward at about 2–3 km/h. In no cases was the calf observed attempting to place its mouth near the underbelly of the mother. The same behavior was recorded during the one bout of peduncle dives performed by the only other calf observed underwater.
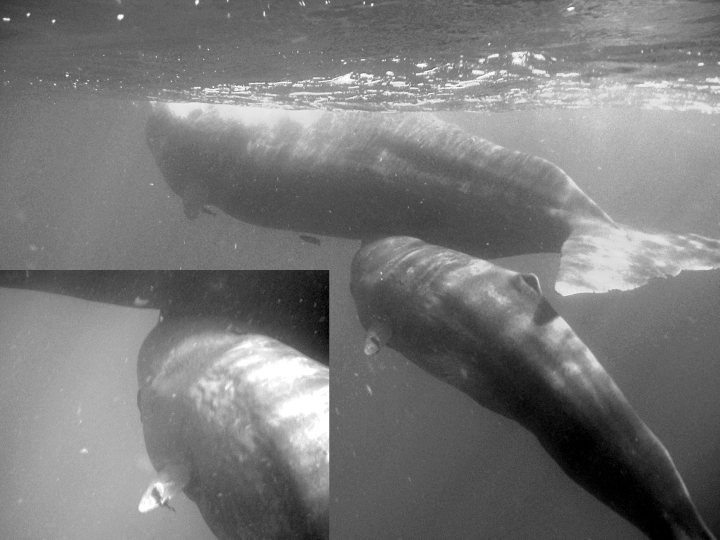
Underwater photograph of the general position taken by the calf while making “nasal suckling” attempts. Inset shows GOS calf pressing its blowhole to its mother's underbelly.
Dive Duration
We recorded dive durations for 981 peduncle dives, the vast majority of which were recorded while in the Caribbean Sea (95%). Of the 940 dives timed in the Caribbean, 712 or 76% were from the GOS calf 5703. Calves with smaller samples (>7 dives) from both the Caribbean (n= 7) and Sargasso Sea (n= 4) were used to compare and contrast results obtained from the GOS calf (Table 1).
| Study area | Calf ID | n | Mean (SD) | Range |
|---|---|---|---|---|
| Caribbean | 5703 | 712 | 14.4 (5.41) | 1.9–97.5 |
| 5725 | 74 | 10.0 (1.89) | 7.1–15.1 | |
| 5701 | 53 | 10.3 (1.72) | 5.9–14.4 | |
| 4001 | 41 | 12.5 (3.05) | 8.0–20.6 | |
| 5719 | 19 | 14.4 (2.75) | 10.7–21.4 | |
| 4002 | 13 | 23.3 (15.5) | 10.0–62.2 | |
| 4003 | 12 | 18.5 (6.12) | 11.9–30.3 | |
| 5718 | 9 | 11.8 (1.85) | 8.8–14.7 | |
| All individuals | 933 | 13.9 (5.52) | 1.9–97.5 | |
| All except 5703 | 221 | 12.3 (5.58) | 5.9–62.2 | |
| Sargasso | 5862 | 20 | 17.1 (16.83) | 10.3–87.6 |
| 3016 | 12 | 30.4 (17.87) | 14.2–65.1 | |
| 3012 | 9 | 20.8 (1.39) | 18.5–23.0 | |
| 5800 | 7 | 15.8 (3.53) | 11.1–21.2 | |
| All individuals | 48 | 20.1 (15.54) | 3.3–87.6 | |
| Both | All individuals | 981 | 14.2 (6.50) | 1.9–97.5 |
| All except 5703 | 269 | 13.8 (8.74) | 5.9–87.6 |
Pooling across all individuals in both study areas, the average duration of a sperm whale peduncle dive was 14.2 s (SD = 6.50). Excluding the large sample obtained from the GOS calf resulted in a similar mean of 13.8 s (SD = 8.74).
Intervals between dives had a mean duration of 5.18 s (SD = 2.95) and varied between 1.3 and 33.6 s. Calves generally took one or two breaths between peduncle dives. The bimodal distribution of dive intervals reflects this bimodal breathing pattern (Fig. 2).
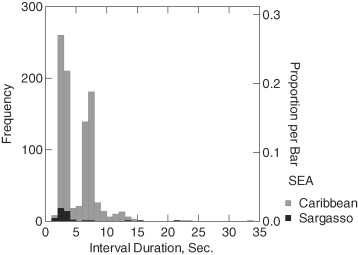
Histogram of surface interval between peduncle dives in seconds. The bimodal distribution is explained by intervals in which sperm whale calves took one or two breaths.
Dive Side
During the great majority of bouts (n= 124 of 139 or 89.2%), dive side was consistent throughout the bout. One would expect that if the side on which calves made peduncle dives was random, the percentages of bouts on each side would be equal (50%:50%). Most bouts of peduncle dives observed took place entirely on the left side of the escort (69.8%), with relatively fewer occurring entirely on the right side (19.4%). When the large amount of data from calf 5703 were excluded, percentages were closer to what one would expect as random but still biased towards the left side (52.7% left; 33.3% right). Calf 5703 showed a clear preference for making peduncle dives on the left side of the escort, as did calves 5725, 4001, 3002, 3016, and 3005. Two calves showed a preference for the right side (5701 and 5862; Table 2).
| Study area | Calf ID | n | % Consistent | % Left | % Right | % Switch | % Left → right | % Right → left | %≥1 switch |
|---|---|---|---|---|---|---|---|---|---|
| Caribbean | 5703 | 82 | 91.5 | 81.7 | 9.8 | 8.5 | 6.1 | 2.4 | 0 |
| 5725 | 13 | 100 | 92.3 | 7.7 | 0 | 0 | 0 | 0 | |
| 5701 | 7 | 100 | 0 | 100 | 0 | 0 | 0 | 0 | |
| 4002 | 6 | 33.3 | 0 | 33.3 | 66.6 | 16.6 | 50.0 | 0 | |
| 4001 | 5 | 80 | 80 | 0 | 20 | 20 | 0 | 0 | |
| 5718 | 3 | 33.3 | 0 | 33.3 | 66.6 | 33.3 | 33.3 | 0 | |
| All | 116 | 88.0 | 71.6 | 16.4 | 12.0 | 6.9 | 5.1 | 0 | |
| Excluding 5703 | 34 | 79.4 | 47.0 | 32.4 | 20.6 | 8.8 | 11.8 | 0 | |
| Sargasso | 3012 | 5 | 100 | 60 | 40 | 0 | 0 | 0 | 0 |
| 3002 | 4 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | |
| 5862 | 4 | 100 | 0 | 100 | 0 | 0 | 0 | 0 | |
| 3009 | 4 | 100 | 50 | 50 | 0 | 0 | 0 | 0 | |
| 3016 | 3 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | |
| 3005 | 3 | 66.6 | 100 | 0 | 33.3 | 33.3 | 0 | 0 | |
| All | 23 | 95.7 | 60.9 | 34.8 | 4.3 | 4.3 | 0 | 0 | |
| Both | All | 139 | 89.2 | 69.8 | 19.4 | 10.8 | 6.5 | 4.3 | 0 |
| Excluding 5703 | 57 | 86.0 | 52.7 | 33.3 | 14.0 | 7.0 | 7.0 | 0 |
Switching sides during a bout of peduncle dives was rare, accounting for 10.8% of all bouts (14% when excluding calf 5703). None of the animals included in this analysis switched sides more than once per bout (Table 2). We did observe one calf in the Caribbean who switched from the left side to the right and then back within a single bout, but this animal was excluded from this analysis because it was identified only once.
The mean dive duration of all left side dives was 14.2 s (SD = 6.34) and the mean dive on the right side was 14.0 s (SD = 7.77) in length. A t-test indicated that there was no difference in mean dive duration between sides (t= 0.441, df = 290.1, P= 0.682).
Discussion
Cetaceans spend the majority of their time underwater where it is difficult for researchers to observe their behavior. As a result, detailed observations and data on complex subsurface behaviors are either incomplete or lacking. In an attempt to accommodate, biologists often consciously or unconsciously make assumptions about the function of certain behaviors based on observations of the whales at the surface, and the occurrence of observable surface behaviors are often used as proxies for specific behaviors presumably being performed subsurface (Whitehead and Dufault 1999).
Sperm whale calves have long thought to be suckling when performing a commonly observed surface behavior of repeated, short dives alongside an adult escort (Beale 1839; Best et al. 1984; Gordon 1987, 1991a; Gordon et al. 1998). Although similar assumptions have been made with regards to humpback whale (Megaptera novaeangliae) suckling (Clapham and Mayo 1987), our recent underwater observations of sperm whale calves performing this behavior question the validity of these assumptions. During repeated underwater observations made by a snorkeller, we did not observe a single apparent suckling dive during which the calf attempted to make oral contact with the mother's nipple. This begs the question, does the surface behavior of many short dives beneath the peduncle of an escorting adult, described in Gordon (1987), generally indicate suckling? Here, we discuss alternative hypotheses for the function of the repeated, short dives beside adult escorts and explore alternatives to the manner in which sperm whale calves could ingest milk.
At this juncture, it is important to make clear three points of terminology before beginning our discussion:
- 1
Gordon et al. (1998) defined suckling attempts as repeated short dives beneath the peduncle of an adult whale but pointed out that it was not always possible to determine if calves were receiving milk. Here, we refer to this observable surface behavior performed by the calf as “peduncle diving” and do not assume that it is an action related to suckling or that suckling is occurring.
- 2
As in Cowie et al. (1951), we use “suckling” to refer to the behavior of a calf whose aim is to ingest milk from an adult female, whatever those actions may be.
- 3
A “bout” of peduncle dives is defined as a series of peduncle dives that began at the first observed peduncle dive when the dorsal fin disappeared beneath the surface, and that ended when the calf stopped making peduncle dives, swam away from the escort at the surface, or if the escort started a fluke-up foraging dive.
To begin the discussion of the function of peduncle diving, let us first assume that the peduncle diving is not related to suckling. If this were the case, what we observed was a different behavior having a different function. One reasonable explanation is that the calf may have been attempting to maintain some form of echelon or infant position as described in bottlenose dolphins, Tursiops spp. (Reid et al. 1995, Gubbins et al. 1999, Mann and Smuts 1999). By peduncle diving, calves may facilitate swimming by positioning itself under its escort to reduce drag (Norris and Prescott 1961, Mann and Smuts 1999). However, if the purpose of echelon or infant position is to reduce the amount of energy spent by the calf, why do sperm whale calves frequently (every ∼14 s) surface during peduncle diving and spend only a few seconds at a time in the preferred position? This is likely more energetically expensive than simply swimming beside of the escort, as seen in bottlenose dolphins (Mann and Smuts 1999).
Other evidence suggests that peduncle dives are related to suckling. As mammals, suckling must occur for sperm whale calves to survive. Peduncle diving behavior is the only behavior currently described for sperm whales that is performed solely by animals within the size range (<7.6 m) of unweaned animals (see Best 1974). Additionally, the social evidence that peduncle diving occurs mostly with one particular adult and occasionally with other females suggests that calves are suckling primarily from their mothers and occasionally from allomothers (Gordon 1987, Gero 2005). For the GOS calf, peduncle diving was only observed with its mother; although the calf was observed escorted by other adult females on many occasions (Gero 2005). These points all support Gordon's (1987) assumption that repeated peduncle dives alongside an adult escort are generally indicative of suckling behavior and that the commonly held assumption is valid.
Nonetheless, we found a complete lack of underwater observations of attempts to make oral contact with the escort's nipples by a particular calf observed over several days. This suggests that it is Best and colleagues' (1984) assumption about the manner in which the sperm whale calves suckle that needs to be examined.
If we assume that Best et al. (1984) were correct in that sperm whale calves suckle by holding their mother's nipple in the gape of their jaw, there might be a number of reasons why this particular calf was making unsuccessful suckling attempts. The most likely explanation is that the calf might have been attempting to initiate suckling by stimulating the female using a behavior similar to the mammary bump observed in Atlantic spotted dolphins, Stenella frontalis (Miles and Herzing 2003), bottlenose dolphins (Peddemors et al. 1992), and killer whales, Orcinus orca (Asper et al. 1988), but was not succeeding. Stimulating the mother to induce the let-down of milk is common among ungulates (reviewed in Lent 1974) and elephants (Langbauer 2000), a species with many social and ecological similarities to sperm whales (Weilgart et al. 1996, Whitehead 2003).
The calf may use this mammary bumping behavior to induce milk let-down, while milk transfer may be occurring out of sight of the observers, either at depth or at night when it is impossible to observe. Based on studies of other cetacean species [bottlenose dolphins: McBride and Kritzler (1951), Eastcostt and Dickinson (1987); belugas (Delphinapterus leucas): Drinnan and Sadleir (1981), Russell et al. (1997); common dolphins (Dephinus delphis): Logan and Robson (1971); and Commerson's dolphins (Cephalorhynchus commersonii): Joseph et al. (1987)], which suggest that many small cetacean calves suckle at least once every 26–40 min, a delay of 12 h for nocturnal suckling in sperm whales seems unlikely. In fact, studies on small cetacean species found no circadian rhythm in suckling behavior (Drinnan and Sadleir 1981, Asper et al. 1988, Russell et al. 1997). Increased nocturnal suckling has been observed in large terrestrial mammals, such as the elephant (Andrews et al. 2005), as well as in bottlenose dolphins, but the authors suggested that it was likely due to reduced human interference by the caretakers and this may not serve as evidence for a circadian rhythm (Eastcostt and Dickinson 1987). The presence of the research vessel or the snorkeller in the water may have resulted in a similar disturbance to the mother–calf pair, which may have prevented the calf from suckling successfully, but unlike the case of this sperm whale calf, suckling was also always observed throughout the day with the dolphins and elephants.
Instead, our inability to observe suckling may have been a result of its occurrence at depths beyond the visibility of the observer. Many marine or aquatic mammalian species, including small cetaceans (McBride and Kritzler 1951, Logan and Robson 1971, Slijper 1979, Drinnan and Sadleir 1981, Eastcostt and Dickinson 1987, Joseph et al. 1987, Russell et al. 1997), mysticetes (Thomas and Taber 1984), manatees (Hartman 1979) and dugongs (Anderson 1984), as well as hippopotami (Brown 1924), nurse their young at or very near (within a few meters) the surface. This is thought to occur because young calves are only able to remain submerged for very short periods of time (Slijper 1979). Unlike the young of small cetaceans, sperm whale calves have been recorded making dives up to 320 m (Gordon 1987). As such, sperm whale calves are able to accompany their mother beyond an observable depth in order to suckle. However, in deep-diving cetaceans like the sperm whale, major physiological systems are shut down at depth, making it unlikely that suckling is occurring at great depths (Kooyman et al. 1981). Thus, it is potentially feasible that sperm whales may be suckling in a unique manner in which milk transfer occurs at depths beyond the visibility of observers but shallower than depths which are physiologically challenging. But why, in contrast to other cetaceans, would sperm whale suckling occur at depth where they must deal with the effects of pressure, breath-holding, and oxygen management when there appears to be little reason for suckling to not occur at the surface?
If suckling is not occurring solely overnight or at depth, the question remains why successful suckling was not observed. As sperm whales are estimated to suckle until they are about 2 yr old (Best et al. 1984), it is extremely unlikely that a sperm whale calf estimated to be 3 mo old based on its size was already weaned, and peduncle diving is only seen from calves.
Alternatively, successful suckling and the ingestion of milk may have occurred during these underwater observations but through the nasal passage rather than the mouth. We saw that the calf repeatedly pushed its blowhole onto the genital area of their mother. Based on these observations, we suggest that sperm whale calves may suckle by placing their blowhole to their escort's nipple and ingesting milk via active ejection by the adult into the nasal passages. Although most cetaceans grasp the nipple between their tongue and palate, it seems unlikely that sperm whales can do so due to the unique shape of their head and reduced, under-slung lower jaw (Slijper 1979). If this proposed suckling method were occurring, it would be an adaptation unique to sperm whales and a novel method of suckling not previously described for any other mammalian species.
Although seemingly unlikely, nasal suckling may be anatomically possible for sperm whales. We suggest that the process proceeds as follows: The calf presses its blowhole firmly against its mother's nipple. Milk is then actively ejected by the mother, as in other cetaceans (Slijper 1979), filling the calf's left nasal passage. As the calf disengages, the blowhole is sealed and milk is retained in the nasal passage between the blowhole and the nasopharynx by the intranarial larynx, often called the “goosebeak” due to its goose head-like appearance (Slijper 1979), and the palatopharyngeal sphincter that surrounds the larynx and separates the nasal passages from the oral cavity (Reidenberg and Laitman 1987). Finally, upon surfacing, the calf is able to allow the passage of milk from the nasal passages to the mouth by either withdrawing the goosebeak from its intranarial position or by dilating the palatopharyngeal sphincter.
It was suggested previously that sperm whales may have a unique ability to take in fluids through their blowhole and into their nasal passages (Clarke 1970, 1978). We suggest that the milk would pass through the left nasal passage as it bypasses the sound production mechanisms and connects directly from the blowhole to the nasopharynx (Wahlberg et al. 2005). Even though the nasal anatomy of the sperm whale is homologous to that of other odontocetes, Wahlberg et al. (2005) found that sperm whales have the ability to use their left and right nasal passage independently of each other, such that they are able to breath through the left nasal passage while producing vocalizations with the right.
The position of the pharynx and larynx in the neck or head is the principal determinant in a mammal's ability to swallow, breath, and vocalize (Crelin 1976, Laitman et al. 1977). The odontocete nasopharynx and intranarial larynx likely evolved in order to separate the digestive and respiratory systems and to allow for the production of vocalizations while ingesting food (Reidenberg and Laitman 1987, Cranford et al. 1996). We suggest that this novel suckling method resulted as a by-product of the selection for the spermaceti organ and the development of a large nasal complex that led to an inability to suckle, or a difficulty in suckling, in a manner common in other cetaceans. Thus nasal suckling became useful or necessary even though the anatomical structures of the nasopharynx and larynx, which existed in a shared ancestor of the sperm whales and the remaining odontocetes (Norris 1975, Cranford et al. 1996, Cranford 1999), evolved to prevent food entering the respiratory system. We suggest that the sperm whale may be able to withdraw the goosebeak, as in the Indus river dolphin (Platanista gangetica minor; Pilleri et al. 1976a, b; Pilleri 1979; Purves and Pilleri 1983), or dilate the sphincter, which surrounds it allowing milk down into the oral cavity.
The need to force the milk down to the esophagus from the sinuses when nasal suckling could explain why sperm whale calves repeatedly surface for air between short “suckling” dives, unlike in bottlenose dolphins (Mann and Smuts 1999), belugas (Drinnan and Sadleir 1981), and killer whales (Asper et al. 1988), which do not have to surface between suckling attempts or when switching mammaries. Other marine mammal calves that can remain submerged for longer periods of time (e.g., manatees, Trichechus manatus; Hartman 1979, Slijper 1979) do not surface after each suckling attempt as seen in sperm whale calves.
This hypothesis should be treated with caution because the data were collected primarily from one calf over a 4-mo span; approximately one-sixth of the period during which sperm whale calves suckle (∼2 yr, Rice 1989). It is possible that sperm whale calves can suckle through either mouth or nose, with the preponderant method varying among calves or with age. Work is ongoing to collect film and photographic evidence of nasal or oral suckling during peduncle diving behavior. To date, much of the data replicates the behavior described here with calves pressing their blowholes to their escort's underbelly whether they were collected in the Azores, Dominica, the Sea of Cortez, or other locations, although a few observations suggest the calves may role onto their side while under their escorts. It is also important to note that we provide no evidence of milk transfer from the escort to the calf via the nasal passage. Attempts were made in the winter of 2006 to collect blow exudate from sperm whale calves to determine if milk proteins were present in the animal's blow. A hand-held 12-m pole with a collector at the tip was used to sample sperm whale calf blow, both while suckling and while resting at the surface for comparison. Unfortunately, no samples were successfully collected during the brief 1-mo field season, thus no advances could be obtained in this regard. Future work will also focus on discounting the disturbance explanation by exploring the correlation between distance from the research vessel and the behavior observed. A towed video array will be used to film subsurface behavior while minimizing disturbance and investigate what occurs after the mother–calf pair dive beyond the reach of human observers.
Whether milk was being transferred successfully or not, individual sperm whale calves showed a preference for making peduncle dives from a particular side of the escort. Most bouts were consistently on one side, and switching sides was relatively rare. Although not quantified, other small cetaceans have been observed switching sides during suckling (Asper et al. 1988, Mann and Smuts 1999). Lateral asymmetries of behavior are common in both humans and non-human primates (Springer and Deutsch 1989, Bradshaw and Rogers 1993, Hopkins and Morris 1993). Behavioral asymmetries have also been identified in other cetaceans (Kasuya and Rice 1970, Hoese 1971, Caldwell and the Dolphin Project 1993, Clapham et al. 1995, Marino and Stowe 1997). This study finds a left-side bias in the apparent suckling attempts in sperm whales in both study areas; however, the duration of a given suckling attempt did not differ between sides. A strong lateral asymmetry could be taken as evidence of nasal suckling, given that the sperm whale's blowhole is displaced to the left; however, a larger data set will be required to show this with any certainty.
Due to obvious problems in observing these animals subsurface in the wild, our understanding of the behavior, development, and mother–calf interactions of these and other large whale calves is plagued by gaps. Here, we have begun to address those gaps while examining their apparent suckling behavior. Due to the sperm whales' complex social structure and intriguing combination of ecological and life-history traits, a more complete understanding of calf behavioral development is important and could give insight into the evolution of the matrilineal social units, deep-diving ability, and the development of the anatomically unique sperm whale nose.
Footnotes
Acknowledgments
Research in Dominica was carried out under a scientific research permit (SCR 013/05-02) provided by the Ministry of Agriculture and Environment of Dominica. We would like to thank all of Balaena's crew members; Paul Brodie for his expert opinion on the anatomical likelihood of nasal suckling and his kind words of encouragement in regards to bringing forward a hypothesis which at first seems unlikely; Tyler Schulz for providing the length measurements from his unpublished work; Dan Engelhaupt for his genetic analysis and Ron Burns at Northwoods DNA Inc. for completing much of the genetic lab work; members of the Read Lab at the Duke University Marine Laboratory for their hospitality while in North Carolina; Andrew Amour and the staff at the Anchorage Hotel and Dive Center for their kind support while in Dominica; the crews of the various whale watching groups in Dominica for their cooperation; and Vivien Hannon from Digital Media Services for her help cleaning up the underwater photographs. Don Bowen, Marty Leonard, Marie Auger-Méthé, Hilary Moors, and Lindy Weilgart each provided helpful comments on drafts. The manuscript was greatly improved by comments from three anonymous reviewers. This project was supported by operating and equipment grants to H. Whitehead from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Whale and Dolphin Conservation Society, to Luke Rendell from the U.K. Natural Environment Research Council, and S.Gero was supported during the course of this study by an NSERC Postgraduate Scholarship (PGS-M).




