ASSESSING KILLER WHALE PREDATION ON STELLER SEA LIONS FROM FIELD OBSERVATIONS IN KENAI FJORDS, ALASKA
Abstract
The behavioral and predatory patterns of Gulf of Alaska (GOA) transient killer whales (Orcinus orca) were studied between 2000 and 2005 using remote video and vessel-based observations near the Chiswell Island Steller sea lion (Eumetopias jubatus) rookery and in the broader Kenai Fjords (KF) region of the northern GOA. GOA transient killer whales were observed on 118 d over the 6-yr period; the median group size was two (range: 1–9). Nine predation events were observed from vessels and an additional sixteen were inferred from remote video studies; all involved Steller sea lions. Estimates from field observations suggest that fifty-nine sea lions were consumed over the summer seasons of 2002–2005; whereas estimates based on published caloric requirements of transient killer whales would suggest a loss of 103 sea lions over the same time period. GOA transients spent a large proportion (43%) of their time resting which may be a strategy for conserving energy. Predation on sea lion pups at the Chiswell Island rookery was greatest during years when a single killer whale was foraging alone and when a 1.5-yr-old calf was evidently being trained to handle prey. Predation on pups was low during years when killer whales were foraging in groups and were observed and presumed to be taking mostly juvenile sea lions. Our study suggests that GOA transients are having a minor effect on the recovery of Steller sea lions in the GOA.
The western stock of Steller sea lions (Eumetopias jubatus) has declined dramatically since the 1970s between Russian far eastern waters through the Gulf of Alaska (GOA) (Braham et al. 1980, Loughlin et al. 1992, Loughlin 1998) and is currently listed as endangered (U.S. Federal Register 62:30772–30773). Leading hypotheses for the decline of the western stock include nutritional stress (Calkins and Goodwin 1988, Alverson 1992, Rosen and Trites 2000), direct or indirect effects of commercial fisheries (Hennen 2006) and predation by killer whales (Orcinus orca; Springer et al. 2003, Williams et al. 2004). The substance and significance of those hypotheses are matters of continuing debate; although it is difficult to test any of them because of the lack of key empirical data. Current field research can, however, lead us to a better understanding of the relationship between killer whales and the potential recovery of Steller sea lion stocks.
Killer whales in the Eastern North Pacific Ocean are divided into three genetically distinct ecotypes: resident, offshore, and transient (Hoelzel and Dover 1991, Barrett-Lennard 2000, Ford and Ellis 2000). Evidence indicates that transient killer whales (Orcinus orca) eat a variety of mammals and occasionally birds (Bigg et al. 1987, Morton 1990, Baird et al. 1992, Ford et al. 1998, Saulitis et al. 2000, Heise et al. 2003, Herman et al. 2005, Vos et al. 2006). Transient killer whales are characterized by a fluid social structure (Baird and Dill 1996, Ford et al. 1999, Baird and Whitehead 2000), small groups of 2–7 individuals, and infrequent vocalizations (Morton 1990, Baird and Dill 1996, Ford et al. 1999, Saulitis et al. 2000, Deecke et al. 2005).
There are three genetically and acoustically distinct subpopulations of transient killer whales identified so far in the Eastern North Pacific (Barrett-Lennard 2000): (1) The AT1 transient group has only been documented in the Prince William Sound/Kenai Fjords (PWS/KF) region (Matkin et al. 2005). (2) The GOA transient subpopulation consists of approximately ninety-three individuals (Angliss and Outlaw 2005). Whales from this group have been photographed from southeastern Alaska to Kodiak Island (Matkin et al. 1999a), and observed preying on or harassing mostly Steller sea lions but also Dall's porpoises and sea otters (Enhydra lutris; Saulitis et al. 2000). (3) West coast (WC) transient killer whales inhabit waters from central California through southeastern Alaska (Ford et al. 1999, Angliss and Outlaw 2005).
We studied the ecology of GOA transient killer whales and the significance of their predation on Steller sea lions using a combination of boat survey and photo identification techniques paired with observations of killer whale activity near a Steller sea lion rookery using remotely operated, real-time video cameras. Our observations of killer whale predation rates and behavior in the field are compared with estimates of potential killer whale predation on Steller sea lions based on estimated metabolic requirements (Kriete 1995, Williams et al. 2004).
Methods
Study Area
Observations of killer whales were made in KF, an area of glacial fjords and inlets on the eastern Kenai Peninsula in south-central Alaska (Fig. 1). The three primary fjords in this area, running north to south, are Resurrection, Aialik, and Harris bays. Resurrection Bay is the largest fjord, extending about 48 km and containing three year-round Steller sea lion haul-outs (Fig. 1). The number of animals using those haul-outs is highest in winter and greatly reduced during late summer.
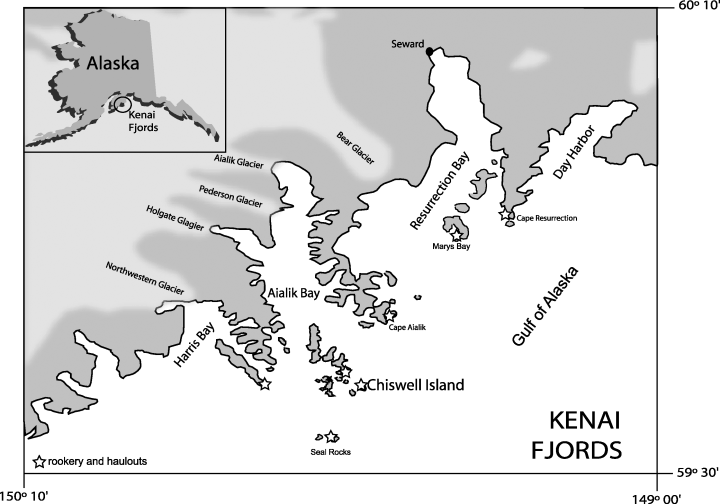
Map of the study area (Kenai Fjords) showing the location of Chiswell Island. The position of the rookery at Chiswell Island and the main sea lion haul-outs are labeled with stars.
Aialik Bay, a deeply forged inlet, extends approximately 41 km from the face of Aialik Glacier to the GOA. Harris Bay forms at the mouth of Northwestern Glacier and Northwestern Lagoon. Neither Aialik Bay nor Harris Bay have regular Steller sea lion haul-outs, but just offshore of Aialik Bay is an archipelago of seven major islands including Chiswell Island (59°36.10′N, 149°34.15′W; <1 km2), home to a small rookery of Steller sea lions (about ninety breeding females) and the focal point of this study (Fig. 1). There are three additional haul-outs in this area with sea lion attendance varying between seasons (Fig. 1).
Field Methods—Chiswell Island Remote Video Observations
Remotely operated cameras were initially installed on Chiswell Island in October 1998 to study sea lion behavior. The six weatherproof cameras overlooking the rookery were equipped with pan, tilt, zoom (18× optical and 12× digital), and windshield wiper/washer functions. The cameras were controlled with ultra-high frequency (UHF) signals sent from the Alaska SeaLife Center (ASLC), while live video signals were returned to ASLC via microwave transmission (Maniscalco et al. 2006).
Camera monitoring of Steller sea lions consisted of 2–4 census counts per day, recording pupping behavior, documenting seasonal use patterns of the rookery by sea lions, and performing individual identification of animals with natural markings such as scars and fungal patches or of tagged/branded animals. These studies were expanded in 2001 to focus on maternal investment, with daily observations conducted from sunrise to sunset during all hours of the day from late May through at least August (Maniscalco et al. 2006).
In 2000, remote cameras were also used to provide information on killer whale predation on Steller sea lions. Typically one camera was used to observe sea lion activity while a second camera viewed the rookery and water adjacent to the rookery from a wide-angle perspective. Activities of killer whales in the vicinity of the Chiswell Island rookery were followed as closely as possible in real time using the remote video cameras and recorded in high quality VHS format for later review. Because positive identification of individual whales was not always possible from video images, identifications were also determined using concurrent data from vessel surveys, general morphology and unique behavioral patterns of the whales, and by composition of whale groups.
Predation of Steller sea lion pups by killer whales was inferred if subsequent to the presence of a transient killer whale(s) at the rookery, the absence of a lactating female's pup could be confirmed for at least a week while the female was observed at the rookery. Pups were also tagged in 2000 and 2001 (thirty and seventeen, respectively) and those that were seen consistently prior to a transient killer whale visit were considered preyed upon if they were consistently missing following the visit. The proportion of pups lost to identifiable lactating females and the proportion of tagged pups preyed upon were then extrapolated to the number of pups on the rookery during periods of killer whale presence to get two independent estimates of total predation. In addition to inferred predation, some pups were observed to be taken from the rookery by killer whales. Those were considered to be among the number of pups estimated to be taken as per the aforementioned methods.
Vessel Surveys
Small research vessels conducted regular surveys of the KF region and also responded to observations of killer whales made with the remote cameras or reports by tour vessel operators between May 2002 and September 2005. Boat-based observations were also used to confirm whale identifications and sea lion kills observed from the remote video cameras and to assess killer whale behavioral patterns.
Individual killer whales were identified from photographs of the left side of their dorsal fin and light saddle patch using high quality cameras and lenses with black and white film. Photographs were checked against an existing photo-catalog (Matkin et al. 1999b) and other unpublished photographs. Biopsy samples of at least one whale in each group were collected whenever possible to genetically verify population affiliation.
From mid May through mid September, approximately sixteen wildlife tour vessels also searched KF for marine mammals and seabirds on tours ranging from 2.5 h to 9.5 h in duration daily. Tour vessel operators were trained to distinguish between the different ecotypes of killer whale. Killer whale sightings from tour vessels were confirmed by researchers aboard their own vessels whenever possible. When researchers were unable to confirm sightings, data collected by trained tour-boat operators were only used when supported with photographs, video footage, and/or a detailed report.
Behavioral Patterns
During vessel-based observations, the whales' locations were plotted at approximately 15-min intervals using a global positioning system (GPS) until an encounter was terminated. Time spent in different behavioral states was determined by noting the time at which a change in behavioral state occurred. Behavioral states were partially derived from definitions of transient killer whale behaviors by Baird and Dill (1996) and by Saulitis (1993). The following basic behavioral states were identified: travel, forage, rest (which included behaviors defined as mill by some authors), and social (also defined as surface-active mill by Saulitis et al. 2005). Standard deviations of the proportion of time spent in different behavioral states were generated by resampling 10,000 times with replacement from a vector of observed behavior, by minute, for all whales (Efron and Tibshirani 1986).
Travel—Whales moved in a line-abreast pattern, surfacing and diving with some synchrony, and in a consistent direction at moderate to high speed. The whales were silent and often moving from one foraging area to another. Dive patterns were regular, with several short (<1 min) dives followed by a longer dive (up to 12 min).
Foraging—Foraging was divided into (1) offshore foraging, (2) nearshore foraging, (3) haul-out foraging, and (4) Steller sea lion harassment. Offshore foraging was defined as all foraging activities occurring farther than 1 km from the shoreline and away from rookeries and/or haul-outs. Foraging whales might travel >1 km underwater. During offshore foraging, whale movements were somewhat erratic or zigzagging while moving in one general direction. Nearshore foraging occurred when whales foraged within 1 km of shore, frequently entering bays and channels and exploring rocky outcrops and shoals. Haul-out foraging occurred within 500 m of a haul-out or rookery. Steller sea lion harassment was most often linked to haul-out foraging and was denoted by a strong behavioral change in the sea lions, such as increased agitation and movement, sometimes accompanied by loud vocalizations. Harassment was usually characterized by the killer whales passing close to the haul-out/rookery and often accompanied by surface charges, tail slaps, or breaches.
All aggressive interactions between whales and potential prey were termed harassments, which included instances where a kill was suspected but not confirmed or when potential prey exhibited a fright or flight response or other strong behavioral reactions to the killer whales. Only events that provided positive evidence of a kill were categorized as predation. Evidence of this behavior included observations of prey in a whale's mouth, or repeated diving in one location in conjunction with the presence of blood, oil, and/or prey remains in the water. For nonpup sea lions it is unlikely that predation went undetected because substantial disturbance occurs when the sea lion is taken. Pup kills were more likely missed, although observations from the remote video system detected them as missing pups.
To approximate energy expenditure, killer whale behaviors were lumped into three categories of increasing intensity following Kriete (1995). Resting was given an activity level of 1; travel and foraging behaviors were level 2; harassment, feeding, and social behaviors were level 3.
Predation Rates
The extent of predation on Steller sea lions in KF was determined from one long-term period of remote video tracking (10 d) and one period of continuous research vessel tracking (36 h) of the killer whales and their observed takes during those periods. Those kills were then extrapolated to all days that GOA transient killer whales were known to be present in the region over the months of May through September for the years 2002 through 2005 when dedicated research vessel surveys occurred in addition to remote video observations.
Energy content for different age-classes of Steller sea lions were applied to the observed prey and an 85% assimilation efficiency by killer whales was assumed (Williams et al. 2004). Our observations of sea lion kills by killer whale predation were compared to estimated predation rates needed to satisfy the energy requirements of killer whales (Williams et al. 2004). Daily energetic requirements of the individual killer whales we observed were based on age, sex, and reproductive status of the whale (Kriete 1995, Williams et al. 2004). Lactating females up to 4 mo postpartum were assumed to have 1.45× the energetic needs of nonpregnant and/or lactating adult females (Kastelein et al. 2003). When the sex of a killer whale was unknown and it was too large to be classified as a juvenile or too small to be an adult male, it was considered to have had the energetic requirements of an adult nonlactating female.
Results
Between January 2000 and September 2005, ASLC remote cameras were monitored in real time for a minimum of 10 min to a maximum of 22 h on 1,874 d (19,980 total h). Monitoring averaged 7.0 h/d (±3.7 h, SD) from September through April in all years and was greatest from May through August in the years 2001–2005 (mean = 15.3 h/d ± 3.4).
Dedicated vessel-based surveys for killer whales were conducted on 574 d in the years 2002 through 2005. Those surveys occurred primarily during summer (May–September) and occasionally during other seasons. A few opportunistic vessel surveys took place in 2000 and 2001.
GOA transients were observed in the vicinity (<1 km) of the Chiswell Island Steller sea lion rookery on 84 d using the remote video cameras. We also encountered GOA transients from research vessels throughout the study area on 37 d. Fifteen of those encounters overlapped with observations from the remote video cameras. An additional 12 d of GOA transient sightings were contributed from experienced tour operators or from researchers on shore for a total of 118 sighting days. The number of sightings varied from year to year (range: 13–35 d) and was not related to observer effort (Pearson coefficient = 0.162, P= 0.760).
Thirteen different GOA transients (ten adults and three calves) identified during the study period were previously identified individuals and twenty-six were new or could not be identified due to quality of the photographs. Photographs or video of six of those twenty-six whales were of sufficient quality to be catalogued as new reidentifiable individuals, resulting in a minimum of nineteen and maximum of thirty-nine individuals observed. Group size ranged from one to nine (median = 2) with a total of 268 GOA transient whale days (number of individuals present per days observed). Most GOA transient sightings (77%) occurred at the Chiswell Island sea lion rookery, often during the months of August and September, just after the peak in sea lion abundance (Fig. 2).
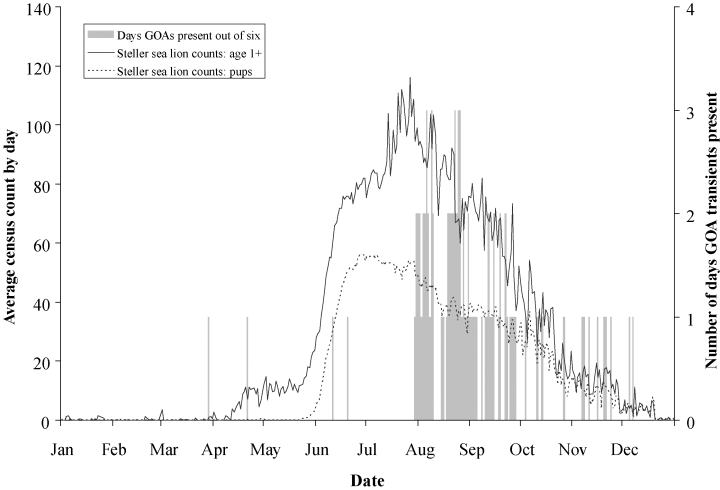
Average daily Steller sea lion census counts for age 1+ (solid line) and pups (dashed line) at the Chiswell Island rookery between 2000 and 2005. The decline in adult and pup counts beginning in August mainly reflects the movements and subsequent abandonment of the rookery by female sea lions and their pups at the end of the summer and is not necessarily related to killer whale presence or predation. The second Y-axis represents the number of days GOA transients were seen at the rookery considering that each specific date occurred six times between 2000 and 2005 (solid gray bars).
Behavioral Budgets
Behavioral budgets for GOA transients were derived from 9,042 min of vessel-based observations. Observations were made throughout the study area, but most were made in the Chiswell Island region. Overall, GOA transients spent the greatest proportion of their time resting (43%± 0.5%) and foraging/feeding (33%± 0.5%). Foraging near shore (8%± 0.3%) and at sea lion haul-outs (14%± 0.4%) was observed much more frequently than offshore foraging (1%± 0.1%). The intensity of behaviors averaged 1.84 on a scale of 1 to 3 and did not change between morning (0600–1200), afternoon (1200–1800), and evening (1800–0000; χ24= 6.00, P= 0.20). However, during one overnight (0000–0600) period of observation GOA transients were observed to rest only.
A typical feature of GOA transients' behavioral pattern near Chiswell Island was the alternation of foraging bouts with periods of resting or social activity away from the rookery, generally within 2 km and, often, on the side of the island opposite the rookery. Boat based follows determined that short-term absences often were characterized by circuitous paths that returned to the rookery. Social activity often included tail slaps, breaches, and rubbing among whales.
Predation
We observed predation by GOA transients on nine Steller sea lions during this study (Table 1). During one continuous 36 h encounter with a group of five GOA transients in 2002 (AT126–AT130; one adult male, two lactating females, and two young-of-the-year), two juvenile Steller sea lions, estimated to be 150 kg each, were killed and consumed. At the Chiswell Island rookery, one juvenile Steller sea lion kill was observed in 2001 in addition to five pups killed during 2003 (Table 1). Sixteen sea lions were also harassed by GOA transients between 2002 and 2005 without direct evidence of a kill. We did not observe GOA transients killing any other species of marine mammal, except an unidentified pinniped killed in Aialik Bay during June of 2005. Three sea otters were also harassed but none were confirmed as kills.
| Date | Location | Number of kills | Age | Whale ID | Observer |
|---|---|---|---|---|---|
| 31 July 2001 | Chiswell Island | 1 | Juvenile | AT109 | Tour vessel |
| 18 May 2002 | Aialik Bay | 2 | Juveniles | AT126–130 | Research vessel |
| 2 August 2003 | Chiswell Island | 1 | Pup | AT109, 111, 125 | Camera and research vessel |
| 4 August 2003 | Chiswell Island | 1 | Pup | AT109, 111, 125 | Camera and research vessel |
| 6 August 2003 | Chiswell Island | 3 | Pups | AT109, 111, 125 | Camera and research vessel |
| 25 June 2005 | Cape Resurrection | 1 | Juvenile | AT109, 111, 125 | Research vessel |
The GOA transient AT109, a postreproductive or infertile female, was the whale observed most often during this study and was present in 80% of our sightings. During 2000 and 2001, AT109 traveled alone, making frequent visits to the Chiswell Island sea lion rookery and was often observed by remote cameras to make repeated passes <25 m from the rookery. Indirect evidence based on the tracking of sea lion mother/pup pairs and tagged sea lion pups (see Methods) suggests that it fed primarily on pups at that time. The period of most intense predation occurred during 10 days beginning 30 July 2001 when an estimated ten pups were taken (Table 2). We could not detect if older sea lions were taken in addition to pups because they may leave the rookery since they are not dependent on a lactating female. If we assume that AT109 took only pups during 2000 and 2001, then based on the number of days she was present, she would have taken fifteen (23%) and twenty-two (42%) pups born on the rookery during those years, respectively.
| Year | GOA transient presence | GOA transient(s) identification | No. pups tracked | No. pups remaining | % taken | Total number pups taken | |
|---|---|---|---|---|---|---|---|
| 2000 | 18–28 Aug | AT109 | 30t | 28t | 7% | 4 | |
| 2001 | 30 Jul–7 Aug | AT109 | 27 and 16t | 22 and 13t | 19% | 10 | |
| 23%b | |||||||
| 21–26 Aug | AT109 | 14 | 13 | 7% | 2 | ||
| 2002 | 30 Jul–7 Auga | None | 23 | 23 | 0% | 0 | |
| 21–26 Auga | None | 13 | 13 | 0% | |||
| 2003 | 31 Jul–10 Aug | AT109, 111, 125 | 36 | 33 | 8% | 5 | |
| 2004 | 27–31 Aug | AT109, 111, 125 | 30 | 30 | 0% | ||
| 8–15 Sep | AT109, 111, 125 | 26 | 26 | 0% | 0 | ||
- a2002 periods were added by way of comparison with 2001 data to express differences in pup loss in years when GOA transients were and were not present.
- bTotal percentage of pups taken based on 12 of 52 pups that were born on the rookery that year.
AT109 was not observed at Chiswell Island in 2002 when she was recorded on only 4 d in KF traveling with AT111 and her newborn calf, AT125. No predation was observed and no pups were recorded as missing from the Chiswell rookery in 2002 during the same time periods that AT109 was present in the previous year (Table 2).
In 2003 AT109 and AT111 returned to Chiswell Island on 18 d between 31 July and 22 September. During that period, they appeared to be training AT125, then 1.5 yr old, to forage. From both remote video cameras and from research vessels, they were observed taking individual sea lion pups from nearby the rookery shoreline and release them alive about 500 m away. All three whales would then lunge at the pups, make occasional tail slaps, and repeatedly pull them under water and set them free. These apparent training bouts lasted approximately 10 min before the pups were consumed. Consumption of older age classes of sea lions was not observed. The loss of five pups determined by the remote video cameras was independently corroborated by observations from the research vessel (Table 1, 2). In addition, at least eight seabirds including common murres (Uria aalge), marbled murrelets (Brachyramphus marmoratus), horned puffins (Fratercula corniculata), and tufted puffins (F. cirrhata) were observed being harassed and either injured or killed by AT125. None of the seabirds were consumed and all were left floating at the surface.
Visits to Chiswell Island by AT109, AT111, and AT125 became less frequent in 2004 (11 d) and 2005 (3 d). However, GOA transient attendance at Chiswell Island in 2004 was similar to 2003 as adult males AT30 and AC13 were present during 5 d in August and 1 d in September. None of these whales were observed preying on pups, nor were pups missing from the rookery following their appearance. They may have targeted older age classes of sea lions in the area, perhaps adult females, as a greater proportion of breeding females were missing after the 2004 breeding season compared to earlier years (ASLC, unpublished data).
Based on our field observations, we estimated the energy intake of an average adult killer whale to be 130,005 kcal/d. Assuming that GOA transients fed only on Steller sea lions while in the KF region during the summer seasons of 2002–2005, a total of five pups, fifty-one juveniles, and three adult female sea lions would have been consumed given the total age/sex ratios of killer whales present during those seasons. If we assume the same amount of predation on pups, previously published estimates of energetic requirements (Williams et al. 2004) would have suggested a total predation loss of five pups, eighty-three juveniles, and six adult female sea lions during the same time period.
Discussion
The nineteen GOA transients observed in this study represent 35% of the fifty-five GOA transients identified in the KF and PWS region since the late 1980s (Matkin et al. 1999b, Barrett-Lennard 2000) and only 3% of all killer whales identified over the years 2002–2004 (Matkin et al. 2005). Other genetically determined killer whale populations that used the area during this study period were AT1 transients (2%), southern Alaska residents (88%), and offshores (8%; Matkin et al. 2005).
The most striking result of this study was that GOA transients were observed to feed only upon Steller sea lions. However, we could not reliably estimate prey preference from October to April because of low observer effort during those months, and we cannot assume that GOA transients fed on Steller sea lions when not in the KF region.
WC transient killer whales from Washington State through Southeast Alaska feed on a variety of marine mammals (Baird and Dill 1995, Ford et al. 1998, Heise et al. 2003, Matkin et al. 2006), and the number of prey species taken has been strongly correlated with the number of predation events observed (Ford et al. 1998). Similarly, AT1 transient killer whales residing sympatrically with GOA transients in KF and PWS have preyed upon harbor seals, Dall's and harbor porpoise (Saulitis et al. 2000), and a northern fur seal (Matkin et al. 2005). We documented nine predation events by GOA transients from research vessels and deduced an additional sixteen from remote video studies. With that many observations, we would have expected more than one prey type to have been taken as suggested by Baird and Dill (1996) and Ford et al. (1998). However, Ford et al. (1998) documented predation events throughout the year and over a much broader area; whereas we observed predation primarily in summer and fall. Furthermore, predation events detected by remote video studies were obviously biased toward predation on Steller sea lions because the cameras were located at a rookery. Yet, over most of the past decade, the GOA transients AT126–AT130 that have preyed upon sea lions during summer in our study have also been observed preying upon and harassing Steller sea lions near Kodiak, Alaska, in winter and spring during the seasonal influx of sea lions,2 which lends some support to the contention that these whales may focus their predation on Steller sea lions in other regions at other times of the year.
The extent to which other GOA transients feed on alternative prey is little known. GOA transients have previously been observed harassing mostly Steller sea lions but also Dall's porpoises and a sea otter in PWS (Saulitis et al. 2000). In addition, the stomach contents of a GOA transient that died in PWS in 1992 contained the flipper tags from 14 Steller sea lions and whiskers from a harbor seal (Heise et al. 2003). Those studies provide additional evidence of a prey preference for Steller sea lions among this genetically distinct group of transient killer whales. However, infrequent sightings of other GOA transients over the past 20 yr (Matkin et al. 1999b, Matkin et al. 2003) suggest that they may forage farther offshore where we lack observations. Steller sea lions rarely travel more than 15–37 km from shore (Loughlin et al. 1998, Loughlin et al. 2003, Raum-Suryan et al. 2004, Briggs et al. 2005). Therefore, GOA transients that feed offshore likely target prey other than Steller sea lions.
The lack of harbor seals in the observed diet of GOA transients in our study is unusual considering harbor seals account for 28% (Straley 2005) to 40% (Matkin et al. 2006) of the transient killer whale diet in northern Southeast Alaska and more than 32% of AT1 transient diet in the KF and PWS region (Saulitis et al. 2000, Matkin et al. 2005). The population of harbor seals in our study area is currently estimated at 800 individuals (ASLC, unpublished data), about twice the local population of Steller sea lions. However, most of the seals aggregate seasonally at the heads of glacial fjords, a habitat that is regularly exploited by AT1 but not GOA transients. It seems likely that there is niche partitioning between the AT1 and GOA transients in this area as sea lions are not seen in the diet of AT1 transients (Saulitis et al. 2000).
Preferential selection of one or a few prey types by some groups in a population may result from cultural traditions and learning. Killer whales have strong matrilineal associations (Baird and Whitehead 2000) and are known to spend years training their offspring to capture prey (Baird 2000). In 2003, AT109 and AT111 engaged intensively in what appeared to be the training of the yearling whale AT125, which included harassing and killing seabirds, harassing sea otters, and prolonged handling times for sea lion pups at the Chiswell Island rookery. Pups taken from near the Chiswell rookery could have been easily and quickly consumed (Williams et al. 2004) if that were the killer whales' only intention. Releasing the pups farther from shore and allowing the year-old calf to lunge, slap, bite, and repeatedly pull them under water strongly suggests the young whale was being trained. Similar observations of mother and yearling killer whales harassing and killing seabirds have been reported in southeastern Alaska (Matkin et al. 2007) and Antarctica (Ballard and Ainley 2005) and in killer whales feeding upon southern sea lions (Otaria flavescens) in Argentina (Hoelzel 1991). Our observations indicate that extensive calf training takes place during the second summer of life and continues less intensively into the next year. In effect, prey preferences may be set at a young age.
With the exception of training offspring, preying upon sea lion pups occurred only when AT109 was foraging alone. Medium to large sea lions may be difficult to handle for a killer whale hunting alone, while hunting in groups allows the taking of larger prey, which can maximize caloric intake (Baird and Dill 1995, 1996). In British Columbia, transient killer whale group size was positively correlated with pinniped prey size (Ford et al. 1998), further suggesting small prey may not be preferred unless hunting alone. Preying upon small animals could be a common strategy for lone, sick, or aging whales as may have been the case with an elderly adult male AT1 transient found dead with remains of five sea otters, a river otter (Lontra canadensis), and two seabirds in its stomach (Vos et al. 2006) and an adult male GOA transient found dead with tags from 14 Steller sea lions that were ≤2 yr old (Heise et al. 2003).
Our estimates of predation by GOA transients on Steller sea lions based on field observations were nearly half of what would be expected based on published estimates of killer whale daily caloric requirements (Williams et al. 2004). It is possible that some predation events during long-term follows of killer whales went undetected, although our observer coverage during those periods was good. In this study, they spent a greater percentage of their time resting (43%) than foraging (23%), which is not consistent with findings from other areas and may account for the low observed rates of prey consumption in this study. Transient killer whales off Vancouver Island spent 63% of their time foraging and 2% resting (Baird and Dill 1995). Similarly, Saulitis et al. (2000) reported foraging and resting to account for 50% and 4%, respectively, of the activity budgets for AT1 transients in the KF/PWS region. If the killer whales spent all of their time in a normal resting state (Kriete 1995), then 93,644 kcal/d or 18.7 fewer juvenile sea lions would need to be consumed for the ratio of male and female killer whales we observed over the 2002–2005 seasons. At the average activity state observed in this study (1.84), the killer whales would need 148,433 kcal/d, which equates to 9.5 juvenile sea lions consumed above our estimate (Fig. 3). Alternatively, if the killer whales had an average activity state of 1.56, which could be accomplished if they spent nighttime hours resting as observed in resident killer whales (Baird et al. 2005) and we observed during one overnight follow, then their consumption would equal our observed estimate of 130,005 kcal/d. However, that assumes a linear relationship between activity level and energy expenditure, which may not be the case (Kriete 1995). We believe that extended rest periods used by GOA transients reduce their metabolic costs, thereby decreasing the need for as large a quantity of prey as would be predicted from the more intense activity budgets of other groups of killer whales such as observed by Baird and Dill (1995).
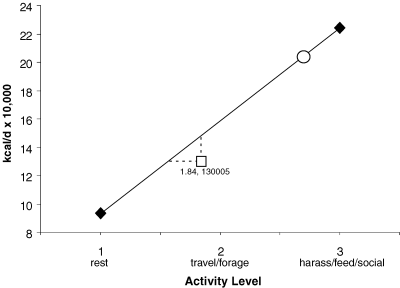
Daily energy expenditure at different activity levels based on Kriete (1995) and adjusted to the proportion of male (10.5%) and female (89.5%) killer whales in this study. The open square represents the average activity state and consumption rate that we observed; the open circle represents the consumption based on previously published work (Williams et al. 2004).
Average predation by killer whales on Steller sea lions in KF accounted for 3% (our field estimates) to 7% (based on published estimates [Williams et al. 2004]) of the local summer seasonal population of sea lions each year (ASLC, unpublished data). This range of mortality due to predation compares to an average annual mortality rate of approximately 20% among Steller sea lions up to 16 yr old in the western stock (Pendleton et al. 2006). However, we observed that predation was concentrated on pups during 2001 and, in later years, on juvenile sea lions, the age-class that may have mortality rates of 42% per year (Pendleton et al. 2006). Pups in the western stock may have a mortality rate of 28% over their first year (Pendleton et al. 2006), and although we estimate a loss of about 42% of the pups born in 2001, that was an atypical year. When predation losses were averaged across the years 2000–2005, an estimated 11% of the pups born at Chiswell Island were lost annually (Maniscalco et al. 2005, 2006).
The GOA transients observed in this study appear to have a selective preference for Steller sea lions as prey during the periods that we observed them. Their rates of predation in KF account for a small proportion of the estimated annual mortality for Steller sea lions and are probably not hindering their recovery in this area. However, caution should be emphasized if comparing these results to other times and areas because the activity budgets and feeding rates of these killer whales may vary during times when not observed in our region. Furthermore, the apparent specialization in predation behavior by this group of transient killer whales should not be extrapolated across transient populations, nor would it be appropriate to extrapolate their effect on Steller sea lion populations to other regions because of differing behaviors between transient groups. Evidence from this study and from other regions in Alaska suggests a complex web of culturally transmitted predatory patterns specific to various groups of transient killer whales complicating the assessment of effects on prey populations.
Acknowledgments
We thank P. Parker, K. Harris, M. Fowler, and E. Teate at the ASLC for the numerous hours spent in front of the Chiswell Island remote observation monitor and L. Albinsson, M. Belk, T. Buckzyna, D. Donovan, S. Lammers, L. Mazzuca, P. Nilsson, A. Ponzo, D. Sullivan, M. Thorsson, M. Robinson, and P. Weiner for assistance with small boat surveys. We also thank E. Saulitis for her contribution of data and expertise in developing and reviewing this paper, and D. Hennen for statistical advice and analysis. This study was conducted under Marine Mammal Protection Act permit number 545–1488–02 issued to the North Gulf Oceanic Society and National Marine Fisheries Service permits (782–1532–00 & 881–1668–00) issued to the Alaska SeaLife Center. Additional Special Use Permits were issued by the United States Fish and Wildlife Service, Alaska Maritime National Wildlife Refuge to conduct this research on refuge lands. This research was supported by the Alaska SeaLife Center Steller Sea Lion Research Program, with funds from the National Marine Fisheries Service.




