Targeting HIV-1 Through Molecular Modeling and Docking Studies of CXCR4: Leads for Therapeutic Development
Abstract
The chemokine receptor CXCR4 is the receptor for several chemokines and major co-receptor for X4 human immunodeficiency virus type-1 strains entry into cell. A three-dimensional model of human CXCR4 was developed by homology modeling using the high-resolution bovine rhodopsin structure as template. Interactions between CXCR4 and flavonoids were investigated using in silico docking studies. The results underscore the potential of these compounds that they may become important new antiviral drugs to combat AIDS. It is worth mentioning also that apart from these existing flavonoids, there are many new compounds that may also be useful as topical agents to inactivate virus, or may act as adjuvants with other antiviral drugs.
Human immunodeficiency virus type-1 (HIV-1) is the causative agent of acquired immunodeficiency syndrome (AIDS), which is characterized as a systemic and fatal disorder (1,2). Since the start of global HIV/AIDS epidemic, more than 30 million people had been infected with HIV (3). Notwithstanding this, by 2003 the Joint United Nations Program on HIV/AIDS estimated that 42 million people worldwide were infected with HIV, and that the virus had claimed the lives of more than 20 million people since the start of the epidemic (http://www.unaids.org; 4). Furthermore, the rate of infection remains on the increase in the developed and developing world. Following the introduction of highly active antiretroviral therapy (HAART) regimes, using combinations of nucleoside/nucleotide reverse transcriptase (RT) and protease inhibitors, HIV has increasingly been considered to be a treatable chronic disease (5). However, there are still needs for new therapeutic agents with improved dosing regimes that are better tolerated with a reduced side-effect burden (6). Side-effects and complicated dosing regimes have led to reduced patient compliance (7,8) and contributed to the emergence of viral resistance. The fundamental difficulties lie in the heterogeneity of HIV and the ability of HIV to evade, through various devices, the hosts’ natural defense mechanisms. Consequently, therapies based on new mechanisms of action are particularly desirable.
HIV gains entry into cells by fusing the lipid membrane of the virus with the host cell membrane which is an event that is triggered by the interaction of proteins between the HIV envelope and cell surface receptors. The virus binds with its gp120 protein to the CD4 receptor forming a complex that undergoes a conformational change creating a co-receptor-binding site for a chemokine receptor (9). Chemokines are a family of proinflammatory cytokines that attract and activate specific types of leukocytes via interaction with their specific receptors and this type of receptor belongs to the superfamily of seven transmembrane (TM) glycoproteins coupled to a G-protein signaling pathway (10). However, these seven TM G-protein-coupled receptors (GPCR) have complex membrane topologies consisting of an N-terminal (Nt) region, three extracellular and intracellular loops, and a cytoplasmic C-terminal tail. CXCR4 is both a chemokine receptor and entry co-receptor for T cell line-adapted HIV-1 (11). A co-receptor is essential on the cellular surface, and the presence of different co-receptors explains the tropism of a strain of HIV isolates for macrophages and lymphocytes (12–21). Each step of this sequential entry process has been suggested as a potential target for developing anti-HIV-1 drugs. Successes in identifying new classes of HIV-1 entry inhibitors targeted to one of the early steps of HIV-1 infection have recently been reported (22–26). The binding of HIV-1 gp120 to the cellular receptor CD4 is critical for HIV-1 entry into cells and has also been suggested as a potential target for developing anti-HIV-1 therapy (27–29). Identification of inhibitors that block gp120–CD4 interactions has been reported, although binding sites of those inhibitors have not been clearly identified (30–40).
Structural information about GPCRs is difficult to obtain using experimental techniques because of enormous difficulties in preparation of samples suitable for subsequent X-ray, NMR, or electron microscopic analysis (41). In contrast to existing antiretroviral agents that work after viral entry, these should have an advantage in that they will not need to access intracellular compartments.
Tremendous efforts had been made already to fully understand the pandemic of the virus and in consequence, to develop an effective therapy to control the spread of the virus. This led to the development of about 20 effective antiretroviral drugs approved by the Food and Drug Administration (FDA) for treating HIV-infected individuals. These drugs have been developed to target important and vulnerable steps in the virus replication cycle. The majority of them are RT and protease inhibitors, and a more recently approved drug is HIV-1 fusion inhibitor (5). In the past, 3′-azido-2′,3′-dideoxythymidine (AZT), 2′,3′-dideoxyinosine (ddI), 2′,3′-dideoxycytidine (ddC), and 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T), are among the drugs that were used for the treatment of individuals infected with HIV-1 (42–46). These pyrimidine and purine nucleoside analogs are potent inhibitors of HIV replication in human T cells and monocyte/macrophages. However, the use of these compounds is associated with adverse side-effects in patients (47) and the emergence of resistant variants of HIV-1 (48). Various attempts have been made to develop new antiretroviral agents (49,50), as reviewed by De Clercq (51,52). Targeting the viral fusion process has become a new focus of research for the next generation of HIV antiretroviral therapies (53). Scientists focused on blocking the interaction of the gp120 protein with CD4, e.g. BMS-806 (54), on developing CXCR4 receptor antagonists such as the bicyclam AMD3100 (55), or on blocking the formation of the necessary rearrangement of the gp41 protein (56). This latter approach resulted in the development of the 36-amino acid peptide Enfuvirtide (T-20), which was approved by the FDA in March 2003. Although this drug validates the viral fusion process as a viable target clinically for the treatment of HIV/AIDS, a complicated manufacturing process results in a high cost (approximately US$20 000 per patient-year) and twice daily subcutaneous injections cause a very high incidence (98% of patients) of local site irritation, both of which will probably limit its clinical utility (57). Moreover, it has been proposed by Charloteaux et al. (58) that the Nt 12 residue long peptide of HIV gp41 is the minimal peptide sufficient to induce significant T cell-like membrane destabilization in vitro. More recently, molecular modeling, design, synthesis, and biologic evaluation of novel 3′,4′-dicamphanoyl-(+)-cis-khellactone (DCK) analogs as potent anti-HIV agents have been proposed (59). An efficient method has been developed for the synthesis of a versatile intermediate bearing azido, hydroxyl, and ester functions as anti-HIV PR inhibitors containing hydroxylethylamino core (60). It has also been shown that conformational flexibility and positional adaptability are important in the design of non-nucleoside HIV-1 reverse transcriptase inhibitors (NNRTI; 61). Based on the elegant design of cyclic ureas as HIV protease inhibitors that was reported by Lam et al. (62) in 1994, cyclic sulfamide has been proposed as potent HIV-1 protease inhibitors (63).
Several different approaches have been used successfully to identify new HIV-1 antagonist candidates. Flavonoids have been investigated for anti-HIV activity (64). Several plant flavonoids such as baicalein, quercetin, quercetagetin, and myricetin have been shown to inhibit HIV-1 RT activity (65). The natural products which can inhibit the growth of disease causing microbes or kill the pathogens and may have no or least and manageable toxicity to host cells are the potential candidates for the systematic studies on the development of antimicrobial drugs to replace antibiotics or other drugs for which microbes have developed resistance (66). Calophyllum longiferum variety austocariaceum contains anti-HIV substance, (+)-calanolide A (67). Flavonolignans which has both flavone and lignan may be exploited for various pharmacologic activities (68). Seed hulls of Hydnocarpus wightiana contains hydnocarpin, neohydnocarpin, and hydnowightin which exhibited hypolipidemic, anti-inflammatory, and antineoplasmic activity (69).
The appearance of drug-resistant virus urged the search for new anti-HIV-1 agents and targets. The present research approach is to discover novel plant-derived natural products through molecular docking as new lead compounds for potential anti-HIV agents, and to modify these compounds to find still more potent anti-HIV agents. Of late, there has been a thrust to employ natural products as medicines.
Materials and Methods
The protocol used to derive the human CXCR4 model is divided into three phases: sequence alignment, model building, and model evaluation. These three phases are reported and discussed in detail.
The primary sequence of human CXCR4 was taken from Swiss-Prot (P61073; 70). The crystal structures of bovine rhodopsin (1F88a, 1F88b) at 2.80 Å resolution (71) were obtained as the modeling template of CXCR4 from the ExPdb database using swiss-pdbviewer (spdbv 3.7b2, http://www.expasy.ch/spdbv/; 72).
A sequence alignment of CXCR4 from bovine rhodopsin and human was constructed using the 3d-pssm program (73) and is illustrated in Figure 1. The alignments between CXCR4 and bovine rhodopsin were generated separately for transmembrane segment (TMS) and loop regions. In the TMS, the alignments were manually adjusted and special care was taken to avoid gaps. Finally, residues in the membrane domain of bovine rhodopsin were replaced by corresponding residues of CXCR4.
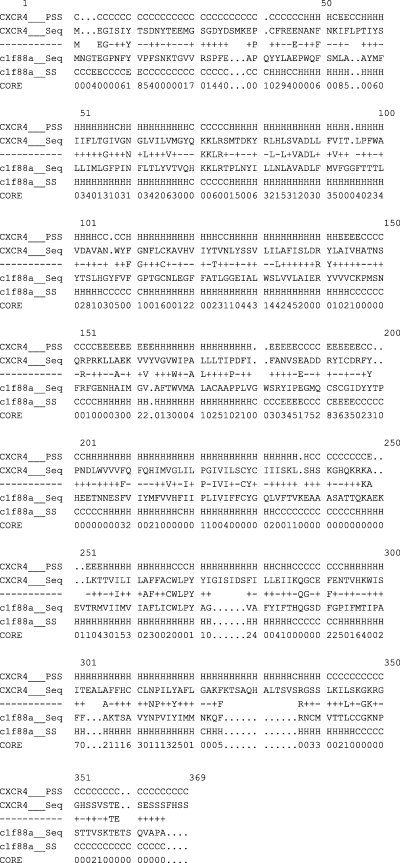
Sequence alignment between CXCR4 and bovine rhodopsin. The potential transmembrane segment of CXCR4 and extracellular loops were labeled. ‘+’ and ‘−’ indicate positive and negative scores for each equivalence, respectively.
Secondary structure prediction
The secondary structure was predicted by using psipred server, Version 2.3 (74).
Homology modeling of CXCR4
The amino acid sequence (P61073) of human was obtained from Swiss-Prot protein sequence database (http://www.expasy.ch/sprot; 70). The homology modeling was performed using crystallographic structure of bovine rhodopsin (1F88; 71) solved at 2.8 Å resolution as template obtained from Protein Data Bank (75). The model was generated using Modeller (76). The program deduces distance and angle constraints from a template structure and combines them with energy terms for adequate stereochemistry in an objective function that is later optimized in Cartesian space with conjugate gradients and molecular dynamic (MD; simulated annealing) methods. The final model was evaluated with procheck (77).
Molecular dynamics (MD) simulations
All simulations were performed using gromacs v 3.0 software (78–80). The receptor protein as well as water parameters were those of the Gromacs force field (81,82). Structural diagrams were prepared using the programs vmd (83), swiss-model (72). Other analyses were performed using scripts included with the Gromacs distribution. The protein is immersed in a box of water of approximately 16 × 11.4 × 9.8 nm3. This approach provides a stable hydrophobic/hydrophilic liquid interface quickly adaptable to the protein structure and has been successfully applied in simulations of helix bundles (84,85) and of ionic channels (86–88). The Particle Mesh Ewald interpolation order was set to 6 and the maximum grid spacing for the FFT was set to 0.12 nm. Total number of atoms is 140 538. The box dimensions are chosen in such a way that the initial minimum gap between periodic images of the protein is 12 Å. The simulation is performed in periodic boundary (PB) water-vacuum-water box. Pressure coupling in vacuo and angular center of mass motion is removed.
But before the simulation, the model was first subjected to energy minimization via 200 steepest descent steps followed by 500 conjugate gradient iterations.
Docking
Natural products provide a large reservoir for screening of anti-HIV-1 agents with novel structure and antiviral mechanism because of their structural diversity. Many compounds with anti-HIV-1 effect have been screened out from natural products. These include alkaloids, sulfated polysaccharides, polyphenolics, flavonoids, coumarins, phenolics, tannins, triterpenes, lectins, alkaloids, phloroglucinols, lactones, iridoids, depsidones, O-caffeoyl derivatives, lignans, ribosome-inactivating proteins (RIPs), saponins, xanthones, naphthodianthrones, photosensitisers, phosholipids, quinines, and peptides (89,90). Flavonoids are widespread in plants and exhibit many biologic properties, including antitumor, anti-inflammatory, and antiviral activities. Acquired immunodeficiency syndrome, which is caused by the human immunodeficiency virus (HIV), has been a life-threatening health problem since 1981 (91) and flavonoids have been investigated for anti-HIV activity (64). Baicalin (BA, 7-glucuronic acid, 5,6-dihydroxyflavone) is a flavonoid compound purified from the medicinal plant Scutellaria baicalensis Georgi, which has been used as an anti-inflammatory and antiallergic agent for the treatment of a variety of inflammatory diseases such as bronchitis, nephritis, hepatitis, asthma, and atopic dermatitis (92,93). It has also been shown to possess anti-HIV activities (94).
Xanthohumol is classified as prenylchalcone flavonoid. Flavonoids have shown anti-HIV-1 activity with emphasis on inhibiting HIV-1 RT (89,90,95). In the present study, the anti-HIV-1 activity of xanthohumol was also investigated. More recently, curcumin (diferuloylmethane) has been reported to inhibit HIV replication and was claimed for anti-HIV-1 and anti-HIV-2 activities in a recent patent application (96). In an attempt to reveal the mechanism in molecular level of curcumin against HIV, the binding mode of curcumin with CXCR4 was computed. Docking studies have been performed. Moreover, the structure of curcumin consists of two o-methoxylated phenols linking with β-diketone function, and they are all conjugated; its rigid and electron-rich structure makes it an interesting candidate as a lead compound for the further development of new antagonist candidates.
The tested flavonoids which include flavones, flavanones, flavans, chalcones, and other derivatives with unique structures along with curcumin and xanthohumol are illustrated in Figure 2. The planarity, aromaticity, and polarity may allow these compounds to bind by stacking with adenine or guanine, or to compete with purine moieties for binding to receptor sites. Many of these molecules also have oxidation–reduction and metal chelation capabilities (97). In addition to these compounds, some new molecules have also been designed and tested (Figure 3).
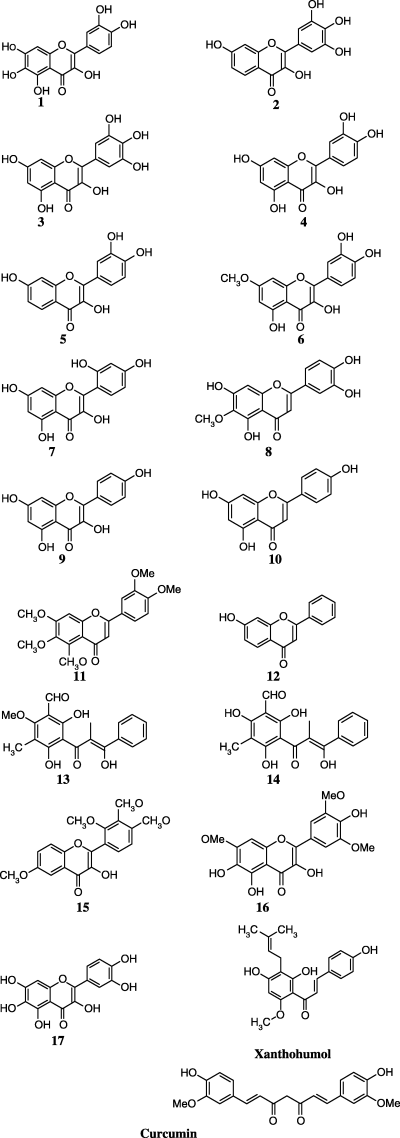
Molecular structure of the compounds studied.
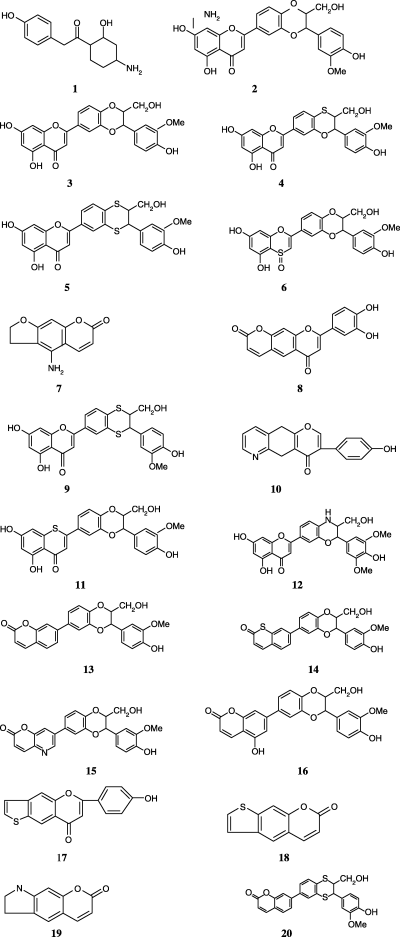
Newer molecular structure of the compounds designed and studied.
The autodock 3.0 program (98) was used to perform an automatic docking exploration for different conformations of the ligand in the CXCR4 model. autodock 3.0 uses three search methods [a genetic algorithm, a local search method, and an adaptive global–local search method based on Lamarckian genetics (LGA)] in conjunction with an empirical force field that allows the prediction of binding free energies for docked ligand. The optimized autodocking run parameters are as follows: the maximum number of energy evaluations was increased to 2 500 000 per run; the maximum number of generation in the genetic algorithm was increased to 100 000; and the number of GA run was 100. All other run parameters were maintained at their default settings.
Molecular structures of new antagonist candidates generated for this study
For every snapshot of protein, the center of the grid was set to the position of the neighborhood of the Phe249 cavity using the average co-ordinates of Cα-atoms of Trp252 and Pro254. The global optimization was started with a population of 100 randomly positioned individuals. The maximum number of iterations of the pseudo Solis and Wets local search was set to 3000. Simulations were performed with a maximum of 1.5 × 106 energy evaluations and a maximum of 27 000 generations. All of the ligands followed the same docking protocol. The ligand conformation with the lowest energy in each class was then used for the analysis of its interaction mode with the interaction site. During each docking experiment, 50 runs were carried out. The rest of the parameters were set as default values.
Results and Discussion
Secondary structure prediction
The secondary structure was predicted by using psipred (74). psipred incorporates two feed-forward neural networks, which perform an analysis on output obtained from Position-Specific Iterated BLAST (PSI-BLAST; 99). The average prediction accuracy for psipred in three structural states is 76.5%, which is higher than any of the other secondary structure prediction methods.
The secondary structure simply tells whether a given amino acid in a given protein is a helix, strand, or coil. Therefore, an amino acid sequence can be mapped into a sequence of helices (H), extended strands (E), and coils (C). This sequence of structural elements is called the secondary structure of the protein and has been illustrated in Figure 4.

Secondary structure of CXCR4 from psipred.
psipred prediction results
The secondary structure prediction is performed using psipred Version 2.3 by David Jones. In each result file, the lines beginning with ‘Conf:’ mean that the numeric value represents the reliability of the result, the larger the more reliable. The lines beginning with ‘Pred:’ are the predict results: ‘H’ represents helices, ‘E’ represents sheets, and ‘C’ represents coil or loop, or other. There are only three states in this program. The lines beginning with ‘AA:’ are the protein's residues, the numbers below them are ruler marks.
The analysis of the homologous models and general structural features
Although bovine rhodopsin has low sequence homology with CXCR4 (the identity is 21.3%), it belongs to the GPCR family and its crystal structure displays an explicit conformational feature of a bundle of seven TM α-helices shared by other GPCRs, and the sequence identity in the TMS between CXCR4 and rhodopsin is approximately 30%, which should be possible to generate models where approximately 80% of the Cα-atoms are within 3.5 Å of their correct positions (100). Thus, it is reasonable to adopt the seven TMS of bovine rhodopsin as template to construct the structure conserved regions (seven TMS) of CXCR4. However, the PRO residue in TMS has long been known to affect helical stability and steric conflict in the helix structure (101). The geometrical and stereochemical features have been calculated for validation by the procheck program at http://pdbdep.protein.osaka-u.ac.jp/validate/, the results show that more than 95.9% torsions angles are within the expected Ramachandran regions, all bond distances and angles lie within the allowable range about the standard dictionary values, and the atom chirality of these two models is also correct, which indicates that CXCR4 model is reasonable in geometry and stereochemistry. Disulfide bonds are playing important roles in maintaining the structural organization of the receptor outer domains. The interaction networks formed by disulfide bonds, hydrogen bonds, salt bridges, and van der Waal contacts make these extracellular segments combine into one well-packed globular domain (Figure 5). The six stereochemical properties viz., Ramachandran plot quality, peptide bond planarity, bad non-bonded interactions, Cα-tetrahedral distortion, main chain hydrogen bond energy, and the overall G factor are good and compares well, with well-refined structures at a similar resolution.
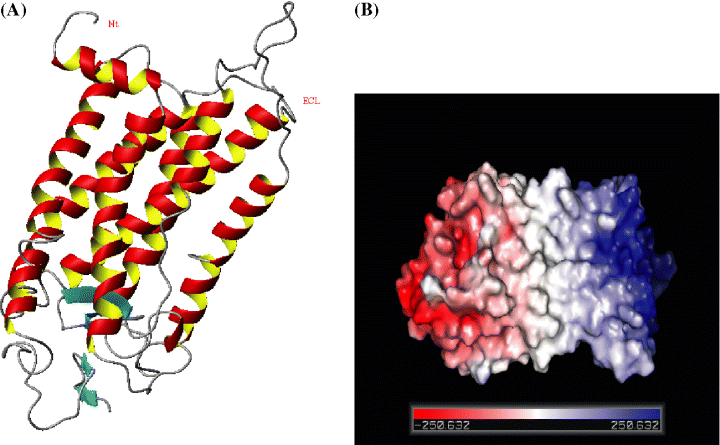
(A) Model CXCR4 shows that N-terminal region extends into outer space of extracellular domain. The figure was generated by using the molmol program (106). (B) Electrostatic surface representation of CXCR4 (color code: blue, positive; red, negative; gray, neutral potential).
Docking
Ligand efficiency (LE), favored by heavy atoms contributions to binding is particularly useful, (102) as it allows the comparison of compounds with quite different affinities, and tends to favor smaller, low-molecular weight compounds. While interaction with a receptor will certainly perturb the conformational energy of a flexible ligand, high affinity would suggest that the ligand be not highly distorted upon binding. Bulky substituents would probably produce significant steric clashes with the residues lining the hydrophobic pocket, altering the docked ligand conformation substantially and lowering the binding affinity, as is the case with compounds 15 and 16. This has been validated experimentally by Souza et al. (103) in 2003. The most potent compound 2-methoxy-3-methyl-4,6-dihydroxy-5-(3′-hydroxy)cinnamoylbenzaldehyde (13), a chalcone is an excellent lead compound for further anti-HIV drug development. This has been tested in vitro by Wu et al. (104) in 2003. Curcumin docked into the interaction site of CXCR4 also showed high binding affinity. The residues which are significantly involved are Trp252, Phe249, Leu253, and Pro254 (Figure 7). The three compounds showing positive result have primary interactive site as extracellular loop (ECL). Due to its unique mode of action, the results from this and other studies may further be used to modify 13, 17 (Figure 6) and curcumin to improve its pharmacologic properties.
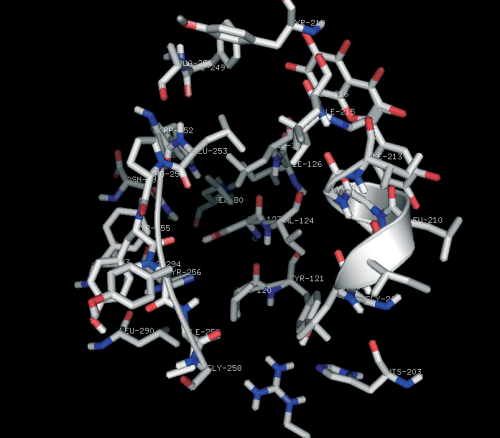
Curcumin docked into the interactive site of CXCR4. Only residues relevant to the discussion are displayed.
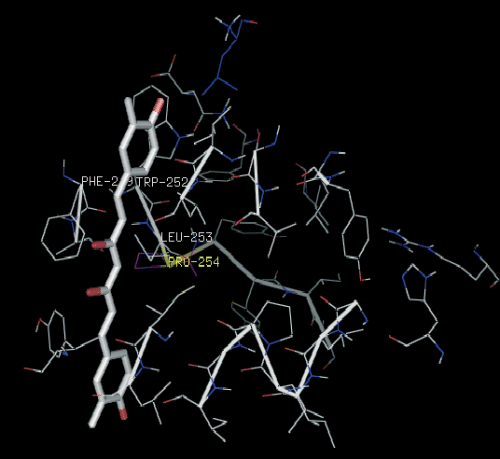
Compound 17 docked into the interactive site of the receptor CXCR4.
In all the newer molecules designed and tested for the study, all the 20 antagonist candidates exhibited a similar docking mode in the interactive site of CXCR4. A final docked representation of the potential binding mode of flavonoids was chosen based on the selection of the compound possessing the lowest docked energy within the most populated cluster of lowest possible energy. Most of the residues interacting with CXCR4 were the same. Ala250 and Phe249 showed significant interaction (Figure 8). The main reason why the process of protein–ligand docking is so difficult is the tremendous complexity of the system; one must take into account hundreds of thousands of degrees of freedom in the two molecules, as well as the not-completely-known combination of energetic forces acting on them (105).
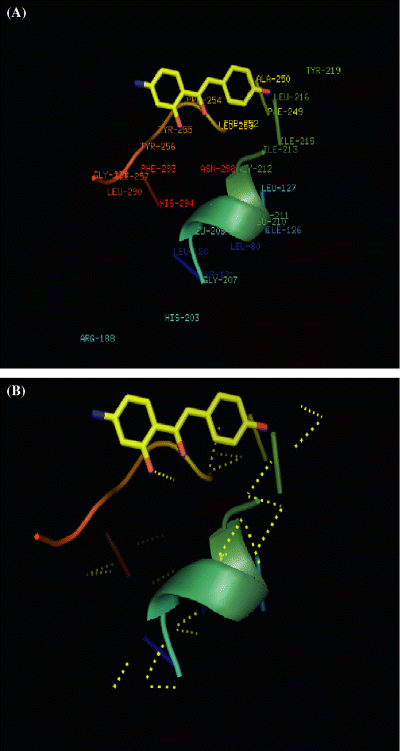
(A) Compound 1 docked. Ala250 and Phe249 are significantly involved. (B) Compound 1 displaying polar contacts (yellow dotted line) with the pocket of CXCR4.
The energy values obtained from autodock are given in Table 1. Ligand docking revealed a consistent set of recurring binding modes. For all MD time scales, well-clustered docking results could be obtained.
| Molecule | Estimated free energy (kcal/mol) | Final docked energy (kcal/mol)a |
|---|---|---|
| 1 | −6.70 | −7.49 |
| 2 | −8.13 | −10.34 |
| 3 | −7.56 | −7.15 |
| 4 | −6.69 | −8.13 |
| 5 | −8.65 | −10.23 |
| 6 | −6.53 | −8.46 |
| 7 | −8.03 | −10.20 |
| 8 | −7.46 | −7.97 |
| 9 | −7.65 | −10.04 |
| 10 | −7.12 | −9.17 |
| 11 | −7.04 | −7.78 |
| 12 | −6.37 | −9.03 |
| 13 | −7.63 | −8.13 |
| 14 | −7.14 | −7.65 |
| 15 | −16.47 | −18.65 |
| 16 | −16.35 | −17.71 |
| 17 | −7.76 | −9.18 |
| 18 | −6.12 | −10.99 |
| 19 | −7.39 | −11.70 |
| 20 | −6.34 | −11.07 |
- aAverage of docking energies of the optimal structure obtained within the binding sites of newer molecules designed for the study.
The system starts at a high temperature T in order to accept most initial steps. The steps are cycled, and at the beginning of each new cycle, the temperature is reduced, making it progressively more difficult for the docking to proceed to a new step. A final low energy-bound conformation is returned.
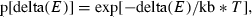
As depicted in Table 1, variation in side chains allow for more energetically stable binding sites, and thus ones that would theoretically be observed more. An energy barrier seemed to be present around true binding sites, based on results which gave high difficulty weights for the ligand leaving and entering the binding site. This excellent result occurred because of the relatively rigid cyclic structure of ligands limiting the choices of docking poses and making the energy difference between the poses very large. The docked conformation with the lowest free binding of energy was probed for further analysis. Docking results from autodock illustrated the molecular interactions between compound 1 and the receptor. Further studies on their binding properties will provide useful information toward the rational design of CXCR4 antagonist candidates.
Molecular dynamics simulations
A three-dimensional model structure of a complex formed by CXCR4 receptor and an agonist ligand is probed and refined using MDs simulations and free energy calculations in a realistic environment. Simulations are complicated because (i) the experimental template structure for GPCRs (bovine rhodopsin) is of low resolution, (ii) the CXCR4 receptor surroundings are irregular (water exposed loops versus lipid exposed TM regions), and (iii) the protonation and solvation states of the inner core receptor residues are unknown.
The 1 nsecond MDs simulation in an explicit membrane environment indicates that both the structure of the receptor and its interactions with the ligand are robust. Results demonstrate that the optimal MD set-up with minimal computational effort is a PB box containing two water shells solvating the extracellular and intracellular loops separated by a vacuum layer surrounding the helical TM regions. It was found that the vacuum layer and water layers are stable under PB conditions during at least 1 nsecond of MD simulation. It is shown that in spite of a partial accounting of solvent screening effects by neutralization of charged side chains, vacuum MD simulations can lead to severe distortions of the loop structures. Whereas the secondary structure of the α-helix bundle is well preserved, the region of the intracellular loops exhibits a significant flexibility. New insight into the structural features of the binding pocket is gained, in particular, the interplay of the ligand with both the receptor and internal water molecules. Water-mediated interactions are shown to participate in the binding, hence, suggesting additional site-directed mutagenesis experiments.
At the beginning of high temperature MD simulation, the Nt part was an isolated structural segment that was not involved in any H-bonding and electrostatic interaction with other ECLs, and the residues within Nt possessed of the large solvent-accessible surface. However, along with the time lapsing, the Nt gradually closed to the ECLs, about 40 pseconds later, the Nt was adsorbed on the surface of ECLs with which a globular domain was formed. Then, the dynamic and complex interactions were detected between Nt and ECLs as well as between ECLs during the whole last 960 pseconds MD.
N-terminal region of CXCR4 formed a compact globular domain with movement scope restricting at the one side of ECLs domain throughout the last 960 pseconds of MD, which explained the narrow vibration amplitude of distance change trajectory of CXCR4 illustrated in Figure 9.
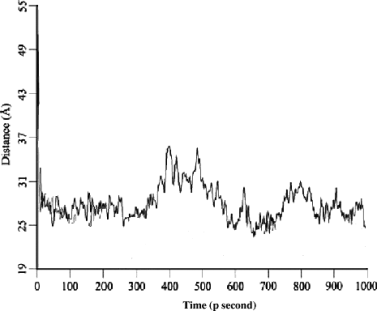
Distance trajectory between geometry center of N-terminal region and that of membrane interface-binding residues for CXCR4 model in 1 nsecond MD simulation.
Thus, clarifying the significances of disulfide bond in maintaining conformational integrity of extracellular domain and avoiding the formation of a separate Nt domain and causing ECLs and Nt together to form a whole extracellular structural domain.
The behavior of the docked receptor–ligand complexes was studied in a dynamic context to account for flexibility and conformational changes. These were performed at a constant temperature of 300 K. In all the simulations, except the case of 15 and 16 (103), the inhibitor–receptor complex remains stable along all the simulation without suffering remarkable structural changes. In the case of compounds 15 and 16 (103), a drift is observed outside the pocket.
Exploitation of inhibitor conformational flexibility (such as torsional flexibility about strategically located chemical bonds) can be a powerful element of drug design, especially for the design of drugs that will be effective against rapidly mutating targets (which is a collection of related targets).
Conclusion
A structure-based ligand design strategy based in conjunction with molecular modeling techniques has been adopted to examine which interactions make a significant contribution to the biologic activity. Interaction network formed by disulfide bonds, hydrogen bonds, van der Waals force, and salt bridges between extracellular segments helped in maintaining the conformation of the docked complex. The study of docking to CXCR4 has opened a new dimension in this regard. New antagonist candidates in addition to the known flavonoids have shown considerable potency to act as anti-HIV drugs and should be validated through in vitro and in vivo results. The structurally and functionally important residues such as inhibitor-binding residues (Phe249 and Ala250) in the interactive site of docked CXCR4-complex were identified. These analyses may provide a basis for probing the structure–function relationship in the CXCR4. The model holds enough essential information about the spatial arrangement of important residues to guide the design of site-directed mutagenesis experiments.
These results may help to provide new insights into CXCR4 inhibition providing a possible explanation of the not well understood differential pharmacologic behavior exhibited by highly potent flavonoids and its derivatives.
Acknowledgment
We are thankful to the referees for painstakingly reading the manuscript and giving valuable suggestions.




