Japanese Society for Dialysis Therapy Guidelines for Management of Cardiovascular Diseases in Patients on Chronic Hemodialysis
Published in J Jpn Soc Dial Ther 2011;44:337–425 (in Japanese). Reprinted with permission from the Journal of the Japanese Society for Dialysis Therapy.
INTRODUCTION
The annual all-cause mortality in chronic dialysis patients in our country is within 10%, indicating that the outcome of dialysis therapy in Japan is one of the best in the world. It is nothing short of extraordinary to maintain favorable survival like this despite challenging conditions such as aging of the patients and increase in the proportion of patients on long-term dialysis and with diabetes mellitus. We can be proud of our achievement. Novel therapeutic strategies for dialysis patients have been developed, such as antihypertensive drugs (e.g. angiotensin II receptor blockers, calcium channel blockers and beta blockers), treatment of anemia (e.g. erythropoiesis stimulating agents), and management of chronic kidney disease-mineral and bone disorder (CKD-MBD) (e.g. activated vitamin D, calcimimetics, and new phosphate binders). While the beneficial effect of these new approaches is well acknowledged, we must not forget that the favorable outcome is also due to the considerable efforts and excellence in management of all the medical staff, including physicians, nurses, and clinical engineers, who are engaged in dialysis therapy in Japan.
While mortality due to infectious diseases is increasing at present, about half of dialysis patients die from cardiovascular disease (CVD). Thus, the management of CVD has become the most challenging clinical issue in dialysis patients. With regard to CVD, the main focus has so far been on blood vessel diseases of the heart and brain; however, peripheral artery disease (PAD) is now also attracting attention because the number of patients with atherosclerotic obstruction of the peripheral arteries in the lower extremities has increased in recent years and endovascular catheter therapy has been introduced and developed. The number of specialists in the field of PAD has increased along with the development of new biomedical technology and expansion of their use. Endovascular catheter therapy is currently offered to patients with chronic dialysis and we expected an increase in the number of patients benefiting from this therapy. Evidence suggests that the pathological process of CVD is also involved in the aggravation of systemic atherosclerosis associated with renal dysfunction, prompting the use of potent anti-atherogenic agents, such as statins in dialysis patients similar to the general population.
With regard to CVD in dialysis patients, unfortunately, there is little clinical evidence to justify the compilation of clinical guidelines. For example, the appropriate blood pressure level in such patients remains unknown, and the target blood pressure level for management of hypertension has not yet been defined even in the guidelines issued by Western countries. Although we discussed this issue in detail in several committee meetings, we only agreed on setting the target blood pressure though we presented this as an opinion rather than guideline by the committee. There is no doubt that we need to validate in the future whether the level is appropriate or not. In fact, we do not know whether any statement on the clinical guideline is right or not especially when evidence is insufficient, and any statement is nothing but “themes of clinical questions”. We need to validate this issue by prospective high-evidence grade studies. The Japanese Society for Dialysis Therapy (JSDT) maintains a patient registry database kept with the standing committee responsible for statistics and investigation. We used the data stored in this database to generate the present guideline. We stress that we should continue to maintain this important registry system in order to revise the clinical guidelines in the future.
The chapters on cardiac failure, ischemic heart disease, arrhythmia, valvular heart disease, cerebrovascular disease, and peripheral artery disease in the guideline are separated into those for “renal dialysis physicians” and “cardiologists (or strokologists)” in order to demonstrate the importance of cooperation between these two specialties. We think that the excellent outcome of dialysis therapy in Japan is in part attributed to the implementation of excellent daily clinical procedures, which are based on “evidence” and/or “experience” in each dialysis facility. We have to validate the daily procedures and present them as treatment guidelines. We hope this guideline is useful in daily clinical practice.
We determined the grading evidence and recommendation levels according to the position statement from Kidney Disease: Improving Global Outcomes (1,2).
REFERENCES
Chapter 1: Dyslipidemia/Atherosclerosis-Arteriosclerosis
I. DYSLIPIDEMIA
Statements
- 1
In dialysis patients, dyslipidemia is an independent risk factor for cardiovascular diseases, particularly incident myocardial infarction (B).
- 2
We recommend measurement of low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), HDL-C, and triglyceride (TG) before dialysis (casual blood sampling) for routine evaluation (1B).
- 3
We suggest the control target levels should be LDL-C < 120 mg/dL or non-HDL-C < 150 mg/dL for the primary prevention, and LDL-C < 100 mg/dL or non-HDL-C < 130 mg/dL for the secondary prevention of ischemic heart disease (2C).
- 4
We suggest that administration of statin should be considered if lipid control cannot be achieved by dietary/exercise therapy (2B).
- 5
We suggest that the evaluation and intervention of undernutrition should be considered if hypolipidemia is present.
Comments
Epidemiology
Observational studies in Japan have demonstrated a close relationship between dyslipidemia (hyper-LDL-cholesterolemia, hypo-HDL-cholesterolemia, hypertriglyceridemia, and/or hyper-non-HDL-cholesterolemia) and the severity of atherosclerosis (1,2) and also the risk of myocardial infarction (3) in dialysis patients. In addition, dyslipidemia is more closely related to coronary artery disease than cerebrovascular disorders. However, observational cohort studies of dialysis patients showed a higher risk of death due to all causes (4) or death due to cardiovascular disease (5) in patients with low total cholesterol (TC) level, reflecting a reverse tendency compared with epidemiological data in the general population. Such relationship is, however, not observed in dialysis patients who are free of inflammation or are not undernourished (as defined by the levels of C-reactive protein [CRP] and serum albumin, respectively) (4,6). In Western countries, the survival curve of dialysis patients who develop acute coronary syndrome is poorer in patients with low body mass index (BMI) than in those with high BMI (7). Similarly, in Japanese dialysis patients, old age, low BMI, and high CRP are reported to be factors that enhance the risk of death after a cardiovascular event (3). These reports suggest that undernutrition, represented by hypoalbuminemia, low BMI, and hypocholesterolemia, correlates with increased risk of death by increasing the risk of death after an event (fatality rate), although hypocholesterolemia per se is not considered to promote atherosclerosis (8).
Causes
Dyslipidemia can be classified into primary and secondary dyslipidemia, depending on the cause. Primary dyslipidemia includes familial hypercholesterolemia (FH) and familial combined hyperlipidemia (FCHL), with a reported respective prevalence of each type of 1:500 and 1:100. Secondary dyslipidemia is caused by various conditions such as diabetes, endocrine (thyroid, adrenal) disorders, liver diseases, kidney diseases, and drugs. Hypercholesterolemia associated with nephrotic syndrome and hypertriglyceridemia and hypo-HDL-cholesterolemia associated with chronic kidney failure are well-known dyslipidemias caused by kidney diseases. Low lipoprotein lipase activity (high apo C-III levels), low hepatic lipase level, and low lecithin cholesterol acyltransferase (LCAT) activity contribute to dyslipidemia in patients with chronic renal failure.
Diagnosis
According to the Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases by the Japan Atherosclerosis Society (9), hyper-LDL-cholesterolemia is defined as LDL-C ≥140mg/dL, hypo-HDL-cholesterolemia as HDL-C <40 mg/dL, and hypertriglyceridemia as TG ≥150 mg/dL in fasting blood samples. However, fasting blood samples are often difficult to obtain from dialysis patients. In general, post-prandial changes in TC or HDL-C level are very small, compared with the increase in TG levels. Thus, LDL-C level calculated by the Friedewald equation decreases while little change is observed in non-HDL-C level (TC minus HDL-C). Also, since non-HDL-C level is the sum of LDL-C and cholesterol present in TG-rich lipoproteins (Fig. 1), it is regarded as an integrated index of the atherogenic lipoprotein level. Therefore, for routine evaluation in dialysis patients, non-HDL-C level in a casual blood sample is considered acceptable in addition to the standard fasting LDL-C level.
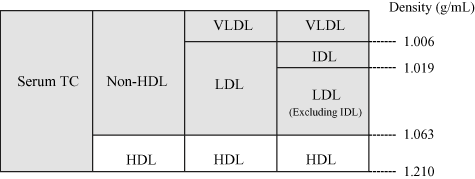
Serum total cholesterol and its components. Serum contains a mixture of lipoproteins of different densities (specific gravity), and the total sum of cholesterol in the various lipoproteins represents serum total cholesterol. Several methods are used to fractionate lipoproteins. HDL has an anti-atherosclerotic properties, and all other fractions apart from HDL (collectively called non-HDL) are atherogenic. The cholesterol present in non-HDL is expressed as non-HDL-C. Thus, non-HDL-C is the sum of cholesterol in atherogenic lipoproteins.
Treatment
In subjects with dyslipidemia in general, secondary dyslipidemia is usually excluded first, followed by recommendations for long-term dietary/exercise therapy. Drug treatment is also considered if the target level cannot be achieved. However, in patients with coronary artery disease, the first option should be drug treatment. A strict target lipid level is set in patients that have not developed coronary artery disease but are at high risk, while a stricter target is set for patients with established coronary artery disease (secondary prevention group).
According to the recent epidemiological study of the Japanese Society for Dialysis Therapy (3), the risk of occurrence of acute myocardial infarction increases 1.24 times (95% confidence interval: 1.14–1.35) with every increase in non-HDL-C level of 1 mmol/L (38.7 mg/dL). Based on the results of this observational study, the present guidelines propose a target level of LDL-C <120 mg/dL or non-HDL-C <150 mg/dL for primary prevention, and LDL-C < 100 mg/dL or non-HDL-C level <130 mg/dL for secondary prevention.
There are only a few randomized controlled trials in dialysis patients regarding whether lipid lowering therapy significantly reduces the risk of cardiovascular events. The 4D (Die Deutsche Diabetes Dialyse) study using atorvastatin (10) and AURORA Study using rosuvastatin (11) suggested that the risk of all cardiovascular diseases (including those not directly related to atherosclerosis such as heart failure and cerebral hemorrhage) can only be reduced slightly even by lipid lowering therapy using statins. However, the risk of ischemic cardiac accidents decreased significantly by 18% in the 4D Study. Taking these results and the results of the observational cohort study in Japan into consideration, it would be reasonable to treat dialysis patients with high LDL-C or non-HDL-C levels with statins to reduce the risk of ischemic heart disease. Furthermore, there is little medical basis for discontinuation of statins therapy, since statin use is reported to associate with better survival in both incident (12) and prevalent dialysis patients (13).
In conducting drug therapy, statins are the first choice. Statins reduce LDL-C level by 25–40% although this effect varies with the drug and dose. In the above 4D (10) and AURORA (11) studies, the frequency of adverse effects were comparable between the statins and placebo groups, suggesting no safety problems with the use of statins. Excluding clinofibrate, fibrates available in Japan are contraindicated in patients with renal failure due to the high risk of rhabdomyolysis based on their excretion via the kidney. Bile acid-binding resins, eicosapentaenoic acid preparations, and intestinal cholesterol transporter inhibitors can also be used in dialysis patients. Niceritrol, a nicotinic acid derivative, reduces serum phosphate levels but could cause anemia and thrombocytopenia and must be administered with caution in dialysis patients. Many patients are treated with more than one drug. For safe treatment, one should monitor symptoms and laboratory tests including serum creatine kinase, aspartate aminotransferase and alanine aminotransferase, and also pay attention to drug interactions.
If the patient develops hypolipidemia, a nutritional disorder should be suspected, and measures to improve the nutritional state should be considered.
The following issues are proposed as topics of future studies; whether a very high TG level is a risk factor of acute pancreatitis in dialysis patients, and whether patients undergoing peritoneal dialysis and children with renal failure should be treated in a manner similar to that of adult hemodialysis patients. We expect further data from sub-analyses and meta-analysis of the 4D, AURORA, and Study of Heart and Renal Protection (SHARP) studies.*
*Supplement
Papers on subanalyses of the 4D Study (14) and AURORA Study (15), and the original report of SHARP (16) appeared during the publication of the guidelines, on which the present simplified guidelines are based, and the preparation of this simplified version. The subanalyses of 4D and AURORA studies suggested that lipid lowering therapy prevents atherosclerotic cardiovascular events in diabetic patients on dialysis, and that it significantly prevents such events more effectively in patients with higher LDL-C levels before the treatment. SHARP also showed that lipid lowering therapy using the combination of simvastatin and ezetimibe significantly reduced the risk of atherosclerotic cardiovascular events and that such reduction showed no significant heterogeneity between the patient groups before and after the initiation of dialysis therapy.
REFERENCES
II. ATHEROSCLEROSIS-ARTERIOSCLEROSIS
Statements
- 1
To evaluate the risk of cardiovascular death in dialysis patients, we recommend the inclusion of risk factors specific to renal failure (e.g. anemia, inflammation, undernutrition, abnormal mineral metabolism), in addition to classic risk factors (1C).
- 2
The extent of arterial wall thickening, arterial wall stiffening, and vascular calcification may be used for the evaluation of cardiovascular risk (Opinion).
Comments
Epidemiology
In dialysis patients, the risk of death due to cardiovascular disease (CVD) such as ischemic heart disease, cerebrovascular diseases, and heart failure is markedly increased, and the relative risk compared to the general population is reported to be 10–30 (1). Dialysis patients are characterized by a high risk of CVD events and low survival rate after the onset (high fatality rate). Compared to the general population, dialysis patients show 2–5 times higher risk of incident acute myocardial infarction and poorer survival rate after acute myocardial infarction (2). This is also true for cerebrovascular diseases (3). The high incidence and high fatality rate are considered to synergistically increase the risk of death due to CVD (4).
Causes
One of the reasons for the high risk of CVD in dialysis patients is advanced atherosclerosis before the initiation of dialysis. About half of the patients have significant coronary artery stenosis at the initiation of dialysis (5,6), and the presence or absence of coronary artery disease at the initiation of dialysis is a strong predictor of cardiovascular events after the initiation of dialysis (7).
Vascular calcification is classified into atherosclerotic calcification affecting the intimal layer of the artery and Mönckeberg's sclerosis affecting the medial layer of the artery, especially the latter is more frequently observed in dialysis patients. Both types of calcification are significant predictors of death in dialysis patients. Abnormal mineral and bone metabolism including vascular calcification associated with chronic kidney diseases (CKD) has been integrated as a new concept named CKD-mineral and bone disorder (CKD-MBD) (8), and it is considered important in clinical practice of dialysis patients.
Because the risk of CVD in dialysis patients is significantly high even after correction for classic risk factors such as old age, hypertension, dyslipidemia, and diabetes, factors specific to CKD are considered to be involved in the elevated risk of CVD (9). Sarnak et al. (10) noted many factors including anemia, inflammation, undernutrition, and abnormal mineral metabolism as non-classic risk factors. Among them, undernutrition (wasting) is diagnosed in daily clinical practice based on the presence of hypoalbuminemia or low BMI. According to reports from Japan, low BMI is a predictor of all-cause death (11,12) and CVD death (11), but not a predictor of future myocardial infarction (12). A report from the United States (13) observed that the survival curve after the onset of acute coronary syndrome was poorer in the low BMI group. In Japan, also, low BMI is independently related to the risk of death after CVD including myocardial infarction, cerebral infarction, and cerebral hemorrhage (12). Thus, certain non-classic risk factors are considered factors that enhance the fatality rate after the onset of CVD.
Diagnosis
Clinically, atherosclerosis-arteriosclerosis can be evaluated quantitatively and qualitatively by examination of the thickness and stiffness of the arterial wall and vascular calcification (Table 1). These measurements may serve as surrogate markers between risk factors and CVD events.
| Arterial wall thickening | Carotid artery intima-media thickness (IMT) | B mode US |
| Presence or absence of plaques | ||
| Arterial wall stiffening | Pulse wave velocity (cfPWV, hfPWV, baPWV) | Pulse wave analysis |
| Cardio-ankle vascular index (CAVI), augmentation index (AI) | ||
| Compliance, stiffness parameter β, etc. | M-mode US (echo-tracking system) | |
| Arterial calcification | Presence or absence of calcification, semiquantification of calcification | Plain X-ray |
| Aortic calcification index (ACI) | Plain CT | |
| Coronary artery calcification score (CACS) | Electron beam CT (EBCT), Multi-detector CT (MDCT) | |
| Vascular luminal narrowing | Presence or absence of narrowing, number of affected vessels, Gensini score | Contrast-enhanced CT, Coronary angiography |
| Myocardial ischemia | ST-T changes | Electrocardiogram (ECG) |
| Ischemic area, coronary blood flow reserve | Myocardial scintigraphy (SPECT) |
- Note that while arterial wall thickening, arterial wall stiffening, and arterial calcification represent changes in the arterial wall itself due to atherosclerosis-arteriosclerosis, vascular luminal narrowing and myocardial ischemia are changes resulting from atherosclerosis-arteriosclerosis.
Carotid artery intima-medial thickness is measured by B mode ultrasonography, which provides quantitative evaluation of arterial wall thickening, and is a predictor of the risk of CVD death and total death in dialysis patients (14).
Aortic pulse wave velocity (cfPWV, hfPWV) is a representative index of arterial stiffness and a predictor of CVD death and total death in dialysis patients (15). While a high baPWV measured in the brachium and ankle is also a prognostic factor in dialysis patients (16), its value falsely decreases in patients with obstructive arteriosclerosis in the lower limbs. Therefore, caution is needed and simultaneous measurement of the ankle brachial pressure index (ABI) may be helpful. The Cardio-ankle vascular index (CAVI) and augmentation index (AI) have also been used as new indices of arterial stiffness.
Various methods are available to evaluate vascular calcification. Among these, electron beam computed tomography (EBCT) has excellent temporal resolution and provides specific assessment of the heart and large blood vessels. Coronary artery calcification is usually evaluated using the coronary artery calcification score (CACS) calculated by Agatston's method. CACS has been reported to be a predictor of cardiovascular events (cardiac death, non-fatal myocardial infarction) in non-dialysis patients with coronary artery disease (17). However, while dialysis patients with high CACS have poor survival, CACS is not necessarily related to cardiovascular events (18). The sensitivity of multi-detector computed tomography (MDCT) has improved in recent years, and this modality has become the mainstay of coronary artery computed tomography (CT). Abdominal plain CT is used to measure the area of aortic calcification, using the aortic calcification index (ACI), which is determined in 10 slices at 1-cm intervals above the origin of the common iliac artery. In dialysis patients, there is a strong correlation between ACI and coronary artery calcification (19). The presence or absence of vascular calcification examined by thoracoabdominal CT (20,21) has also been shown to be an independent predictor of all-cause death and CVD death in dialysis patients and is considered to be useful in daily clinical practice.
Although evaluation of these non-invasive surrogate indices may help estimate the individual CVD risk, the criteria used for their evaluation or appropriate frequency of their use have not been established. Longitudinal changes in these measures are not well known in dialysis patients. We propose that the evaluation method(s) should be selected taking into consideration the characteristics of individual patients and availability in the medical facilities, and to perform the measurement once every year, if possible.
Treatment
We do not describe here the treatment for each risk factors and their preventive effects on atherosclerosis-arteriosclerosis because they are discussed in detail in relevant chapters. As for other matters, lifestyle modifications, including smoking cessation and regular exercise at an intensity appropriate for each patient are considered important. Furthermore, early detection and, if possible, early treatment of CVD are particularly important in dialysis patients.
REFERENCES
Chapter 2: Blood Pressure Abnormalities
I. HYPERTENSION
Statements
- 1
In dialysis patients, we recommend blood pressure should be evaluated not only in the dialysis room but also at home (1B).
- 2
In patients under stable long-term maintenance dialysis with no impairment of the cardiac function, we suggest the target of antihypertensive treatment should be blood pressure <140/90 mm Hg before dialysis at the beginning of the week (Opinion).
- 3
We recommend dry weight (DW) should be appropriately set in achieving the target blood pressure (1B).
- 4
We recommend antihypertensive agents should be administered when the reduction in blood pressure is inadequate even after achievement/maintenance of DW (1B).
Comments
Epidemiology
According to reports on the present state of chronic dialysis therapy in Japan published at the end of 2005, 74.5% of all dialysis patients were hypertensive based on systolic blood pressure measured at the initiation of dialysis and the criteria of the Japanese Society of Hypertension (JSH 2004) (1). Persistent hypertension is a major cause of left ventricular hypertrophy, ischemic heart disease, heart failure, and death, and thus the control of hypertension is important in dialysis patients (2).
However, a rapid fall in blood pressure during dialysis does not only have serious effects on outcome (3), it may also affect the quality of life or shunt insufficiency. In dialysis patients, aortic calcification also has a marked impact on outcome, as does pulse pressure, and systolic and diastolic pressures (4–7). The outcome is poorer in patients with low diastolic pressure but normal systolic pressure and also in patients with high systolic pressure with normal diastolic pressure (5). Also, the mortality rate is reported to rise significantly if the average weekly blood pressure of the pulse pressure measured before and after dialysis three times a week and at home twice daily at awakening and before going to bed exceeds 70 mm Hg (7). This is also true for various other indices, including the predialysis (8) and postdialysis (9) blood pressures, ambulatory blood pressure monitoring (ABPM) (10), and average weekly blood pressure (7,11). At least, it is important to include home blood pressure in the evaluation (7,11–13). It is more important to base any clinical or therapeutic decision on the mean of multiple measurements than a single casual blood pressure measurement (7,11).
Many cohort studies demonstrated poorer prognosis of patients with high predialysis blood pressure compared with hypotensive patients. This observation is probably due to the inclusion among the hypotensive group of patients with malnutrition or those with severe chronic heart failure (so-called reverse epidemiology). Future prospective interventional studies are needed to further evaluate hypertensive patients (14–16).
Causes
Many factors are suspected to be responsible for hypertension in dialysis patients. Since blood pressure is reported to normalize in more than 60% of patients following strict management of body fluid volume (17,18), optimization of DW is important, and the present guidelines propose how DW should be determined.
Diagnosis
Standardization of blood pressure measurement is necessary for the diagnosis of blood pressure abnormalities.
Standardization of blood pressure measurement:
- 1
Blood pressure should be measured under fixed conditions although it can be measured in either the seated or supine position depending on the setup at each facility. Before the commencement of dialysis therapy, blood pressure should be measured after a period of rest of at least 5 min before the start of dialysis. Subjects should refrain from drinking caffeine 30 min before the measurement and from smoking during the measurement.
- 2
Blood pressure should be measured with the heart rate at least once every hour.
- 3
At the end of dialysis, the blood pressure should be measured in a similar manner immediately before returning of blood and within 5 min after the end of returning of blood, needle removal, and hemostasis. Blood pressure measured immediately before returning of blood at the end of dialysis is called “blood pressure at end of dialysis”.
- 4
After setting or changing DW, blood pressure should be measured also in the standing position at the end of dialysis.
- 5
Home blood pressure should be measured as recommended by the guidelines of the Japanese Society of Hypertension. Measurements before going to bed at night and at awakening in the morning are recommended.
- 6
While ABPM has also been reported to be useful (10,19), it cannot be strongly recommended, due to the restricted use of one upper limb for shunting.
- 7
In dialysis patients with repetitive periodic changes in body fluid volume, it is important to evaluate blood pressure not only in the dialysis room but also at home (7,11–13). In dialysis patients, evaluation on a weekly basis is important, because dialysis is performed after 1–2 rest days.
- 8
Blood pressure decreases progressively from the beginning to the end of the week. Reports on how the blood pressure should be evaluated or used are very scarce. The weekly average blood pressure (WAB) represents the mean blood pressure measured before and after dialysis three times a week and daily home blood pressures in the morning and night. Prospective observational studies demonstrated that WAB is a more significant predictor of left ventricular hypertrophy and cardiovascular disorders than casual predialysis or postdialysis blood pressure measured at the beginning of the week (7,11). Blood pressure measured at awakening on a non-dialysis day in the middle of the week could be also used since it correlates with WAB (R2 = 0.71).
Treatment
Target of antihypertensive treatment—clarification of subjects and objectives
In dialysis patients, a U-shaped relationship is observed between blood pressure and prognosis (20). However, this relationship needs proper interpretation, that is, it is important to clarify the subjects and objectives when determining the target blood pressure of antihypertensive treatment.
The aim of antihypertensive treatment is to reduce the long-term risk of cardiovascular diseases in patients on chronic maintenance dialysis, rather than reduce all-cause mortality (21). Therefore, patients with cardiac dysfunction, for example, are excluded. The target level should be set after comprehensive evaluation of cardiac function in both patients with markedly reduced left ventricular ejection fraction and those with reduced diastolic function due to severe left ventricular hypertrophy. In particular, since the outcome is reported to worsen in patients with increased aortic stiffness due to aortic calcification, by excessive decrease in diastolic blood pressure and increase in pulse pressure, caution against excessive reduction in blood pressure is necessary in consideration of the effects of the diastolic blood pressure on various pathologic conditions such as chronic heart failure and coronary blood flow. For these reasons, the criteria for blood pressure control should not be applied uniformly to all patients but applied selectively by excluding patients with clearly reduced cardiac function, for example, and further evaluated at follow-up.
While it is difficult to propose specific target blood pressure values in the present guidelines due to the scarcity of evidence, a dialysis blood pressure less than 140/90 mm Hg at the beginning of the week is recommended as a provisional target. However, a rapid decrease in blood pressure (30 mm Hg or greater fall in systolic pressure) during dialysis (3,22) and orthostatic hypotension after dialysis are reported to worsen prognosis, and further studies are necessary. On the other hand, observational studies indicated that predialysis blood pressure does not correlate with the effect of falls in blood pressure during dialysis (23).
The target blood pressure is set to reduce the long-term risk of cardiovascular diseases in patients on maintenance dialysis, and should not be applied to patients with pre-existing cardiovascular disorders. In high-risk patients, the lowest blood pressure recorded during dialysis (≤110/60 mm Hg) correlates significantly with the risk of death within 5 years (3).
Algorithm of antihypertensive treatment
One precondition of antihypertensive treatment is securing appropriate amount of dialysis, and thus the conditions of dialysis such as duration, frequency, blood flow volume, and dialysis membrane need to be re-evaluated. Only then should an appropriate DW be set, achieved, and maintained. This should be followed by administration of appropriate antihypertensive drugs when necessary. If a fall in blood pressure is observed during dialysis, antihypertensive medication should be suspended, or its dose reduced, the DW should be set again, the patient should be followed up, and antihypertensive medication resumed, if necessary.
- 1
Weight control, control of salt intake, and cessation of smoking are the most important basic items of guidance during dialysis.
- 2
To achieve the target of antihypertensive treatment, the DW must be set appropriately first (this issue is discussed in a different chapter).
- 3
It is imperative to control changes in body fluid volume between dialyses (interdialysis weight gain) to prevent any fall in blood pressure during dialysis, and guidance should be given to control it within 3% of the DW when the interval of dialysis is one day and within 5% when the interval is 2 days.
- 4
Attempts should be made to control the inter-dialysis fall in blood pressure due to increased DW. Physicians should be aware that a rise in blood pressure leads to worsening of long-term outcome.
- 5
Antihypertensive drugs should be administered when the target blood pressure cannot be achieved after achievement of the DW.
Principles of selection of antihypertensive drugs
Antihypertensive drugs should be administered if appropriate control of blood pressure cannot be achieved by maintaining DW alone with the following points in mind:
- 1
Large-scale clinical studies or randomized controlled trials are lacking on this topic.
- 2
Angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors, which are reported to prevent left ventricular hypertrophy, are the preferable first line of antihypertensive drugs (24–30). Particularly, ARBs are easy to use, because they are excreted primarily in bile, are not dialyzable, and have only a few adverse effects such as cough.
- 3
A history of myocardial infarction and significant coronary artery lesion warrant the use of β-blockers, but caution should be applied when using these agents in patients with heart failure (31,32).
- 4
Calcium antagonists are also recommended. Prospective observational studies indicate that these drugs significantly reduce total death and cardiovascular death rates (33–35).
- 5
Since dialysis patients may also have sympathetic hyperactivity, the use of central sympathomimetic drugs and α-blockers should be considered if blood pressure cannot be controlled with the above drugs. However, since there is little or no information on the subject, and since such drugs can cause various adverse effects such as orthostatic hypotension, they should be regarded as second choice drugs.
Guidelines for appropriate setting of the DW
Definition of DW DW is defined based on the following three criteria: (i) body weight with appropriate body fluid volume, (ii) no rapid and excessive decrease in blood pressure during dialysis, and (iii) lack of marked long-term burden on the cardiovascular system.
The following criteria are widely applied when determining DW:
- •
No marked fall in blood pressure during dialysis.
- •
No hypertension (predialysis blood pressure at the beginning of the week <140/90 mm Hg).
- •
No peripheral edema.
- •
No pulmonary congestion on chest X-ray.
- •
Cardiothoracic ratio ≤50% (≤53% in females).
Evaluation of body fluid volume DW should be determined by taking the following items into consideration in addition to the cardiothoracic ratio.
- 1
Physical findings
Edema may be observed even without volume overload in the presence of hypoproteinemia or in bed-ridden patients.
- 2
Atrial natriuretic peptide (hANP)
hANP is used to evaluate body fluid volume and should be measured monthly (covered by medical insurance). However, the criteria used vary among reports. Plasma hANP concentration is generally 50–100 pg/mL or less when DW is achieved (36), although the level is higher in the presence of cardiac diseases. Therefore, the use of hANP as an index is difficult.
- 3
Diameter of the inferior vena cava
The inferior vena cava (IVC) is delineated by sagittal upper abdominal ultrasonography, and its diameter is measured at 2 cm distal to its junction with the hepatic vein. The IVC diameter changes with respiration but its absolute value and collapsibility index (CI = IVCi/IVCe) (where IVCe represents the maximum diameter during expiration and IVCi is the minimum diameter during inspiration) are measured (37). In many patients, the IVC diameter decreases with water removal, and this is associated with complete collapse of IVCi about 2 h after dialysis. Thereafter, the IVCe stabilizes around 7 mm and shows a plateau. In individual patients, the IVC diameter and CI reflect changes in body fluid volume and circulating blood volume.
- 4
Others
The CRIT-LINE Monitor is reported to show changes in blood volume, while body impedance analysis provides a measure of body fluid volume including intracellular and extracellular fluid (38).
II. DIALYSIS-RELATED HYPOTENSION
Statements
- 1
Dialysis-related hypotension can be divided into orthostatic hypotension, chronic sustained hypotension, and intradialytic hypotension (IDH, crash) (Opinion).
- 2
A rapid drop in systolic blood pressure (≥30 mm Hg) during dialysis and orthostatic hypotension after dialysis are two factors associated with poor prognosis (B).
- 3
Undernutrition (hypoalbuminemia) hampers maintenance of blood pressure by reducing the plasma refilling rate (Opinion).
- 4
A recent history of rapid intradialytic fall in blood pressure necessitates evaluation of cardiac function by echocardiography. We suggest consultation with a cardiologist (Opinion).
- 5
We recommend that the amount of water removed per unit time should be mitigated to avoid drop in blood pressure during dialysis, and prolongation of the duration of dialysis should be considered.
Comments
Classification of dialysis-related hypotension
Dialysis-related hypotension can be classified into the following types:
- •
Intradialytic hypotension (IDH, crash)
- •
Orthostatic hypotension
- •
Chronic sustained hypotension
Causes
The fall in blood pressure during dialysis is usually considered to be caused by setting of DW at an unnecessarily low level or low circulating blood volume due to excessive removal of fluid. However, blood pressure cannot be maintained appropriately if the interdialysis body weight gain is large and the amount of the fluid removed per unit time is high. Any decrease in blood pressure should be managed by prolongation of duration of dialysis or increase in DW. Another important factor is hypoalbuminemia associated with malnutrition. Hypoalbuminemia can induce the following changes: (i) reduce the colloid osmotic pressure, and thus reduce the plasma refilling rate, (ii) prevent appropriate body fluid movement from the interstitial tissue into blood vessels due to excessive fluid removal, (iii) reduce the circulating blood volume. These changes hinder the control of blood pressure. Therefore, sufficient serum albumin levels must be maintained. With regard to intradialytic hypotension, it must be remembered that blood pressure may fall during dialysis even without a decrease in the circulating blood volume. In addition, it must be emphasized that myocardial infarction or rapid progression of aortic stenosis must be considered in patients who develop inexplicable intradialytic hypotension.
Reduced cardiac function. In patients with cardiac dysfunction, the blood pressure falls immediately after the initiation of dialysis or following removal of water. Few methods have been described recently to estimate the levels and changes in circulating blood volume, thus allowing the detection of early changes. Any rapid falls in blood pressure occurring during dialysis necessitates evaluation of cardiac function by echocardiography. Aggressive examination and treatment of coronary artery disease and aortic stenosis are important, and consultation with a cardiologist is advised.
Abnormalities of the autonomic nervous system. Abnormalities of the autonomic nervous system are observed frequently particularly in diabetic patients. Low circulating blood volume following excess water removal stimulates the autonomic nervous system to reverse the condition, through contraction of peripheral vessels, although hypotension can occur in patients with autonomic nervous system dysfunction.
Others. A high dialysis fluid temperature, anemia, acetate dialysate, food intake during dialysis, anaphylactic shock due to drugs (e.g. nafamostat mesilate and angiotensin converting enzyme [ACE] inhibitors), first use syndrome related to the dialysis membrane, and ethylene oxide gas used for disinfection, could also reduce blood pressure.
Diagnosis
Intradialytic hypotension is defined as symptomatic sudden drop in systolic blood pressure (by 30 mm Hg or more) during dialysis or a decrease in the mean blood pressure (by 10 mm Hg or more).
Treatments
Drug therapy
Droxidopa, a noradrenergic nerve function improving agent (39,40), is reported to be effective in the treatment of dizziness, lightheadedness, and malaise in dialysis patients with orthostatic hypotension. Amezinium metilsulfate enhances the noradrenergic activity at nerve terminals (41) and is effective in preventing any decrease in blood pressure during dialysis. However, since many of such patients have problems with the setting of DW, nutritional state, and/or cardiac function, the cause of dysdialysis syndrome should be identified, and measures as those mentioned above should be undertaken.
Treatment of dysdialysis syndrome (Instability of hemodialysis) Dysdialysis syndrome is a condition in which necessary dialysis-water removal is difficult due to a fall in blood pressure.
Hypoalbuminemia, malnutrition, and anemia should be treated. Excessive burden to the body by hypoalbuminemia due to undernutrition, and removal of a large water volume per unit time leads to dysdialysis syndrome. Also, since a slight fall in hemoglobin level can exacerbate depression of cardiac function and render the maintenance of blood pressure difficult in patients with low cardiac function.
Other measures are listed below, but the most important is evaluation of cardiac function. Echocardiography is used for this purpose, and treatment against cardiomegaly, valvular heart disease, and ischemic heart disease should be conducted.
- 1
Slow removal of water
The K/DOQI Guidelines recommend maintaining the maximum rate of water removal at 15 mL/kg per h or below.
- 2
Programmed water removal
The volume of water to be removed over a 4-hour dialysis session can be programmed to be achieved as follows: 40% of the total volume of water to be removed during the first hour, 30% during the second hour, 20% during the third hour, and 10% during the last hour. Using such a program, the circulating blood volume decreases rapidly during the first hour but is stabilized thereafter, and the percent fall in circulating blood volume can be reduced even if the same total volume of water is removed.
- 3
DW alteration system
If dialysis is performed on Mondays, Wednesdays, and Fridays, the body weight gain is large on Mondays, DW can be determined as the body weight to be achieved after dialysis on Friday, permitting DW+1.0 kg on Monday and DW+0.5 kg on Wednesday.
- 4
Mid-dialysis discontinuation of water removal
Two hours after the beginning of dialysis, water removal may be interrupted for about 15 min to stimulate plasma refilling.
- 5
Prevention of hypoglycemia
A fall in blood glucose level at 2 h after the start of dialysis is seen in a proportion of diabetic patients. In such patients, the hemodynamics may be stabilized by infusion of 20–40 mL of 50% glucose solution.
- 6
Stimulation of plasma refilling
Since plasma refilling is suppressed in patients with hyponatremia, infusion of 10% NaCl is a possible treatment, but its effect is transient. Albumin preparations can be administered for the management of hypoproteinemia to maintain blood pressure during dialysis. Hydroxyethyl starch, glycerol, and mannitol can also be administered continuously to stimulate plasma refilling. Dextran sulfate, the molecular weight of which is the largest next only to albumin, is also effective although its effect is also transient. Necessary water removal is secured while these drugs are infused continuously at a rate of 100 mL/h or above during dialysis.
- 7
Administration of pressor agents
The blood pressure can fall in diabetic patients due to autonomic nervous system dysfunction despite no change in circulating blood volume. Oral pressor drugs such as droxidopa (39,40) and amezinium metilsulfate (41) are often used in such patients. On the other hand, other drugs such as dopamine and etilefrine can also be used out of necessity, but it is important to try to identify the cause without using them exclusively.
- 8
Low temperature dialysis
Systematic meta-analysis of 22 research studies that included 408 patients showed that the frequency of intradialytic hypotension is 7.1 (95% CI: 5.3–8.9) times higher in the control group (dialysis fluid temperature: 36.5–38.5°C) than in the low temperature dialysis group (dialysis fluid temperature: 34.0–35.5°C) and that the mean blood pressure after dialysis is 11.3 mm Hg higher (95% CI: 7.7–15.0) in the low temperature dialysis group (42).
- 9
Method of dialysis
Although the reason for the effectiveness of hemodialysis filtration (HDF) in preventing a decrease in blood pressure remains unclear, the blood pressure is stabilized in some patients by HDF with 4–6 L of fluid replacement (43). Hemofiltration (HF) and acetate-free biofiltration are effective in preventing falls in blood pressure in acetate-intolerant patients (44,45).
REFERENCES
Chapter 3: Heart Failure
Statements
- 1
Heart failure is a complex clinical syndrome based on structural and functional disorders that impair the systolic and diastolic functions of the ventricle. The primary sign of heart failure is congestion in various organs (A).
- 2
Congestion is diagnosed through medical interview, physical examination, and chest radiography, we recommend it should be evaluated before the beginning of dialysis is recommended (1C).
- 3
Although non-cardiac edema is not a rare cause of congestion, ischemic heart disease, in particular, is a frequent cause of heart failure (B).
- 4
We recommend body fluid volume should be carefully managed based on restriction of salt intake in the treatment of heart failure in dialysis patients (1A).
- 5
We recommend aggressive treatment with renin-angiotensin inhibitors and β-blockers should be considered as the mainstay of medical treatment for disorders causing heart failure (1B).
Comments
Epidemiology
Heart failure is a complex clinical syndrome associated with structural and functional disorders of the heart that impair ventricular filling (diastolic) and ejection (systolic) functions. According to the report by the Statistical Survey Committee of the Japanese Society for Dialysis Therapy (JSDT) (1), the most frequent cause of death in chronic dialysis patients is heart failure, accounting for about 25% of all deaths. Structural/functional disorders of the heart are observed more frequently in dialysis patients than in non-dialysis patients, and only 16% of patients have normal cardiac function at the initiation of dialysis (2). Furthermore, volume overload is also noted in dialysis patients. Based on these two factors, about 30% of patients present with congestive heart failure at the initiation of dialysis.
Causes
Dialysis patients exhibit a wide variety of cardiac disorders such as ischemic heart disease, valvular heart disease, hypertensive cardiomyopathy, metabolic cardiomyopathy, bradycardiac/tachycardiac arrhythmias of long duration, and pericarditis, and if they develop heart failure, differentiation of these conditions becomes important. On the other hand, dialysis patients can also develop non-cardiac edema, which is not accompanied by a clear organic or functional disorder of the heart but is caused by relative excess of body fluid. The most common causes of non-cardiac edema are: (i) volume overload due to excessive salt intake, (ii) severe anemia, (iii) arteriovenous fistula with high blood flow, and (iv) hyperglycemia (3). One report estimated that about 25% of symptoms of congestion in dialysis patients are due to non-cardiogenic edema (4). These conditions used to be known as high output heart failure, but since this condition is not accompanied by cardiac dysfunction, in principle, the term non-cardiac circulatory failure is increasingly being applied to this condition (5). It must be emphasized that volume overload is an important risk factor of cardiovascular death in dialysis patients (6).
Diagnosis
The diagnosis of heart failure requires careful evaluation of congestion of major organs based on medical interviews and physical findings. In dialysis patients, these examinations should be conducted before dialysis, at the peak of body fluid volume. The protocol used for the diagnosis of heart failure in dialysis patients is similar to that applied to non-dialysis patients (7,8).
Medical interviews and physical examinations
The clinical signs of heart failure include pulmonary congestion and a decrease in blood pressure associated with left-side failure, peripheral edema associated with right-side failure, hepatomegaly, jugular vein distension, and peritoneal or pleural effusion. Patients with mild pulmonary congestion complain of dyspnea on exertion. As the condition advances, dyspnea at rest or paroxysmal nocturnal dyspnea and orthopnea appear. Acute heart failure is suspected if only the clinical signs of left-side heart failure are observed, whereas chronic heart failure is suspected when clinical signs of both-side heart failure are noted. The clinical findings that are specific to hemodialysis patients include repeated attacks of hypotension during dialysis, difficulty in achieving DW due to decreases in blood pressure during dialysis, and rapid widening of the cardiothoracic ratio. In such events, heart failure should be suspected even if the clinical signs of heart failure are not clear, and cardiac function should be evaluated (9).
Significance of brain natriuretic peptide
Human brain natriuretic peptide (BNP) or N-terminal fragment of proBNP (Nt-proBNP) is also useful for the diagnosis of heart failure (10–12), evaluation of the severity of heart failure (10,13), prediction of future cardiovascular events (14,15), and prognosis (15,16). For the diagnosis of heart failure in dialysis patients, it is important to establish a standard value based on values measured during appropriate DW and lack of clinical signs of heart failure. In symptomatic patients, the cardiac load is estimated by evaluating the relative changes compared with the standard (17).
Risk factors
Age, diabetes, history of coronary artery disease, reduced left ventricular systolic function, high diastolic pressure, hypoalbuminemia, and low hemoglobin concentration are important risk factors of de novo occurrence of heart failure in dialysis patients (18,19).
Diagnosis of causative disorders To determine the cause of heart failure, the patient should be examined for the presence (or absence) and type of heart murmur(s), arrhythmias, 12-lead electrocardiogram (ECG) abnormalities, regional chest wall motion abnormalities and valvular disease by echocardiography. Coronary artery disease is observed in 40–60% of dialysis patients (20–24), and acute coronary syndrome is very likely to be manifested as heart failure (25). In chronic heart failure, factors that cause non-cardiac edema must be evaluated first. In patients with pulmonary congestion due to volume overload, the clinical symptoms reappear with increases in body fluid volume. The appearance of clinical symptoms and signs of congestion despite appropriate control of DW and the presence of only a mild increase in body fluid volume necessitates thorough examination to differentiate organic cardiac disorders.
Treatments
General management
The principle of treatment is management of body fluid volume based on strict restriction of salt intake (5 g/day), and guidance to control interdialysis body weight gain at less than 3% of the DW when the interdialysis interval is 1 day and less than 5% when the interval is 2 days. In patients with clinical signs of congestion, treatment is started with downward adjustment of the DW for fluid overload, but the correction of anemia, optimization of the arteriovenous fistula flow, and management of blood glucose level are also important.
Medical treatment
Left ventricular remodeling is often observed in patients with chronic heart failure and impairment of left ventricular systolic or diastolic function, in order to compensate for the decrease in cardiac output. However, compensation by left ventricular remodeling may eventually worsen systolic and diastolic functions. Breaking this vicious cycle is the most important step to improve prognosis. Left ventricular remodeling is promoted primarily by marked activation of the neuroendocrine systems such as the sympathetic nervous system and renin-angiotensin (RA) system (26). Thus, inhibitors of the RA system and β-blockers are used to suppress the activation of these neuroendocrine systems (8).
Digitalis
Treatment with digoxin does not improve the prognosis of non-dialysis patients with left ventricular systolic dysfunction (27). Based on reports demonstrating that digoxin treatment increases the risk of death by 28% in dialysis patients and that the risk of death increases with higher blood digoxin levels or with plasma potassium level of 4.3 mEq/L or less (28), any aggressive administration of digoxin for the treatment of heart failure should be avoided in dialysis patients.
Inhibitors of the renin-angiotensin system (RA system inhibitors)
In non-dialysis patients, aggressive treatment of heart failure using RA system inhibitors is recommended regardless of the disease causing left heart dysfunction (8). However, there is only little information on the effectiveness of RA system inhibitors in dialysis patients with heart failure. In dialysis patients with myocardial infarction, treatment using angiotensin converting enzyme inhibitors was reported to reduce the risk of death within 3 months by 42% (29), and treatment using ACE inhibitors and angiotensin receptor blockers (ARBs) reduced the risk of death within 1 year by 30% (30). However, these beneficial effects could not be observed in other studies (31). At present, there is little evidence that RA system inhibitors prevent exacerbation of heart failure in dialysis patients, but there is also no report that the same drugs enhance the development heart failure or cardiovascular events.
β-blockers
The beneficial effects of β-blockers in patients with heart failure are well documented in non-dialysis patients (32,33). Cice et al. (34) compared the effect of carvedilol (which includes an α-blocking action) with that of placebo in dialysis patients with dilated cardiomyopathy complicated by NYHA grade II–III heart failure and reported significantly fewer cardiovascular events in the carvedilol group. Berger et al. (29) also reported that β-blockers reduced the risk of death of dialysis patients after myocardial infarction by 22% in an observational cohort study. In Japan, Nakayama et al. (35) reported that low-dose (5 mg) carvedilol improved left ventricular systolic function, morphological abnormalities of the left ventricle, and significantly decreased plasma BNP levels in dialysis patients with asymptomatic left ventricular systolic dysfunction. Thus, β-blockers are also expected to improve the prognosis of dialysis patients with heart failure.
Indication of ultrafiltration
Ultrafiltration may be employed in patients with unsatisfactory response to drug therapy, in addition to usual dialysis to alleviate preload.
REFERENCES
Chapter 4: Ischemic Heart Disease
Statements
- 1
Asymptomatic myocardial ischemia is not uncommon in patients on maintenance hemodialysis. We recommend active screening for ischemic heart disease starting from the initiation of dialysis (1B).
- 2
We recommend evaluation of possible myocardial ischemia in the presence of shortness of breath, heart failure, fall in blood pressure during dialysis, or changes in electrocardiogram (ECG) and chest X-ray (1C).
- 3
We recommend echocardiography should be performed when myocardial ischemia is suspected. Non-invasive techniques should be used for examination, including myocardial scintigraphy (1B).
- 4
We recommend the application of cardiovascular drug therapy and correction of coronary risk factors by non-invasive treatment (1B).
- 5
We recommend acute coronary syndrome should be excluded in the acute phase of heart failure (1B). Biomarkers of myocardial ischemia often show false positive results, and caution is necessary for the diagnosis (B).
Comments
Epidemiology
Cardiovascular deaths are 10–20 times more frequent in dialysis patients than in the general population (1). The prevalence of coronary artery disease is also high in this population (2). Coronary artery disease in dialysis patients is often asymptomatic or presents non-specific symptoms. Significant coronary stenosis was noted in 50% or more of asymptomatic dialysis patients who underwent coronary angiography within 1 month after the introduction of dialysis (3), and significant coronary artery stenosis of more than 50% narrowing on coronary angiography was reported in 53% of asymptomatic patients at the introduction of dialysis (4). The prevalence of coronary artery disease is markedly higher in dialysis patients than in non-dialysis patients, and marked asymptomatic coronary artery stenosis may be present already at the initiation of dialysis therapy, possibly leading to serious cardiac events or cardiac death. We consider dialysis patients as a high-risk group for coronary artery disease and recommend aggressive screening of these patients for coronary artery disease.
Diagnosis
In the presence of clear symptoms of angina pectoris
The appearance of symptoms of angina pectoris (e.g. precordial pain, pain radiating to the left shoulder, back pain, and epigastric pain) warrants prompt referral to a cardiologist.
In the absence of clear symptoms of angina pectoris
Many dialysis patients show no clear symptoms or signs of myocardial ischemia. When shortness of breath on exertion, palpitation and signs of heart failure are observed, ischemic heart disease should be suspected. Changes in ECG and chest X-ray findings may indicate myocardial ischemia. Such changes in symptoms or signs and test findings may also be caused by inappropriate setting of DW, excessive internal shunt flow, valvular heart disease advanced by valve calcification, pericardial fluid retention from uremic pericarditis, pulmonary hypertension, or left ventricular hypertrophy. Therefore, it is important to differentiate the causes of these changes in symptoms and signs, ECG, or chest X-rays (Table 1) by performing resting echocardiography, evaluating cardiac function and circulating blood volume, and examining the presence or absence of valvular disease, pulmonary hypertension and pericardial/myocardial disorders. Abnormalities of circulating blood volume require modification of the DW. For patients who show no improvement in signs or symptoms and abnormalities of ECG or chest X-ray findings even after modification of the DW, we recommend referral of the patient to a cardiologist. Referral to a cardiologist is also recommended if cardiac dysfunction, pulmonary hypertension, and disorders of the heart valves, pericardium, or myocardium are considered possible. Moreover, when no abnormality is detected by echocardiography, referral to a cardiologist is necessary to exclude possible ischemic heart disease (Fig. 1).
| 1. Symptoms |
| a. Non-specific symptoms |
| Shortness of breath on exertion, palpitation, discomfort of the chest, epigastric region, and back, languidness of lower limbs, etc. |
| b. De novo heart failure |
| c. Heart failure not responding to a reduction in the DW |
| d. Repeated hypotension during dialysis |
| e. Sustained hypotension |
| 2. New abnormalities detected by regular ECG |
| a. ST-T changes (including non-specific changes) |
| b. Appearance of Q waves |
| c. Arrhythmias |
| 3. New abnormalities detected by regular chest radiography |
| a. An increase in the cardiothoracic ratio (≥5%) |
| b. Pulmonary congestion |
| c. Interstitial pulmonary edema (Kerley's A, B, C lines) |
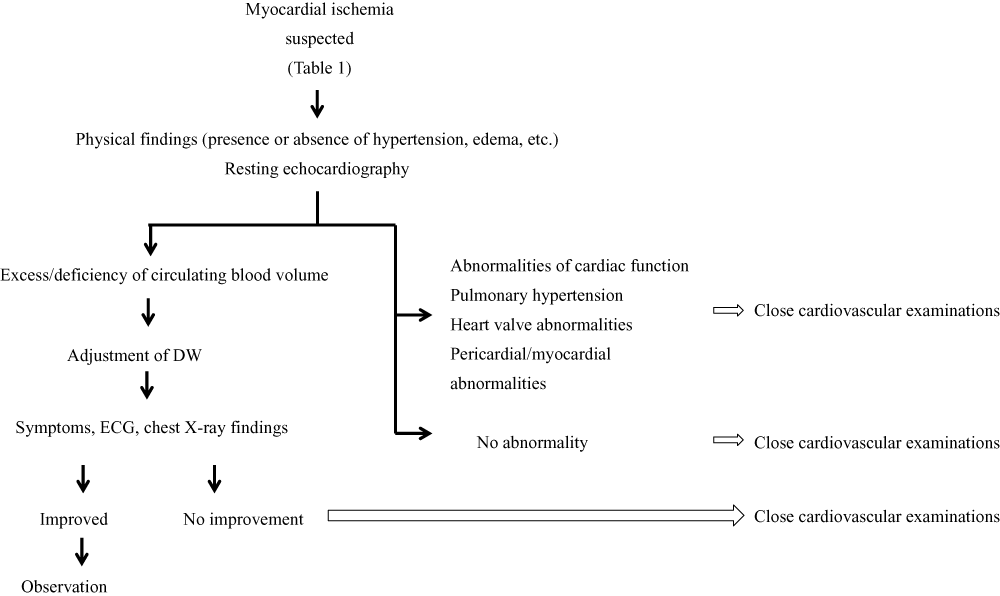
Diagnostic processes for ischemic heart disease in dialysis patients.
Diagnostic procedure
ECG. Changes in resting 12-lead ECGs are important in the diagnosis of myocardial ischemia. Myocardial ischemia should be suspected in patients with ECG findings of acute/old myocardial infarction or non-specific ST-T changes. Disorders of peripheral arteries, such as arteriosclerosis obliterans, dialysis-related amyloidosis, bone/joint disorders, such as vertebral canal stenosis, and low exercise tolerance are often encountered in patients on dialysis. In patients with left ventricular hypertrophy, ST-T changes are difficult to detect, and exercise stress ECG is often difficult to evaluate.
Echocardiography. Resting echocardiography is useful for the evaluation of circulating blood volume, diagnosis of systolic/diastolic cardiac dysfunction, and evaluation of valvular heart disease, pulmonary hypertension, and pericardial/myocardial disorders. Stress echocardiography may be recorded during exercise or drug loading such as dobutamine and dipyridamole, but caution is needed since paroxysmal atrial fibrillation frequently occurs in dialysis patients.
Radionuclear studies of the heart. First, we recommend drug-loaded myocardial perfusion scintigraphy using adenosine. In addition, myocardial scintigraphy using fatty acid analogue without any loading may be useful for the detection of myocardial ischemia in dialysis patients (5). Myocardial scintigraphy is useful for the evaluation of risk of cardiac events. The incidence of cardiac events is 4% in those with a normal adenosine-loaded myocardial perfusion scintigram but high at 67% in those with abnormal scintigraphy (6). Also, abnormalities of myocardial fatty acid metabolism detected by fatty acid imaging may indicate the risk of cardiac death in dialysis patients (7).
Coronary artery computed tomography (CT) angiography. Contrast-enhanced coronary artery CT angiography is inappropriate for dialysis patients due to the associated problem of volume loading by the contrast medium, and because the diagnosis of coronary artery stenosis/obstruction is difficult in patients with marked coronary artery calcification.
Coronary artery magnetic resonance angiography (MRA). Very little information is available on the application of coronary artery MRA for coronary artery diseases in dialysis patients, and its usefulness is unclear. In principle, however, the use of the magnetic resonance imaging (MRI) contrast medium is contraindicated in dialysis patients.
Diagnosis of acute coronary syndrome. Development of acute myocardial infarction in a dialysis patient is often associated with the primary symptoms of heart failure such as dyspnea and cough rather than left shoulder pain, chest pain, or back pain, and Q waves are observed infrequently on ECG (8). Acute heart failure in dialysis patients should be differentiated from acute coronary syndrome. Biomarkers of acute myocardial ischemia include myocardial troponin T, myocardial fatty acid-binding protein, and CPK-MB fraction, but care must be exercised to avoid false positive dialysis patients based on measurement of marker levels (9,10).
Treatment
Non-invasive treatments
Dialysis as well as non-dialysis patients are treated with antiplatelet agents, β-blockers, nitrites, Ca channel blockers, angiotensin I converting enzyme inhibitors, angiotensin II receptor antagonists, and nicorandil. Dose modification may be necessary in using β-blockers and angiotensin I converting enzyme inhibitors.
Reports on the preventive effect of cardiovascular drugs on cardiac events are scarce. Angiotensin II receptor antagonists are reported to have reduced the incidence of cardiovascular events in dialysis patients (11), but one meta-analysis study casted doubt on their effectiveness in this population (12). Nicorandil is reported to reduce cardiac events in asymptomatic dialysis patients (13) and cardiac events/cardiac deaths after coronary artery interventions (14,15).
Correction of coronary risk factors such as anemia, hyperlipidemia, and hypertension is also important. The 2008 Guidelines for the Treatment of Renal Anemia of the Japanese Society for Dialysis Therapy recommend a target Hb level of 10–11 g/dL for dialysis patients in general and 11–12 g/dL for active and relatively young patients. We suggest managing the Hb at a stable level without allowing it to fall below 10–11 g/dL, the lower limit proposed by the guidelines. Very little evidence is available concerning the effectiveness of lipid lowering therapy using HMG-coenzyme A reductase inhibitor (statins). There are two large-scale interventional studies in dialysis patients (German Diabetes and Dialysis Study (16) and AURORA Study (17)), and neither reported a significant decrease in the risk of cardiovascular diseases. However, in the former study, which included many patients with hyper-LDL-cholesterolemia, statins significantly reduced the incidence of ischemic cardiac events, which was the secondary end-point. Consideration of statin therapy may be necessary in dialysis patients with hyper-LDL-cholesterolemia or hyper-non-HDL-cholesterolemia.
Coronary revascularization
Invasive revascularization is effective against coronary artery diseases. Revascularization may be achieved by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), and the therapeutic outcome has improved following the use of drug-releasing stents in the former and off-pump bypass surgery in the latter.
Treatment of acute coronary syndrome
Acute coronary syndrome often leads to sudden death, and, upon its development, the patient should be immediately transported by ambulance to a hospital with a special cardiology unit. The outcome is poorer in dialysis patients than in non-dialysis patients (18), and emergency PCI is usually considered.
REFERENCES
Chapter 5: Arrhythmias/Valvular Heart Disease
I. SUDDEN CARDIAC DEATH AND ARRHYTHMIAS
Statements
- 1
We recommend performing exercise electrocardiogram (ECG) and Holter ECG for induction of arrhythmias and evaluation of the effects of therapy, respectively (1B).
- 2
Since dialysis patients with arrhythmias are likely to have organic heart diseases, we recommend echocardiography, stress nuclear imaging tests, and, when necessary, coronary angiography (1B).
- 3
We recommend aggressive treatment of ventricular fibrillation/flutter, sustained ventricular tachycardia, sick sinus syndrome, sinoatrial block, and marked atrioventricular bock (1A).
- 4
We suggest that warfarin should not be used in the treatment of atrial fibrillation without careful evaluation, but, if warfarin therapy is judged to be beneficial, the prothrombin time-international normalized ratio (PT-INR) should be maintained at less than 2.0 (2C).
- 5
We recommend measurement of PT-INR in directly sampled blood from a blood vessel (1C).
Comments
Epidemiology
Sudden cardiac death (SCD) and fatal ventricular arrhythmias occur in 5–7% of dialysis patients (1,2), and this incidence rate is 25–70 times higher than in the general population (3). SCD occurs frequently 12 h after the initiation of dialysis and 36–48 h after previous dialysis (4) and is thought to be related to heart failure, coronary artery disease, diabetes, fluid overload, activation of the sympathetic nervous system, hyperkalemia, and hypokalemia. Fatal ventricular arrhythmia is a collective term for cardiac arrest, ventricular fibrillation, ventricular flutter, and ventricular tachycardia.
Important bradycardia includes sick sinus syndrome, sinoatrial block, and marked atrioventricular (AV) block, but there is no evidence that they are observed frequently in dialysis patients.
Atrial fibrillation (AF) and atrial flutter are important causes of acute congestive heart failure, chronic congestive heart failure due to tachycardia-induced cardiomyopathy, and dysdialysis syndrome. AF can be classified into (i) paroxysmal AF, (ii) persistent AF, and (iii) permanent AF. AF is noted in about 12% of patients at the initiation of dialysis (5), and this incidence increases with age and history of dialysis (6). The risk of death is significantly higher in dialysis patients with AF than in those with sinus rhythm (5).
Pathological features
Arrhythmias observed in dialysis patients are caused secondarily by ischemic or other cardiomyopathy, valvular heart disease, electrolyte abnormalities, and rapid changes in circulating blood volume, and differential diagnosis from organic heart diseases is important.
Diagnosis
Clinical symptoms
The primary clinical features of fatal arrhythmia are sudden cardiopulmonary arrest, syncope, convulsion, and acute congestive heart failure; those of severe bradycardiac arrhythmia are syncope, convulsion, and acute congestive heart failure; while those of tachycardiac arrhythmia are palpitation, skipped beat, and congestive heart failure. However, more than a few patients remain asymptomatic.
Examinations
Standard resting 12-lead ECG is important for the detection of organic heart diseases, arrhythmias, and electrolyte abnormalities. The ECG should be performed at the introduction of dialysis and periodically thereafter. Exercise ECG should be used to induce and diagnose arrhythmias, while Holter ECG should be used to evaluate the risk of arrhythmias and the effectiveness of treatment. In dialysis patients with arrhythmias, organic heart diseases should be detected by echocardiography and, stress nuclear imaging tests. If the results of these examinations strongly suggest coronary artery disease, it is necessary to perform coronary angiography (7).
Treatment
Preventive treatments against fatal ventricular arrhythmia
β-blockers are recommended as the mainstay for drug therapy, because they are safe and effective in suppressing any ventricular extrasystoles and other arrhythmias and in preventing SCD (8). In dialysis patients with left ventricular systolic dysfunction (left ventricular ejection fraction <35%), administration of carvedilol significantly reduces not only total cardiovascular deaths but also sudden cardiac deaths (9). According to a report that evaluated the drugs used before cardiac arrest and the outcome after cardiac arrest, β-blockers and RAS inhibitors dose-dependently improved the outcome (10) Group I antiarrhythmic drugs should be used carefully, because they are likely to increase the mortality rate associated with fatal arrhythmias (11,12). Catheter cauterization as a treatment for ventricular tachycardia is useful in non-dialysis patients (13) and is also considered useful in dialysis patients, but there is no evidence to support this conclusion.
Emergency treatment of fatal ventricular arrhythmias
The automated external defibrillator (AED) is a device that can save patients if it is applied immediately after the onset of ventricular fibrillation. However, evidence of the benefit of equipping dialysis facilities with AEDs is insufficient (14).
Implantable cardioverter-defibrillator for fatal ventricular arrhythmias
The implantable cardioverter-defibrillator (ICD) is reported to improve the outcome of fatal ventricular arrhythmias in dialysis patients (2). However, the risk of death after treatment in patients with stage 4–5 CKD including dialysis patients is about 40 times higher than in patients with normal renal function (15), and this must be explained to the patients themselves and their families when attempting treatment with the ICD.
Treatment of marked bradycardiac arrhythmia
The indications for permanent pacemaker implantation in patients with progressive second- or third degree AV block are as follows: (i) presence of clear clinical symptoms related to bradycardia, (ii) bradycardia caused by irreplaceable drugs, (iii) cardiac arrest sustained for 3 s or longer or ventricular pulse rate less than 40/min during wakefulness, (iv) history of catheter ablation of the AV junction, (v) history of heart surgery, and (vi) presence of progressive neuromuscular disease (16).
Warfarin therapy for atrial fibrillation
Warfarin is recommended for the treatment of non-dialysis patients with AF to prevent thrombotic complications (17). However, in a study that followed up 1671 dialysis patients with AF over a mean period of 16 years, the risk of de novo stroke was 193 times higher in warfarin users than in non-users, and the risk of stroke increased 279 times in patients using warfarin without monitoring the PT-INR compared with patients not using warfarin (18). The Dialysis Outcomes and Practice Patterns Study (DOPPS) also reported that warfarin was administered in 16% of patients with AF but the risk of stroke increased 217 times in warfarin users aged 75 years and above (19). However, the above results were those of observational studies, and thus larger-scale interventional studies are necessary in the future to evaluate the usefulness of warfarin. Until then, warfarin must be used carefully in dialysis patients with AF. False elevations of the PT-INR are observed frequently during dialysis, and they are reportedly caused by inappropriate blood sampling using an indwelling catheter (20). We recommend that blood used for the determination of PT-INR be collected directly from a blood vessel.
Defibrillation treatment of atrial fibrillation
Defibrillation is attempted by medication or electric shock. The indications for electric defibrillation are the following: (i) severe tachycardiac atrial fibrillation not responding to medications accompanied by progressive myocardial ischemia, symptomatic hypotension, angina pectoris, or heart failure, (ii) severe tachycardiac atrial fibrillation or unstable hemodynamics, and (iii) intolerable clinical symptoms due to atrial fibrillation despite stable hemodynamics (17). Antiarrhythmic drugs described in the present guidelines are used for defibrillation, but monitoring of the dose, blood concentration of the drug during treatment, and ECG recording are necessary in treated patients, because all such drugs have arrhythmia-inducing effects by prolonging the QT interval or extending the QRS duration. One meta-analysis that compared the rhythm control and heart rate control in non-dialysis patients with AF showed no significant difference in mortality rate, incidence of non-CNS bleeding, and incidence of ischemic stroke except that the proportion of patients who required hospitalization was significantly lower in the heart rate control group (P < 001), and ventricular tachycardia or bradycardia and QT prolongation were observed frequently in the rhythm control group (21). In dialysis patients treated with various antihypertensive drugs and who show marked changes in potassium concentration associated with dialysis therapy, rate control should be the basic treatment.
Rate control therapy for atrial fibrillation
Rate control therapy using β-blockers and non-dihydropyridine Ca-antagonists should be the primary treatment. In dialysis patients, treatment with digoxin results in immediate digoxin toxicity (22). Thus, it is desirable to avoid digoxin because it has a long disappearance time from blood and interacts with many other drugs. The target heart rate is 90–100 beats/min or below in the acute period, a resting heart rate of 60–80 beats/min eventually, and a heart rate of 90–110 beats/min during moderate exercise (7). Catheter ablation is a reasonable alternative for drug therapy in patients with symptomatic recurrent atrial fibrillation not accompanied by left atrial dilation (17). While there is no evidence of the usefulness of catheter ablation in dialysis patients, there is also no reason to exclude it.
REFERENCES
II. VALVULAR HEART DISEASE
Statements
- 1
In dialysis patients with asymptomatic heart murmur or heart murmur accompanied by congestive heart failure, syncope, arterial embolism, or dysdialysis syndrome on auscultation, we recommend differential diagnosis of valvular heart disease (1A).
- 2
We recommend the diagnosis of valvular heart disease and evaluation of its severity by Doppler echocardiography (1A)1.
- 3
In dialysis patients with acute valvular heart disease, we suggest that infective endocarditis should be differentiated (2B).
- 4
We recommended periodic performance of echocardiography in patients with mild or moderate valvular heart disease and not to postpone surgical treatment in patients with severe valvular heart disease (1A).
Comments
Epidemiology
In general, the mean left ventricular-aortic pressure gradient in patients with aortic stenosis (AS) increases by 7 mm Hg per year, in association with a mean annual narrowing of the valvular area narrows of 0.1 cm2(1). In dialysis patients, AS occurs at a younger age and deteriorates more rapidly with progression of calcification. The incidence of AS in dialysis patients is 1.5–8.0%/year, and the rate of narrowing of the valvular area is 0.23 cm2/year (2). Reduced mobility and contact insufficiency of the aortic valve due to calcification are major causes of aortic regurgitation (AR). AS occurs more frequently with AR than alone. The progression of aortic valve calcification correlates with the following factors: (i) aging, (ii) male sex, (iii) an increase in the Ca×P product, (iv) period of dialysis, (v) progressive increase in CRP, (vi) oral intake of calcium preparations (2–5). A proportion of dialysis patients shows no adhesion in the commissural region but mitral stenosis (MS) with marked valvular ring calcification. The progression of MS, similar to AS, is characteristically rapid in dialysis patients. Infective endocarditis due to bacterial infection from shunt puncture or venous catheter placement, occurs 17 times more frequently in dialysis patients compared with non-dialysis patients (6).
Pathological features
The cause of valvular heart disease accompanied by severe calcification in dialysis patients is age-related degeneration. The mechanism of degeneration is destruction of the most external endothelial cell layer of the valve, followed by infiltration of inflammatory cells, such as monocytes and T-lymphocytes, into the interior of the valve, uptake of low-density lipoprotein, release of inflammatory cytokines, proliferation of interstitial cells of the valve, and remodeling and calcification of the extracellular matrix (7,8). In addition to the age-related degeneration, infective endocarditis, aortic dissection, and bicuspid aortic valve may also cause AR. On the other hand, mitral regurgitation (MR) occurs when the valve cusps fail to close tightly due to tethering of the papillary muscle, which pulls the cusps, in addition to the widening of the mitral ring due to enlargement of the left ventricular cavity as a result of infective endocarditis or ischemic cardiomyopathy, in association with age-related degeneration (9). To determine the cause of acute AR or MR in dialysis patients, differentiation of infective endocarditis is desirable (10). Furthermore, infective endocarditis in patients who receive artificial valve replacement may lead to more serious acute dysfunction of the heart (cardiogenic shock and congestive heart failure). The condition may not respond to treatment with antibiotics and may cause valvular ring and endocardial abscesses. The causative bacteria are frequently staphylococci, streptococci, or Gram-negative bacilli. Functional MR may also occur when the setting of dry weight (DW) is inappropriate.
Diagnosis
Clinical symptoms
Chronic valvular disease is characterized by a long asymptomatic period, although heart murmur is heard from an early stage. Clinically, heart murmur is heard before the appearance of symptoms. The commonest clinical features are anginal attacks, syncope, congestive heart failure, palpitation (arrhythmias), and arterial embolization (1), and dysdialysis syndrome is specific to dialysis patients. These symptoms are due to low cardiac output and pulmonary hypertension. In dialysis patients with asymptomatic heart murmur and those with heart murmur accompanied by congestive heart failure or syncope, angina pectoris, arterial embolism, or dysdialysis syndrome, differentiation of valvular heart disease is recommended (1). In dialysis patients with MR, symptoms of congestion can be resolved or alleviated by lowering the DW, and accordingly, it is important not to postpone surgical treatment in patients with severe valvular heart disease
Examinations
The area of the normal orifice of the aortic valve is 2.5–3.5 cm2, and that of the mitral valve is 4.0–6.0 cm2. Doppler echocardiography should be used for the diagnosis of valvular heart disease and evaluation of its severity (1). The valve orifice area can be either determined by direct tracing the valve orifice in a short-axis tomogram of echocardiography or estimated by the continuous wave Doppler method from the pressure halftime (PHT). However, since it is impossible to accurately trace the valve orifice in dialysis patients with severe valve ring calcification, estimation of the valve orifice area from the PHT is more appropriate. Since functional MR can occur if the DW is set inappropriately, Doppler echocardiography should be performed on the achievement of the DW.
Treatments
While there is no effective drug therapy for AS, MS, or MR in dialysis patients, patients with valvular diseases accompanied by calcification treated with calcium carbonate as a phosphorus-binding agent should be switched to sevelamer hydrochloride or lanthanum carbonate (11). β-blockers and non-dihydropyridine Ca-antagonists are effective against any associated tachycardia (1). Also, valvuloplasty using a balloon is not recommended for severe calcification, and surgical valve replacement is indicated instead for such patients (1).
REFERENCES
Chapter 6: Surgical Treatments
I. ISCHEMIC HEART DISEASE
Statements
- 1
Chronic kidney disease is a poor prognostic factor of coronary artery bypass grafting (CABG) (B).
- 2
In dialysis patients, the long-term survival rate is higher after CABG than after percutaneous coronary intervention (PCI) (B).
- 3
In dialysis patients, we suggest that use of artificial heart-lung machine should be avoided to alleviate perioperative complications (2B).
- 4
In dialysis patients, the greater saphenous vein used as a graft may deteriorate rapidly, and we suggest use of the internal thoracic artery for CABG (2B).
Comments
Coronary artery revascularization
In Japanese dialysis patients who had undergone coronary artery bypass grafting (CABG), the reported hospital death rate is 9% (1), and the 5-year survival rate is 40–60% (1). Furthermore, in dialysis patients who had undergone CABG using an artificial heart-lung machine, the mortality rate is 3.1 times higher, the incidence of mediastinitis is 2.4 times higher, and incidence of cerebral infarction is 2.1 times higher, than in patients with normal kidney function (2). Therefore, safety management during the CABG perioperative period is the most important in dialysis patients.
In the field of percutaneous coronary intervention (PCI), postoperative re-stenosis has markedly diminished following the advent of drug-eluting stents (DES) and the development of new antiplatelet agents, making PCI the first choice treatment for coronary artery disease. In dialysis patients, however, the re-stenosis rate has been reported to be high even after PCI using DES (3). While the benefits of DES in dialysis patients including the long-term follow-up results remain unclear, the long-term survival rate of dialysis patients is presently higher after CABG than after PCI (4,5). In Japan, the long-term survival rate is reported to be comparable between the two procedures (6). However, since diabetes is often the primary disease in dialysis patients, many patients are considered to require repeated coronary artery revascularization, and a higher priority may be placed on CABG as long as the general condition permits it.
Coronary artery bypass grafting
Dialysis patients often develop generalized advanced arteriosclerosis. Therefore, off-pump CABG without the use of the artificial heart-lung machine is needed to reduce the risk of release of atheromatous emboli by procedures such as insertion of a blood feeding tube into the ascending aorta for extracorporeal circulation and to facilitate perioperative hydration management. In fact, perioperative complications including deaths have reduced following the introduction of off-pump CABG (7–11). Shroff et al. (11) reported that the hospital death and 3-year postoperative mortality rates were significantly lower in the group of dialysis patients treated with the internal thoracic vein. Therefore, it is recommended to use the internal thoracic vein as long as possible in bypass surgery of the left anterior descending artery.
Percutaneous coronary intervention should be considered first for patients with acute coronary syndrome. Also, left ventriculoplasty must be considered for patients with ischemic cardiomyopathy. However, in dialysis patients, many of whom have myocardial infarction with lesions in multiple areas of the coronary artery and/or left ventricular dysfunction, the surgical procedure (e.g. off-pump CABG or on-pump beating CABG alone) should be selected after sufficient evaluation of whether postoperative death associated with the initial surgery can be avoided.
REFERENCES
II. VALVULAR HEART DISEASE
Statements
- 1
We suggest valvular heart disease in dialysis patients should be regarded as part of generalized ectopic vascular calcification (2B).
- 2
In surgical treatment for valvular disease in dialysis patients, we recommend preoperative evaluation of arterial calcification by coronary angiography, whole body computed tomography (CT) and ultrasonography of the carotid artery (1C).
- 3
Frequent intradialytic hypotension suggests valvular heart disease, and we recommend echocardiography in such patients (1C).
Comments
Since dialysis patients often have generalized severe arteriosclerosis and calcification lesions, it is important to preoperatively evaluate not only the cardiac and valvular functions but also head and neck lesions by imaging procedures such as coronary angiography, ultrasonography of the carotid artery, head CT, and head magnetic resonance imaging (MRI). Other pathologies such as calcification of the aorta and atherosclerotic lesions of the abdominal organs should be assessed by thoracic and abdominal CT, and peripheral obstructive arteriosclerosis by using indices such as the ankle brachial index (ABI). Then, it is necessary to judge whether an artificial heart-lung channel can be established (particularly, when a blood feeding tube should be placed) or whether aortic clamping is possible (1). More importantly, the state of the ascending aorta must be checked, and the position of insertion of the blood feeding tube and the site of aortic clamping must be determined carefully by intraoperative examinations such as ultrasonography. Blood feeding via a vessel other than the ascending aorta must be considered in patients with porcelain aorta, and deep hypothermic circulatory arrest, blocking with, for example, a balloon, must be considered if aortic clamping is impossible.
Dialysis patients readily develop septicemia and are likely to develop infective endocarditis due to bacteria attached to degenerated areas of the valvular cusps. Acute valvular disease must always be treated with infective endocarditis in mind.
Aortic valve
Aortic stenosis. The clinical features of aortic stenosis (AS) are angina pectoris, syncope, and heart failure, and these are absolute indications of surgical treatment. However, dialysis patients often exhibit fewer symptoms because of low ADL level. The progression of calcification, on the other hand, is very rapid, and when calcification of the ascending aorta progresses to marked calcification involving the valve ring to the aortic wall, valve replacement becomes difficult with a rise in intraoperative complications. Therefore, surgery should be considered as early as possible in dialysis patients with maximum blood flow rate of 4.0 m/s or higher, or valve orifice area of 0.6 cm2 or smaller (2), as determined by echocardiography, after taking into consideration the general condition of the patient.
In valve replacement, maximum resection of the calcified areas of the valve ring is necessary. Any remaining calcified tissue can induce regurgitation around the artificial valve and cause hemolysis. Also, if an artificial valve of a sufficient size cannot be used due to hardening of the sinus of Valsalva or valve ring, widening of the valve ring is dangerous, and the use of an artificial valve for a small annulus may be a practical alternative.
Mitral valve
Mitral stenosis. In dialysis patients, calcification is often observed in the valvular cusps, papillary muscle, and tendinous cords, and mitral stenosis (MS) is frequently induced by mitral annular calcification (MAC). Since cardiac dysfunction occurs more slowly in patients with mitral valve abnormalities than those with aortic valve disorders, internal treatment such as adjustment of dry weight is attempted first. If body fluid management is still difficult, surgery is considered if the valve orifice area diminishes to 1.0 cm2 or less. However, valve replacement is technically difficult in patients with severe MAC, and the risk of surgery is high.
Mitral regurgitation. Close examination is needed in patients with calcification- and ischemic-related mitral regurgitation (MR). If degeneration of the valve is mild, valvuloplasty may be possible, but, in this situation, coronary bypass grafting or left ventriculoplasty must be considered also.
Warfarin administration after mechanical valve replacement
Since there are racial differences in warfarin control after mechanical valve replacement, careful evaluation of the results in Japanese patients must be considered (3–5). Also, careful monitoring according to the prothrombin time-international normalized ratio (PT-INR) by integrating conflicting risks of bleeding and thromboembolism is necessary. The Guidelines for Anticoagulant/Antiplatelet Therapies for Cardiovascular Diseases list the criteria for warfarin therapy (Table 1) (6). Hemorrhagic complications and cerebral hemorrhage are frequently encountered in dialysis patients (7,8). These are due to various abnormalities such as low blood vitamin K level, excess administration of warfarin, anemia, and defective clotting mechanisms. Therefore, it is necessary to maintain the PT-INR at a low level in dialysis patients. Also, since the risk of embolism is high during the first few months after surgery, during which sufficient endothelialization of the sutured areas of the valve is necessary (9), warfarin control by a specialist is desirable for at least 3 months after surgery.
| Class I |
| 1. Warfarin therapy for patients within 3 months after artificial valve replacement with PT-INR of 2.0–3.0 |
| 2. Warfarin therapy for patients within 3 months after mitral valvuloplasty with PT-INR of 2.0–3.0 |
| 3. Warfarin therapy for patients within 3 months or longer after surgery under the following conditions |
| Mechanical valve |
| AVR (bicuspid or Medtronic Hall valve), no risk factor† |
| PT-INR = 2.0–2.5 |
| Other disk valves or Starr-Edwards valve, no risk factor |
| PT-INR = 2.0–3.0 |
| AVR + risk factors |
| PT-INR = 2.0–3.0 |
| MVR + risk factors |
| PT-INR = 2.0–3.0 |
| Biological valve |
| AVR + risk factors |
| PT-INR = 2.0–3.0 |
| MVR + risk factors |
| PT-INR = 2.0–3.0 |
| Valvuloplasty |
| Mitral valvuloplasty + risk factors† |
| PT-INR = 2.0–2.5 |
| Class Iia |
| 1. Warfarin administration with PT-INR of 2.5–3.5 for patients with thromboembolism despite appropriate warfarin therapy. |
| 2. Combination of aspirin or dipyridamole coadministration with warfarin for patients with thromboembolism despite appropriate warfarin therapy. |
| Class III |
| 1. No warfarin for patients with mechanical valve replacement |
| 2. Administration of aspirin alone for patients with mechanical valve replacement |
| 3. Administration of neither warfarin nor aspirin for patients with biological valve replacement |
- † Risk factors: atrial fibrillation, history of thromboembolism, reduced left heart function, hypercoagulation state. AVR, aortic valve replacement; MVR, mitral valve replacement; PT-INR, prothrombin time-international normalized ratio.
The table used in this chapter was reproduced from the Guidelines for Non-Drug Therapies of Valvular Disease (2007 revised edition) published by the Japanese Circulation Society. Reproduction was made with permission by the Scientific Committee of the JCS.
REFERENCES
Supplement: Perioperative management for heart surgery in dialysis patients
Preoperative management
- 1
Preoperative evaluation: Before surgery for coronary artery or valvular disease, it is important to evaluate not only cardiac and valve functions but also head and neck lesions by various imaging modalities such as coronary angiography, ultrasonography of the carotid artery, head CT, and head MRI. It is also important to assess calcification of the aorta and arteriosclerotic lesions of abdominal organs by thoracoabdominal CT, as well as peripheral obstructive arteriosclerosis by indices such as the ankle brachial index (ABI). It is also necessary to establish the artificial heart-lung channel (particularly, the site of blood feeding) and judge whether aortic clamping is possible.
- 2
Perioperative dialysis must be planned carefully in cooperation with related departments.
- 3
Dialysis should be performed on the day before surgery as much as possible. Hypotension can occur in dehydrated patients following the induction of anesthesia due to a decrease in circulating blood volume.
Intraoperative management
- 1
The shunt flow must be checked at each entry into the operation room and at the end of each operation. During surgery, utmost care should be exercised to avoid mechanical compression of the shunt. Obstruction may be caused by hypotension, low cardiac output, and other mechanisms. The duration of such events should be shortened.
- 2
Since water excretion is impaired during surgery, care should be taken to avoid excessive fluid overload since it may cause congestive heart failure. However, excessive fall in circulating blood volume is likely to invite fatal complications. Therefore, circulating blood volume should be critically monitored intraoperatively.
- 3
It must be remembered that dialysis patients often suffer from anemia and hypoproteinemia before surgery. Moreover, the release of inflammatory mediators following bleeding, surgical stress, and excess interstitial edema, transfusion or the use of colloid solution may be necessary. Also, the use of artificial colloid solution, which is excreted via the kidney, should be avoided in dialysis patients.
- 4
In surgery using an artificial heart-lung apparatus, the water load should be kept at minimum during extracorporeal circulation by hemodialysis and extracorporeal ultrafiltration method (HD, ECUM). Also, hyperkalemia should be corrected.
Postoperative management
- 1
Restoration of body weight to preoperative baseline level shortly after surgery results in a decrease in circulating blood volume, often causing hypotension or low cardiac output. Therefore, water should be removed slowly over a period of about one week after surgery. The use of drugs with a peripheral vasoconstrictor activity such as noradrenaline is likely to induce fatal complications such as non-occlusive mesenteric ischemia (NOMI). Also, the myocardium of dialysis patients often shows diastolic dysfunction, and a decrease in preload is likely to trigger hypotension or low cardiac output. Therefore, it is necessary to maintain a sufficient circulating blood volume early after surgery.
- 2
If necessary, daily dialysis, CHDF and other procedures should be considered early after surgery. For such procedures, anticoagulants with a short half-life, such as nafamostat mesilate, which is inactivated rapidly in the channel, should be used for hemorrhage.
- 3
Emergency dialysis should be considered when the patient develops hyperkalemia, congestive heart failure due to hypervolemia, pulmonary congestion, or severe acidosis.
- 4
Since the immunological capacity is depressed in dialysis patients, greater emphasis should be placed on clean manipulations. In using antibiotics, changes in their pharmacokinetics should be considered, and their dose and administration period should be modified.
Chapter 7: Cerebrovascular Disorders
I. CEREBRAL HEMORRHAGE
Statements
- 1
Dialysis should be avoided within 24 h after the onset (1C).
- 2
Shortly after the onset of cerebral hemorrhage, we recommend dialysis methods that cause only a mild increase in the intracranial pressure such as continuous hemodiafiltration, peritoneal dialysis, and hemodialysis with restricted blood flow should be selected (1B). We also recommend that glycerol should be administered during dialysis and nafamostat mesilate should be used as an anticoagulant (1B).
- 3
We suggest that blood pressure should be aggressively controlled from the acute period of cerebral hemorrhage (2C).
- 4
We suggest that surgery should be considered for the treatment in the acute period of major cerebral hemorrhage accompanied by an increase in the intracranial pressure (2C).
- 5
We recommend that blood pressure should be strictly controlled to prevent cerebrovascular accident and recurrence (1B).
Comments
Epidemiology
The annual incidence of cerebral hemorrhage in dialysis patients (3.0–10.3/1000 people) is significantly higher than in the general population (1.2 in the Hisayama Study) (1–7). Cerebral hemorrhage in dialysis patients is mainly caused by hypertension, produces larger hematoma than in non-dialysis patients, and is associated with poor prognosis. The associated mortality rate (27–83%, mean: 53%) is also higher than in the general population (19%) (7), and the prognosis is particularly poor in patients with a hematoma volume of 50 mL or greater and those with ventricular rupture (1,2,4–6,8–28).
Pathological features
The pathological changes in the acute period of cerebral hemorrhage are classified into primary damage, which is destruction of the brain parenchyma due to hematoma, and secondary damage caused by subsequent intracranial hypertension, impairment of cerebral circulation and metabolism, and brain edema.
Initial treatment and diagnosis
In patients with provisional diagnosis of stroke, highest priority should be placed on securing respiration and circulation, followed by neurological evaluation. Detection of a high density area in the cerebral parenchyma on head computed tomography (CT) establishes the diagnosis of cerebral hemorrhage. If differentiation from hemorrhagic infarction is necessary, or if cerebral aneurysm, cerebral arteriovenous anomaly, or tumoral bleeding is suspected, evaluation by magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), or cerebral angiography becomes necessary (29). Also, a small hematoma detected soon after the onset may increase in size thereafter, and thus periodic follow-up examination of the hematoma size by CT is important (29). In this regard, nephrogenic systemic fibrosis (NSF) in patients with renal failure due to gadolinium contrast media used for MRI has attracted attention, and warning not to use gadolinium contrast medium in patients with glomerular filtration rate of less than 30 mL/min per 1.73 m2, in principle, has been issued (30).
Treatments
Management of brain edema during the acute period
Glycerol is effective for acute treatment of large cerebral hemorrhage associated with intracranial hypertension (31–33). Since glycerol is not expected to increase urinary volume in dialysis patients and causes volume overload, its administration during dialysis, when excretion of glycerol with water removal is expected, is desirable.
Neurosurgical emergency procedures (removal of hematoma, ventricular drainage) are necessary in patients with serious disturbance of consciousness, those in whom the estimated hematoma volume exceeds 30 mL, and those who subsequently develop hydrocephalus due to ventricular rupture of hematoma (33). While the results of craniotomy are unfavorable in dialysis patients (1), the results of stereotactic removal of hematoma for putaminal hemorrhage are comparable to those in non-dialysis patients especially when the hematoma volume is 30–50 mL (34).
Blood pressure control during the acute period
During the acute period of cerebral hemorrhage, it is very important to control blood pressure to prevent re-bleeding, expansion of hematoma, and exacerbation of brain edema. Although it is important to maintain systolic pressure at 180 mm Hg or below (mean blood pressure ≤130 mm Hg), it is also recommended to slowly reduce the blood pressure at 80% of the pretreatment value to avoid the risk of cerebral ischemia due to excessive decrease in blood pressure (Table 1) (35,36).
| Blood pressure management in patients with acute-phase cerebral infarction | ||
| No indication for thrombolytic therapy | SBP <220 mm Hg or DBP <120 mm Hg | No aggressive antihypertensive treatment (except when there is a systemic complication such as hypertensive encephalopathy, aortic dissection, acute renal failure, acute pulmonary edema, and acute myocardial infarction) |
| SBP >220 mm Hg or DBP >120 mm Hg | Target of antihypertensive treatment: 85–95% of the previous value | |
| Antihypertensive agents (i.v. drip infusion of nicardipine, diltiazem, nitroglycerin, or nitroprusside) | ||
| With indications for thrombolytic therapy | SBP >185 mm Hg or DBP >110 mm Hg | Target of antihypertensive treatment: <180/105 mm Hg |
| Antihypertensive agents (i.v. drip infusion of nicardipine, diltiazem, nitroglycerin, or sodium nitroprusside) | ||
| Blood pressure management in patients with acute-phase cerebral hemorrhage | ||
| SBP >180 mm Hg or MBP >130 mm Hg | Target of antihypertensive treatment: 80% of the previous value | |
| Antihypertensive agents (i.v. drip infusion of nicardipine, diltiazem, nitroglycerin, or nitroprusside) | ||
- DBP, diastolic blood pressure; MBP, mean blood pressure; SBP, systolic blood pressure.
Brain examination for asymptomatic microbleeds
Microbleeds appearing as low-signal-intensity areas by T2*-weighted MRI are frequently detected in patients with long-term hypertension and those with a history of cerebrovascular disorders, but they are also noted frequently in dialysis patients (37,38). While microbleeds have been reported to affect the occurrence of cerebral hemorrhage (39–41), whether the risk of cerebral hemorrhage is increased by antithrombotic therapy is unclear.
Management of renal failure during the acute period
Since the risk of expansion of hematoma is high within 24 h after the onset of cerebral hemorrhage (42), it is desirable to avoid dialysis. Since dialysis exacerbates intracranial hypertension by removing solutes and water, it is important to select a procedure that has minimum effect on intracranial pressure. Peritoneal dialysis, continuous hemodiafiltration, and hemodialysis with restricted blood flow, which are less likely to increase intracranial pressure compared with usual intermittent hemodialysis, are recommended as the dialysis techniques to be used during the acute period (43–45).
Blood pressure control for primary and secondary prevention
Management of blood pressure is extremely important for the secondary prevention of cerebral hemorrhage. The recurrence rate is high among patients with inadequate blood pressure control, and controlling the diastolic pressure at 90 mm Hg or below is recommended to prevent the recurrence (33,46,47). With regard to primary prevention, blood pressure has also been shown to markedly affect the risk of de novo cerebral hemorrhage (48). It has also been reported that the incidence increases three times at systolic pressure of 160 mm Hg or higher compared with less than 140 mm Hg (49) and that the pre-dialysis systolic pressure correlates significantly with hematoma volume (4).
REFERENCES
II. CEREBRAL INFARCTION
Statements
- 1
Soon after the onset of cerebral infarction, we recommend dialysis methods that result in only mild increase in intracranial pressure such as continuous hemodiafiltration, peritoneal dialysis, and hemodialysis with restricted blood flow should be selected (1B).
- 2
We suggest that measures should be taken during administration of antithrombotic agents to prevent hemorrhagic complications such as using low-dose heparin during dialysis (2C).
- 3
We suggest that warfarin therapy should not be performed routinely for atrial fibrillation; however, if considered beneficial, it is desirable to use such therapy by maintaining the prothrombin time-international normalized ratio (PT-INR) at <2.0 (2C).
- 4
We recommend that indications for carotid endarterectomy and endovascular treatments for severe carotid artery stenosis should be carefully evaluated (1C).
Comments
Epidemiology
Among cerebrovascular disorders in dialysis patients, cerebral hemorrhage has long been considered more frequent than cerebral infarction compared with the general population. However, the incidence of cerebral infarction has increased in recent years (1–7).
Pathological features
The pathogenic mechanisms of cerebral infarction are classified into thrombotic, embolic, and hemodynamic, and clinically, it is divided into atherothrombotic infarction, cardiogenic infarction, lacunar infarction, and others (8). Since treatment during the acute period and prognosis differ among the clinical types, it is extremely important to differentiate the disease type at the onset (9). Cerebral infarction often occurs within 6 h after the end of dialysis (1), and the decrease in blood pressure has been reported to be milder in patients who develop the infarction soon after the end of dialysis than those who develop it 6 h or longer after the onset (10). The mechanisms of hemodialysis-induced cerebral infarction include reduction of cerebral blood flow due to increased blood viscosity and fall in blood pressure associated with water removal or orthopedic hypotension following sit up or stand up after dialysis (11). The cerebral blood flow is reported to decrease before a rapid fall in blood pressure during dialysis, and impairment of the mechanism of auto-regulation of cerebral blood flow may also be a causative factor (4,12).
Initial treatment and diagnosis
Clinical management of patients suspected with cerebrovascular disorders includes examination of the consciousness level, securing the airway, evaluation of respiration and circulation, and evaluation of neurological symptoms. The presence of disturbance of consciousness warrants exclusion of other causes, and head CT should be performed. If head CT suggests cerebral infarction, MRI and MRA should be performed to determine the type of cerebral infarction, and the therapeutic strategy should be determined. If necessary, carotid artery ultrasonography, echocardiography (transthoracic, transesophageal), Holter electrocardiogram (ECG), and cerebral angiography, should be added, and the pathogenic mechanism should be clarified (Fig. 1) (13).
Algorithm for the diagnosis of cerebrovascular disorders.
Treatments
Treatments during the acute stage
Blood pressure control. During the acute phase of cerebral infarction, regional cerebral blood flow at the lesion and surrounding penumbra region may decrease further due to treatment with antihypertensive agents, which may cause expansion of the lesion (14). Therefore, in principle, aggressive antihypertensive treatment is not recommended. However, if systolic pressure is 220 mm Hg or higher or diastolic pressure is 120 mm Hg or higher, antihypertensive treatment is recommended to reduce the risk of hemorrhagic complications (15,16). To perform thrombolytic therapy, however, the systolic and diastolic pressures must be reduced to less than 185 and 110 mm Hg, respectively. It is important to slowly reduce the blood pressure by setting the target at about 85–90% of the pre-treatment level (15,16).
Management of edema. Intravenous administration of glycerol is effective in alleviating brain edema and may save patients with large cerebral infarction associated with intracranial hypertension (17). In dialysis patients, it is important to manage azotemia and electrolyte balance with attention to exacerbation of brain edema due to dialysis.
Antithrombotic therapy. Antiplatelet agents (aspirin, ozagrel sodium) and anticoagulants (heparin, argatroban) are used for antithrombotic therapy (Table 2) (18–20). Since the urinary excretion rate of ozagrel sodium is high in the unchanged form, its dose must be reduced in dialysis patients to about half the recommended dose (21).
| Drug name | Commercial names | Excretion rate as urinary unchanged drug (%) | Ccr (mL/min) | Dialyzablity | |||
|---|---|---|---|---|---|---|---|
| >50 | 10–50 | <10 or dialysis | |||||
| Antiplatelet agents | Aspirin | Biaspirin | 2–30% Excretion increases with alkalization of urine | 100 mg | Careful administration at the same dose as patients with normal renal function | ○ | |
| Bufferin 81 mg | 81 mg | ||||||
| Ticlopidine hydrochloride | Panaldine | 0.01–0.02% | 200–600 mg | Same as patients with normal renal function | × | ||
| Clopidogrel bisulfate | Plavix | Urinary excretion 41% | 50–75 mg | Same as patients with normal renal function | × | ||
| Fecal excretion 51% | Same as patients with normal renal function | ||||||
| Cilostazol | Pletaal | 3.47% | 200 mg | Same as patients with normal renal function | × | ||
| Sodium ozagrel | Cataclot | 61.1% | 80–160 mg | 40–80 mg | 20–40 mg | Unknown | |
| Xanbon | |||||||
| Anticoagulants | Argatroban | Novastan | 22.8% | 60 mg/day × 2 days + 20 mg/day × 5 days | Same as patients with normal renal function | × | |
| Slonnon | |||||||
| Heparin sodium | Heparin Sodium | 0–50% | Appropriate dose | Same as patients with normal renal function | × | ||
| (2–3 time prolongation of APTT) | |||||||
| Warfarin potassium | Warfarin | ≤2% | Appropriate dose | Careful administration at the same dose as patients with normal renal function | |||
| (determined according to PT-INR) | × | ||||||
| Thrombolytic agent | Alteplase | Activacin | 0% | 34.8 × 104 IU/kg | Same as patients with normal renal function | × | |
| Grtpa | |||||||
| Brain protecting agent | Edaravone | Radicut | 0.68% | 30 mg/administration | Same as patients with normal renal function | Contraindicated, in principle | × |
- PT-INR, prothrombin time-international normalized ratio; Ccr, creatinine clearance rate.
Argatroban, a selective antithrombin, is excreted primarily via the biliary system, and thus reduction in its dose is not considered necessary in patients with renal failure (19). However, since about half the usual dose of argatroban results in the optimal activated partial thromboplastin time (APTT) in many dialysis patients (21), its dose must be fine-tuned by frequent measurements of APTT.
During the first 3 h of the onset, thrombolytic therapy using recombinant tissue plasminogen activator (rt-PA, alteplase) may be indicated. It is recommended that the patients should be transported to expert facilities early after the onset.
Management of renal failure during the acute-phase treatment. Since intracranial pressure autoregulation collapses immediately after the onset of cerebral infarction (12), and due to the high risk of rapid progression of intracranial hypertension and exacerbation of brain edema, dialysis should be avoided on the day of the onset. Thereafter, also, the need for dialysis should be evaluated carefully, and, if it is performed, procedures such as peritoneal dialysis, continuous hemodiafiltration, and hemodialysis with restricted blood flow, which have mild effects on intracranial pressure and maintain cerebral perfusion pressure, should be selected. Rapid and massive removal of water should be avoided, because it exacerbates brain ischemia.
Treatments during the chronic phase/prevention of recurrence (secondary prevention)
During the chronic phase (more than 1 month after the onset of cerebral infarction), patients are treated with antiplatelets, anticoagulants and antihypertensive agents to prevent the recurrence of cerebrovascular disorders. Antiplatelets and anticoagulants are effective in the prevention of non-cardiogenic and cardiogenic cerebral infarction, respectively (9). Antiplatelets include aspirin, ticlopidine, clopidogrel, and cilostazol, which can be used as in non-dialysis patients (Table 2) (18–20).
Primary prevention
Antithrombotic therapy for atrial fibrillation. The incidence of atrial fibrillation in dialysis patients is extremely high (22–31), but the involvement of atrial fibrillation in stroke is unclear based on the effects of platelet dysfunction and the use of heparin during dialysis (24–26,29–32). There is little evidence concerning the preventive effect of warfarin against cerebral infarction, and as it increases the risk of hemorrhagic complications, many authors have discouraged its use (32–34). Recent large-scale studies also reported the danger of warfarin administration (35,36). If warfarin is used as a beneficial drug, it is important not to increase the risk of hemorrhagic complications by periodically measuring the PT-INR and maintaining it at <2.0 (36).
Carotid endarterectomy and endovascular treatment. Poor prognosis is reported for carotid endarterectomy (CEA) conducted for severe carotid artery stenosis in patients with renal failure (37–39), but CEA is also reported to be more effective in patients with renal failure than in those without renal failure (40). Since the results of carotid artery stenting (CAS) are also unsatisfactory in patients with renal failure (41), the indications for these procedures must be evaluated carefully.
Blood pressure control for primary and secondary prevention
Since a J-curve relationship is observed between the blood pressure and recurrence of stroke (42), a warning against excessive decrease in blood pressure was issued, but recently, this view has been refuted by some (43). Usually, it is important to initiate antihypertensive treatment more than 1 month after the beginning of treatment and to gradually reduce blood pressure over a period of 1–3 months. Blood pressure of less than 140/90 mm Hg is recommended as the final target of blood pressure control except in patients with marked stenosis of both carotid arteries and those with obstruction of a major artery (15). Also, with regard to primary prevention, hypertension is reported to be a risk factor of cerebral infarction, but its role in cerebral infarction is smaller and more limited than that in cerebral hemorrhage (44).
REFERENCES
Supplementary note
Guidelines for the management of cerebrovascular disorders in dialysis patients were prepared in relation to cerebral hemorrhage and cerebral infarction. The preparation of guidelines concerning subarachnoid hemorrhage was deferred, because there is little evidence in dialysis patients.
Chapter 8: Peripheral Artery Disease
Statements
- 1
Dialysis is an independent risk factor for peripheral arterial disease (PAD) regardless of the presence or absence of diabetes (B).
- 2
In PAD patients, we recommend that cardiovascular disorders should be simultaneously evaluated (1B).
- 3
In dialysis patients, severe calcification lesions are often observed distal to the knees (B), but since symptoms are inconspicuous, efforts for early detection are important (Opinion).
- 4
We suggest that ankle-brachial systolic pressure index (ABI) should be examined, at least once a year (Opinion).
- 5
We recommend that treatment of PAD should include careful evaluation of the pathological features of ischemia (1B).
Comments
Peripheral arterial disease (PAD) can be divided into arteriosclerosis obliterans (ASO) of the lower limbs and Buerger's disease or thromboangitis obliterans (TAO). Since ASO is the main entity, PAD is often considered to mean ASO in a narrow sense. Also, ASO frequently shows a chronic course. This chapter deals with chronic ASO of the lower limbs.
Epidemiology and risk factors
Incidence. Chronic kidney disease is regarded as an independent risk factor for PAD, and the possibility of PAD should be considered when the GFR is less than 30 mL/min per 1.73 m2 regardless of the presence or absence of diabetes (1). However, since PAD is associated with only few symptoms and progresses rapidly, it is often detected after the appearance of critical limb ischemia (CLI) when it becomes intractable. Also, the lesions are located distal to the knee and are often accompanied by severely calcified lesions.
Only a few large-scale studies are available on the prevalence of PAD in patients with chronic kidney disease (CKD). In 2004, O'Hare et al. (2) investigated the prevalence of PAD in non-dialysis CKD patients and reported that renal dysfunction is an independent risk factor for the occurrence of de novo PAD. In a study by Koch et al. (3), CLI coupled with angiographically-confirmed significant stenosis or ulcerated/necrotic lesions of the lower limbs was noted in 34 (10.5%) of 322 patients at the initiation of dialysis, and, on a 5-year follow-up, de novo CLI developed in 25 (8.9%) of 288 patients with no lesion at the initiation of dialysis. With regard to reports on Japanese patients, the cross-sectional study of Okamoto et al. (4,5) involving patients on maintenance hemodialysis using the ABI and skin perfusion pressure (SPP), reported ABI values less than 0.9 in 16.7%, and SPP values less than 50 mm Hg in 41.4% of the patients, and that 37.2% of hemodialysis patients had PAD based on the diagnostic sensitivity and specificity of the SPP and that about half of these patients were asymptomatic.
The above studies point to a higher prevalence of PAD in dialysis patients than in the general population (6). PAD has been diagnosed in 15–23% of the patients according to clinical symptoms (7–9) (Table 1) and in 33.0–38.3% in Western countries (10–12) and 16.6–16.7% in Japan (4,13) using an ABI <0.9 as a criterion (Table 2).
| Authors | Number of patients | Subjects | Percentage of patients with PAD |
|---|---|---|---|
| USRDS (2000) (7) | 35,438 | Patients who initiated dialysis | 15.0 |
| Webb et al. (1993) (8) | 325 | Patients on maintenance dialysis | 19.0 |
| Hemo study (2000) (9) | 936 | Patients on maintenance dialysis | 23.0 |
- Diagnosis of PAD: History of PAD (amputation/revascularization), pain during walking, rest pain, ulcer/gangrene. USRDS, United States Renal Data System.
Risk factors in dialysis patients
O'Hare et al. (14) evaluated the pathogenic factors of PAD in patients started on hemodialysis and those on maintenance dialysis and reported that coronary artery disease elevates the incidence of PAD in dialysis patients 2.85 times, that the incidence of PAD is 4.18 times higher in diabetic than in non-diabetic dialysis patients, that the duration of dialysis and undernutrition correlated positively with the incidence of PAD but negatively with the pre-dialysis diastolic pressure and blood levels of parathyroid hormone.
Diagnosis
Diagnosis of PAD
Clinical examination of the feet (inspection, palpation). Inspection and palpation are the most basic diagnostic techniques. However, it must be remembered that PAD cannot be excluded even if the dorsal artery of the foot or posterior tibial artery are palpable (Fig. 1) (15).
Ratschow's test. (reproduced from Peripheral Artery Diseases in Dialysis Patients, Iyaku (Medicine & Drug) Journal, Co., Ltd.). Feet hanging test. Lift the feet in the supine position and rotate the ankles 20–40 times (until the soles become pale). Sit up immediately and observe changes in the color of the insteps. A: Lift the feet and rotate the ankles. B: Immediately after hanging the feet. C: 2 min after hanging the feet. If normal, redness returns to the instep, and veins stand out.
Ankle-brachial systolic pressure index (ABI). The ABI is the most important screening test. We recommend that ABI be measured in dialysis patients at the initiation of dialysis and once annually thereafter regardless of the presence or absence of symptoms. However, as dialysis patients often have severe vascular calcification and peripheral lesions, it is inappropriate to directly apply the criteria for non-dialysis patients.
In dialysis patients, the normal range of ABI is reported to be shifted to the right to 1.02–1.42 (4,5), and cutoff PAD value for confirmation of the diagnosis is less than 0.9.
There are also other physiological indices of skin microcirculation including the SPP, toe-brachial pressure index (TBI), and transcutaneous PO2 (tcPO2). Each has been reported to be useful in the evaluation of ischemia of peripheral tissues (Table 3) (5). The SPP, which was reported by Castronuovo et al. (16), is determined by measuring the influx of red blood cells at the capillary level using laser, and it is 79 ± 14 (SD) mm Hg in healthy individuals. With regard to morphological diagnostic methods, imaging of the blood flow waveform using vascular ultrasonography, multidetector-row computed tomography (MDCT), and magnetic resonance imaging (MRI) is useful, but angiography is important for the localization of lesions. Sufficient delineation to the ankle is necessary for localization.
| Test | Cut-off value | Sensitivity | Specificity |
|---|---|---|---|
| ABI | 0.9 | 29.9 | 100 |
| TBI | 0.6 | 45.2 | 100 |
| tcPO2 | 50 mm Hg | 61.1 | 70.0 |
| SPP | 50 mm Hg | 78.6 | 91.6 |
- ABI, ankle brachial pressure index; SPP, skin perfusion pressure; TBI, toe-brachial pressure index.
Staging of PAD
The severity of PAD is evaluated using the Fontaine classification (Table 4). In the general population, the Fontaine stage is evaluated on a treadmill at an inclination of 12% and a speed of 2.4 km/h (Japanese). However, since dialysis patients often suffer bone and joint disorders, causing difficulty in the evaluation of the walking distance, it must be remembered that evaluation of intermittent claudication may be difficult. CLI means stage III or IV PAD according to the Fontaine scale.
| Stage | Clinical findings |
|---|---|
| I | No symptom |
| II | Intermittent claudication |
| IIa | Induced by walking ≥200 m |
| IIb | Induced by walking <200 m |
| III | Rest pain |
| IV | Ulcer/gangrene |
Treatments
General treatments
If the clinical evaluation excludes the presence of CLI, treatment should be started with foot care, medication, and exercise therapy. If improvement is not clear or exacerbation is observed, the stage of treatment should be elevated by evaluating ischemia by techniques such as angiography. Exercise therapy three times/week, 30–45 min/session, over 12 weeks is reported to be effective in non-dialysis patients (17–19). Guidance to quit smoking is particularly important (20,21). It is important to continuously observe the condition of the feet and evaluate the presence or absence of ischemia and infection. It is also important to treat wounds by appropriate debridement or dressing technique. While it is important to attempt limb conservation by these conservative approaches, caution is necessary not to let prognosis deteriorate by ruling out timely limb amputation. However, the prognosis after limb amputation is extremely poor in dialysis patients, and careful evaluation of the extent of amputation is necessary.
Drug therapy
There is no large-scale randomized controlled trial of any drug in dialysis patients. In Japan, the antiplatelet agents covered by medical insurance are cilostazol, sarpogrelate, beraprost, ticlopidine, eicosapentaenoic acid, and juvela nicotinate. All have been approved based on the results obtained in non-dialysis patients and have been reported to be effective for alleviating symptoms due to ischemia. In general, cilostazol is reported to be effective in non-dialysis patients, not only in alleviating symptoms but also improving the distance of walking and mitigating intermittent claudication (22–24). However, cilostazol is contraindicated in patients with heart failure, because it increases the heart rate, and caution is necessary in dialysis patients showing a tendency of volume overload.
A meta-analysis study demonstrated the effectiveness of intravascular administration of prostaglandin E1 for the treatment of ulcer and alleviation of pain in CLI patients (25).
Treatment should be conducted taking into consideration limb conservation, prognosis, and quality of life. No single treatment is complete, and a multi-disciplinary approach is important. Foot care must be provided persistently.
The Trans-Atlantic Inter-Society Consensus (TASC) Guidelines (26) are useful for evaluation of the therapeutic effects, but the evaluation criteria for treadmill exercise are impractical, because they are often difficult to achieve in dialysis patients due to complications such as bone and joint disorders.
In patients diagnosed with CLI, the earliest possible revascularization surgery is necessary. However, the indications for such surgery and whether surgical or endovascular treatment should be provided depends on overall assessment including consideration of limb conservation, prognosis, and quality of life in connection with cardiovascular complications, sites, length, and distribution of the lesions, whether the lesion is stenosis or obstruction (TASC classification) (26). The final decision is usually left to the specialists.
In general, treatment of PAD in dialysis patients (indications, endovascular treatment, revascularization) can be the same as that in non-dialysis patients. However, it must be understood that dialysis patients tend to have more severe calcification, lesions in more distal areas, relative immunodeficiency, undernutrition, and compromised host immunity against infections. The general principles for the treatment of PAD in dialysis patients are as follows:
- 1
Endovascular treatment (primary stenting) should be the first choice for iliac artery lesions.
- 2
Endovascular treatment should be the first choice for TASC A and B lesions with a length of less than 15 cm in the femoropopliteal region. Surgery is the first choice for long obstructive lesions and complicated lesions, but the revised TASC II Guidelines mention: Since techniques, such as subintimal angioplasty using the subintimal recanalization technique, have been developed, even a long completely obstructed lesion can also be an indication of endovascular treatment (26).
- 3
In CLI, endovascular treatment should be considered for localized infrapopliteal lesions. While surgery should be considered for chronic long lesions and obstructive lesions, endovascular treatment may be selected in high-risk patients for surgery.
- 4
For patients with CLI complicated by wound infection, some treatment that improves blood flow must be considered along with treatment for infection.
- 5
Revascularization by endovascular or surgical procedure should be followed by continuous treatment with an antiplatelet agent as adjuvant drug therapy (26,27). Also, the concomitant use of other procedures including low-density lipoprotein (LDL) apheresis and hyperbaric oxygen therapy should also be considered.
Revascularization should be considered in patients with PAD, particularly, severe intermittent claudication or CLI. The therapeutic principles concerning revascularization in infrapopliteal arteries are not discussed in detail even in the latest guidelines. According to the BASIL trial (28), which is the only comparative study on the treatment for CLI, the patency and survival rates between endovascular treatment and surgical revascularization were comparable, but the study was not restricted to infrapopliteal arterial lesions.
The reported initial success rate of endovascular treatment for PAD in dialysis patients was 92–97%, the 30-day mortality rate was 6.3–10%, 1-year limb conservation rate was 53–73%, and 1-year survival rate was 80–94%, suggesting that percutaneous transluminal angioplasty (PTA) is useful as a treatment for PAD in dialysis patients (29–31).
In surgical revascularization for CLI in dialysis patients, the reported mortality within 30 days is high at 18% (32,33). Causes of death are often related to heart diseases such as acute myocardial infarction and heart failure (34,35), and sufficient preoperative evaluation of cardiac complications is important in surgical revascularization. The reported patency rate after surgical revascularization is relatively favorable, being 62–85% after 1 year and 56–81% after 2 years. The reported limb conservation rate was 56–77% after 1 year and 50–71% after 2 years, and the survival rate was 39–73% after 1 year and 33–65% after 2 years (32,35,36).
While there have been few studies that compared the results of PTA and surgical revascularization in dialysis patients with PAD, Jaar et al. (37) reported that the mortality rate was also higher in the surgical revascularization group. However, the study was retrospective, and the possibility that the condition was more severe in the surgical revascularization group cannot be excluded.
Other treatments
In addition to endovascular treatment or surgical revascularization for limb conservation, other treatment modalities such as LDL apheresis and hyperbaric oxygen (HBO) therapy may be indicated as supplementary treatments. LDL apheresis is indicated for abnormal lipid metabolism, and its effect on lipid metabolism is not considered to be directly related to LDL removal (38). The procedure is reported to have vasodilating, rheology-improving, and anti-inflammatory effects. LDL apheresis is more likely effective in relatively early cases (38). HBO therapy is indicated for PAD patients with refractory ulcer. While it has been reported to be effective in diabetic patients (39,40), there is no report about its effectiveness in dialysis patients. Bathing in a carbonate spring is reported to be effective, and bathing in hot water at a CO2 concentration higher than a certain level (1200 ppm) is considered to improve blood flow (41). Also, even in patients with one limb amputation, continuation of close observation, prevention, and treatment of the unamputated limb is important.
Prognosis
Limb amputation and prognosis
In Japan, the percentage of dialysis patients who have undergone lower limb amputation was 1.6% at the end of 2000 but increased progressively to 2.2% at the end of 2003 and 2.6% (4755/183 492) at the end of 2005. Of these patients, 70% were diabetic (42).
The prognosis of dialysis patients after lower limb amputation is extremely poor (43,44), and the hospital death rate is high (43). Dossa et al. (43) reported that the hospital death rate after lower limb amputation was 7% in non-dialysis patients but was very high at 24% in dialysis patients. The 2-year survival rate after lower limb amputation was 79% in non-dialysis patients but was markedly lower at 27% in dialysis patients. Aulivola et al. (44) also reported extremely poor prognosis of dialysis patients after lower limb amputation (particularly major amputation).
Life prognosis
In dialysis patients with PAD, the reported hospitalization rate (45) and risk of death (3,10,12) are high, and the death rates within 6 months after initiation of dialysis (46) and after acute myocardial infarction (47) are also high.
As for the relationship between ABI and life prognosis, the reported total risk of death is 7.09 and 2.20 times higher in those with an ABI of less than 0.9 and 1.3 or above, respectively, compared with those with an ABI of 1.1–1.3. The risk of death due to cardiovascular disorders was also reported to be 10.6 and 3.1 times higher in the respective groups (48). Therefore, it is necessary to examine patients for not only PAD but also other cardiovascular disorders and to treat them as much as possible.
REFERENCES
Chapter 9: Use of Cardiovascular Drugs in Dialysis Patients
Statements
- 1
In dialysis patients, excretion of renally excreted drugs is likely to be delayed, causing high drug concentrations and adverse effects due to their toxicity. Therefore, we suggest that hepatically metabolized drugs should be selected as much as possible (Opinion).
- 2
In administering a renally excreted drug, we recommend assessment of renal function and urinary excretion rate of the drug, we recommend that the dose should be appropriately adjusted, and periodic monitoring of adverse reactions should be conducted (1A).
- 3
While administration of contraindicated drugs is necessary in some situations, we recommend avoidance of such drugs except when the estimated benefit of administration surpasses the risk of adverse effects (1A).
- 4
We recommend that gadolinium contrast agents for magnetic resonance imaging (MRI) should not be used in dialysis patients, since they can induce nephrogenic systemic fibrosis (NSF) (1C).
Comments
Understanding the pharmacokinetics (absorption, distribution, metabolism, excretion) of drugs, which is the most significant determinant of their blood levels, is important for effective and safe administration. Since renal excretion is remarkably reduced in dialysis patients, the absorption, distribution, and metabolism of drugs are likely to be affected, and their blood concentrations are likely to rise, causing adverse effects due to their toxicity. Therefore, in using drugs, their doses should be adjusted in consideration of their urinary excretion rates in active forms (often the unchanged drugs), total clearance, bioavailability, protein binding rate, volume of distribution, and dialyzability, and those that are metabolized primarily by the liver and metabolites of which are inactive should be used when possible (Table 1).
| Drug | Major elimination route | Dialyzability | Dose for normal renal function (mg/day) | Adjustment for dialysis patients (mg/day) | ||
|---|---|---|---|---|---|---|
| Generic name | Administration route | |||||
| Ia | Quinidine Sulfate | Oral | Liver (kidney15–40%) | − | Maintenance dose: 200–600 | Usual dose |
| Disopyramide Phosphate | Oral | Kidney 50–60% | ± | 300 | 100–150 | |
| Oral, sustained release | 300 | Contraindication | ||||
| Injection | 50–100 mg/administration | 100 | ||||
| Cibenzoline Succinate | Oral | Kidney 60% liver 40% | − | 300–450 | Contraindication | |
| Injection | 1.4 mg/kg per administration | |||||
| Pirmenol Hydrochloride Hydrate | Oral | Liver (kidney 17–31%) | − | 200 | 100–150 | |
| Procainamide Hydrochloride | Oral | Kidney 50–60% (Active metabolites: 80%) | + | 750–2000 | Administration interval extended 4 times | |
| Injection | i.v. injection at 200–1,000 mg/administration or i.m. injection at 500 mg/administration every 4–6 h | 200–400 mg/administration 1–2 times/day | ||||
| Ib | Aprindine Hydrochloride | Oral | Liver | − | 40–60 | Usual dose |
| injection | Up to 100 mg/administration | Usual dose | ||||
| Mexiletine Hydrochloride | Oral | Liver | ± | 300–450 | Usual dose | |
| Drip infusion | See the package insert | Usual dose | ||||
| Lidocaine | Injection | Liver | − | Up to 50–100 mg/administration or 300 mg/h | Usual dose | |
| Ic | Pilsicainide Hydrochloride Hydrate | Oral | Kidney 80% | ± | 150–225 | 25 |
| Injection | Maximum dose: 1.0 mg/kg | Unknown | ||||
| Flecainide Acetate | Oral | Liver 70% kidney 30% | − | 100–200 | 50–100 | |
| Injection | 1.0–2.0 mg/kg per administration with a maximum of 150 mg/day | Administration interval extended 2 times | ||||
| Propafenone Hydrochloride | Oral | Liver | − | 450 | Usual dose | |
| II | Acebutolol Hydrochloride | Oral | Kidney 36% | − | 200–600 | 100–400 |
| Atenolol | Oral | Kidney 90% | + | 25–100 | 25 mg after dialysis 3 times/week | |
| Alprenolol Hydrochloride | Oral | Unknown | Unknown | 75–150 | Unknown | |
| Arotinolol Hydrochloride | Oral | Liver | − | 20–30 | Usual dose | |
| Esmolol hydrochloride | Injection | Liver (kidney <10%) | − | 0.15 mg/kg per min | Usual dose | |
| Oxprenolol Hydrochloride | Oral | Liver | Unknown | 60–120 | Usual dose | |
| Carteolol Hydrochloride | Oral | Kidney 60–70% | − | 10–30 | 2.5–15 | |
| Nadolol | Oral | Kidney 90% | − | 30–60 | 30–60 mg after dialyses 3 times a week | |
| Bisoprolol Fumarate | Oral | Kidney 50% | − | 5 | 2.5 | |
| Pindolol | Oral | Kidney 35–54% | + | 5–15 | 5–10 | |
| Bufetolol Hydrochloride | Oral | Unknown | Unknown | 15 | Unknown | |
| Propranolol Hydrochloride | Oral | Liver | − | 30–120 | Usual dose | |
| Metoprolol Tartrate | Oral | Liver | − | 60–240 | Usual dose | |
| Landiolol Hydrochloride | Injection | Liver (kidney 8.7%) | + | See the package insert | Usual dose | |
| III | Amiodarone Hydrochloride | Oral | Liver | − | 400 mg at the initiation, 200 mg during the maintenance period | Usual dose |
| Injection | See the package insert | |||||
| Nifekalant Hydrochloride | Injection | Liver (kidney 28–37%) | − | Single administration at 0.3 mg/kg per 5 min, maintenance dose 0.4 mg/kg per h | Single administration at 0.1 mg/kg per 5 min, maintenance dose 0.15–0.2 mg/kg per h | |
| Sotalol hydrochloride | Oral | Kidney 80% | + | 80–320 | Contraindication | |
| IV | Diltiazem Hydrochloride | Injection | Liver | − | 10 mg/about 3 min/administration | Usual dose |
| Bepridil Hydrochloride Hydrate | Oral | Liver | − | 100–200 mg/day divided into 1–2 doses | Usual dose | |
| Verapamil Hydrochloride | Oral | Liver | − | 120–240 | Usual dose (also reported to be reduced to a half due to depression of non–kidney CL) | |
| Injection | 5 mg/administration if necessary | |||||
| Digoxin | Oral | Kidney 75% | − | Maintenance dose 0.25–0.50 | 0.125 mg as a maintenance dose 2–4 times/week | |
| Injection | Maintenance dose 0.25 | 0.1–0.125 mg as a maintenance dose 2–4 times/week | ||||
| Deslanoside | Injection | Kidney 60% | − | Maintenance dose 0.2–0.3 mg once a day | Dose reduction needed, but details unknown | |
| Metildigoxin | Oral | Kidney 40% (Active metabolites 45%) | − | Maintenance dose 0.1–0.2 | 0.05 mg as a maintenance dose 2–4 times/week | |
| No indication | Adenosine Triphosphate Disodium Hydrate | Injection | Each cell | +? | i.v. injection: 5–40/administration 1–2 times i.v. drip infusion: 4–80 | usual dose |
- Since antiarrhythmic drugs such as aprindine, cibenzoline (contraindicated for dialysis patients), flecainide, mexiletine, procainamide, and propafenone and many β–blockers are metabolized by CYP2D6, their blood levels may be increased rapidly by strong CYP2D6 inhibitors such as cinacalcet hydrochloride, paroxetine hydrochloride hydrate, and quinidine sulfate. Particularly, as aprindine and propafenone show nonlinear pharmacokinetic curves with saturation of the enzyme that metabolizes them, monitoring (TDM) of the blood levels of these antiarrhythmic drugs is recommended in their concomitant use.
- * This table presents information obtained from package inserts and by a review of the literature available at the time of editing in a simplified form. Therefore, we ask the readers to understand that the data may not be universal and to check the latest package inserts and literature for details.
Points of caution in the use of major drugs in dialysis patients are described below.
Antiarrhythmic drugs
Antiarrhythmic drugs pose the greatest problems among cardiovascular drugs. In dialysis patients, optimal adjustment of their blood levels is extremely difficult due to the effects of both their excretion and dialysis. Since careless administration of antiarrhythmic drugs may allow the appearance of serious adverse effects and exacerbate the outcome, the need for their use should be carefully evaluated (1,2).
When the use of antiarrhythmic drugs is important in dialysis patients, a full 12-lead electrocardiogram (ECG) should be recorded before the initiation of drug therapy. This should be repeated when four to five times the period of the half-life of the drug after its first use has passed and its concentration is considered to have reached a stable state. Furthermore, the ECG should be recorded again at least once a month after stabilization of the condition.
Antihypertensive drugs
In case of using renin-angiotensin system inhibitors, though it is important to confirm their excretion routes, dose reduction is basically unnecessary in terms of angiotensin II receptor blockers (Table 2). Although almost all angiotensin converting enzyme inhibitors are excreted through the kidney, whether dose reduction is necessary or not is not clear at present, because a dose-related adverse reaction is rare in these drugs. Dose adjustment is also unnecessary for calcium channel blockers and α1-receptor blockers. β-blockers have various routes of elimination, including hepatic metabolism, renal excretion, or both. Considering their indication, they should be used carefully (Table 2) (3–6). If possible, drugs that are eliminated primarily by hepatic metabolism should be selected in dialysis patients.
| Classification | Drug | Major elimination route | Dialyzability | Dose for normal renal function (mg/day) | Adjustment for dialysis patients (mg/day) | |
|---|---|---|---|---|---|---|
| Generic name | Administration route | |||||
| Angiotensin–converting enzyme inhibitors (ACE–I) | Alacepril | Oral | Kidney (59.2% in active forms) | + | 25–100 | 25–50 |
| Imidapril Hydrochloride | Oral | Kidney 25.5% | + | 2.5–10 | 5 | |
| Enalapril Maleate | Oral | Kidney (88% in active forms) | + | 2.5–10 | Reduced to 25–50% | |
| Captopril | Oral | Kidney 40–75% | + | 12.5–150 | 12.5–75 | |
| Quinapril Hydrochloride | Oral | Kidney (30–96% in active forms) | – | 5–20 | 2.5–10 | |
| Cilazapril Hydrate | Oral | Kidney (70–80% in active forms) | + | 0.25–2 | 0.25 | |
| Temocapril Hydrochloride | Oral | Kidney ≤ 35% | − | 2–4 | Reduced to 75% | |
| Delapril Hydrochloride | Oral | Unknown | − | 15–120 | Started at 7.5 | |
| Trandolapril | Oral | Kidney (14–29% in active forms), feces 66% | – | 1–2 | Started at 0.5 | |
| Benazepril Hydrochloride | Oral | Kidney (17–21% in active forms) | − | 2.5–10 | Started at 2.5 | |
| Perindopril Erbumine | Oral | Kidney (3–10% in active forms) | + | 2–4 | Reduced to 50% | |
| Lisinopril Hydrate | Oral | Kidney 88–100% | + | 5–20 | Reduced to 25–50% | |
| Angiotensin II receptor antagonists (ARB) | Dose reduction is unnecessary, as all are metabolized by the liver and disappear. | |||||
| Renin Inhibitors (DRI) | Aliskiren Fumarate | Oral | Fecal | − | 150–300 | Usual dose |
| Calcium channel blockers (CCB) | Dose reduction is unnecessary, as all are metabolized by the liver and disappear. | |||||
| Preparations containing angiotensin II receptor antagonists (ARB)/calcium channel blockers (CCB) | Dose reduction is unnecessary, as all are metabolized by the liver and disappear. | |||||
| Preparations containing angiotensin II receptor antagonists (ARB) /hydrochlorothiazide (HCTZ) | Contraindicated for anuric and dialysis patients | |||||
| Beta blockers non–β1 selective ISA(–) | Propranolol Hydrochloride | Oral | Liver | − | 30–120 | Usual dose |
| Bufetolol Hydrochloride | Oral | Unknown | Unknown | 15 | Unknown | |
| Nadolol | Oral | Kidney 90% | − | 30–60 | 30–60 after dialysis 3 times/week | |
| Beta blockers β1 selective ISA(+) | Alprenolol Hydrochloride | Oral | Unknown | Unknown | 75–150 | Unknown |
| Oxprenolol Hydrochloride | Oral | Liver | Unknown | 60–120 | Usual dose | |
| Carteolol Hydrochloride | Oral | Kidney 60–70% | − | 10–30 | 2.5–15 | |
| Pindolol | Oral | Kidney 35–54% | + | 5–15 | 5–10 | |
| Penbutolol | Oral | Liver | − | 20–40 | Usual dose | |
| Bopindolol Malonate | Oral | Liver | + | 1–2 | Usual dose | |
| Beta blockers non–β1 selective ISA(–) | Atenolol | Oral | Kidney 90% | + | 25–100 | 25 after dialysis 3 times/week |
| Bisoprolol Fumarate | Oral | Kidney: 50% | − | 5 | 2.5 | |
| Betaxolol Hydrochloride | Oral | Kidney 26–27% | − | 5–20 | 2.5–15 | |
| Metoprolol Tartrate | Oral | Liver | − | 60–240 | Usual dose | |
| Landiolol Hydrochloride | Injection | Liver (kidney 8.7%) | + | See the package insert | Usual dose | |
| Beta blockers non–β1 selective ISA(+) | Acebutolol Hydrochloride | Oral | Kidney 36% | − | 200–600 | 100–400 |
| Esmolol hydrochloride | Injection | Liver (kidney <10%) | – | 0.15 mg/kg per min | Usual dose | |
| Celiprolol Hydrochloride | Oral | Liver (kidney 10–20%) | Unknown | 100–400 | Usual dose | |
| Beta blockers with vasodilating action | Tilisolol Hydrochloride | Oral | Kidney 50% | Unknown | 10–30 | 5–15 |
| Nipradilol | Oral | Liver | − | 6–18 | Usual dose | |
| Mixed Alpha + Beta blockers | Amosulalol Hydrochloride | Oral | Liver (kidney 30%) | − | 20–60 | 10–40 |
| Arotinolol Hydrochloride | Oral | Liver | − | 20–30 | Usual dose | |
| Carvedilol | Oral | Liver | − | 2.5–20 | Usual dose | |
| Bevantolol Hydrochloride | Oral | Liver | Unknown | 100–200 | Usual dose | |
| Labetalol Hydrochloride | Oral | Liver | − | 150–450 | Usual dose | |
| Diuretics | Generally, the doses are not reduced while some thiazide and loop diuretics are excreted via the kidney. However, diuretics are contraindicated for anuric patients. Spironolactone is contraindicated for anuric, hyperkalemic patients. Eplerenone is contraindicated for moderate or severer kidney dysfunction and hyperkalemia. | |||||
| Alpha blockers: | Dose reduction is unnecessary, as all are metabolized by the liver and disappear. | |||||
| Centrally acting adrenergic drugs | αMethyldopa Hydrate | Oral | Kidney 20–60% | + | 250–2000 | Reduced to 50% |
| Guanabenz Acetate | Oral | Liver | − | 4–8 | Usual dose | |
| Clonidine Hydrochloride | Oral | Kidney 40–62% | − | 225–450 µg | Reduced to 50% | |
| Vasodilators | Hydralazine Hydrochloride | Oral | Liver | − | 30–200 | Usual dose |
| Budralazine | Oral | Unknown | Unknown | 90–180 | Unknown | |
- In this table, the dose of ACE–I is adjusted according to the urinary excretion rate of the active drug, but there is the opinion that the maintenance dose need not be adjusted, because no marked adverse effect occurs without a dose reduction in dialysis patients.
- * This table presents information obtained from package inserts and by a review of the literature available at the time of editing in a simplified form. Therefore, we ask the readers to understand that the data may not be universal and to check the latest package inserts and literature for details.
Lipid-lowering drugs
Several types of lipid-lowering drugs are available in the clinical setting. However, renally excreted fibrates (e.g. fenofibrate, bezafibrate) are contraindicated for dialysis patients. Clinofibrate is an exceptional fibrate excreted in bile, but it should be administered carefully in patients with renal failure. Niceritrol has been reported to reduce serum phosphate levels (7). However, since it is also reported to cause thrombocytopenia (8), it must be administered carefully. Although probucol can be administered at the usual dose, it may cause QT prolongation on ECG, and caution is needed (9).
Statins, with the exception of rosuvastatin, and other drugs can also be administered at the usual dose even in dialysis patients.
Contrast media and other contraindicated drugs
Patients with severe renal dysfunction may develop NSF after the use of a gadolinium-based contrast medium (10–12). Since there is no established treatment for this adverse effect, prevention is most important, and the Japanese Society of Nephrology, Japan Radiological Society, and Japanese Society for Dialysis Therapy jointly issued guidelines for the use of gadolinium contrast media (13). In principle, the use of such contrast media should be avoided. However, if the benefit of their use is expected to surpass the risk of NSF, an informed consent should be obtained, and contrast media with fewer reports of NSF should be selected.
Iodinated contrast media and fluorescent contrast agents can be used in dialysis patients usually without any problems despite a delay in excretion.
In principle, cardiovascular drugs contraindicated for dialysis patients should not be used. However, if the benefit of their use surpasses the risk of avoidance, they should be administered carefully. Other drugs like warfarin and cilostazol, which are used frequently, should also be used carefully in dialysis patients after sufficient evaluation of indications. Concerning drugs that can be administered to dialysis patients, also, the dose must be determined with attention to adverse effects after sufficient consideration of the routes of metabolism, excretion, dialyzability, and benefit (Table 3).
| Classification | Drug | Major elimination route | Dialyzability | Dose for normal renal function (mg/day) | Adjustment for dialysis patients (mg/day) | |
|---|---|---|---|---|---|---|
| Generic name | Administration route | |||||
| Coronary Vasodilator | Isosorbide Mononitrate | Oral | Liver | + | 40–80 | Usual dose |
| Dipyridamole | Oral | Liver | – | 75 | Usual dose | |
| Dilazep Hydrochloride Hydrate | Oral | Liver | – | 150–300 | Usual dose | |
| Trapidil | Oral | Liver | Unknown | 300 | Usual dose | |
| Isosorbide Dinitrate | Oral, sustained release | Liver | ± | 40 | Usual dose | |
| Cutaneous application (skin patch) | Liver | ± | 40 (1 patch) | Usual dose | ||
| Oral | Liver | ± | Appropriate dose | Usual dose | ||
| Nicorandil | Oral | Liver | + | 15 | Usual dose | |
| Nitroglycerin | Sublingually | Liver | − | Appropriate dose | Usual dose | |
| Cutaneous application (skin patch) | Liver | − | 2 patches | Usual dose | ||
| Transdermal therapeutic system | Liver | − | 1 patch | Usual dose | ||
| Injection | Liver | − | Appropriate dose | Usual dose | ||
| Cardiotomic, Cardiac Glycoside | Digoxin | Oral | Kidney: 75% | − | Maintenance dose 0.25–0.50 | 0.125 mg as a maintenance dose 2–4 times/week |
| Injection | Maintenance dose 0.25 | 0.125 mg as a maintenance dose 2–4 times/week | ||||
| Metildigoxin | Oral | Kidney: 40% (Active metabolites: 45%) | − | Maintenance dose 0.1–0.2 | 0.05 mg as a maintenance dose 2–4 times/week | |
| Deslanoside | Injection | Kidney: 60% | − | Maintenance dose 0.2–0.3 mg once a day | Reduction is needed, but details of the pharmacokinetics are unknown. | |
| Cardiotomic catecholamine | Adrenaline | Injection | Liver | + | See the package insert | Usual dose |
| l–Isoprenaline Hydrochloride | Injection | Kidney 40–50% | + | 0.2–1.0 | Reduced to 1/2–2/3 | |
| Denopamine | Oral | Non–kidney | − | 15–30 | Usual dose | |
| Docarpamine | Oral | Liver, small intestine, blood content | + | 2250 | Usual dose | |
| Dopamine Hydrochloride | Injection | Liver | + | 1–5 µg/kg per min (up to 20 µg/kg per min) | Usual dose | |
| Dobutamine Hydrochloride | Injection/drip infusion | Liver (bile) | + | 1–5 µg/kg per min | Usual dose | |
| Noradrenaline | Injection | Liver (kidney ≤ 16%) | + | See the package insert | Started at a low dose | |
| Cardiotomic Phosphodiesterase III Inhibitor | Olprinone Hydrochloride Hydrate | Injection | Kidney 70–80% | Unknown | Initially at 10 µg/kg per 5 min, continued at 0.1–0.4 µg/kg | Reduced to 1/3 |
| Pimobendan | Oral | Kidney (active drug 20–40%) | − | 2.5–5 | Started at 1.25–2.5 | |
| Milrinone | Injection | Kidney 95% | − | See the package insert | Started at 0.25 µg/kg/min | |
| Cardiotomic Cyclic AMP | Bucladesine Sodium | Injection | Kidney 16.6% | Unknown | 0.005–0.2µg/kg per min | Usual dose |
| Cardiotomic, others | Colforsin Daropate Hydrochloride | Drip infusion | Kidney 10.8–17.8% | Unknown | 0.5–0.75µg/kg per min | Usual dose |
| Others, α–hANP preparations | Carperitide (genetical recombination) | Injection | Non–kidney | Unknown | 0.1–0.2µg/kg per min | Reduced to 1/2 |
| Thrombolytic Drug | Alteplase (genetical recombination) | Injection | Liver | − | See the package insert | Usual dose |
| Urokinase | Injection | Liver | − | Initially at 6 × 104 units (up to 24 × 104 units), continued for about 7 days with gradual dose reductions | Usual dose | |
| Batroxobin | Drip infusion | Non–kidney (kidney <1%) | − | 10–20 BU/h at a time every other day | Usual dose | |
| Pamiteplase | Injection | Non–kidney | − | 35 000 IU/kg per min | Usual dose | |
| Monteplase (genetical recombination) | Injection | Non–kidney | – | 13 750–27 500 IU/kg | Usual dose | |
| Antiplatelet Drug | Aspirin | Oral | Liver | + | 100 | Usual dose |
| Aluminum Glycinate | Oral | Liver | + | 81 | Usual dose | |
| Ozagrel Sodium | Injection | Kidney 61.1% | + | 80–160 | Reduced to 1/2, administered after HD on days with HD | |
| Clopidogrel Sulfate | Oral | Liver | − | 50–75 | Usual dose | |
| Sarpogrelate Hydrochloride | Oral | Liver | − | 300 | Usual dose | |
| Cilostazol | Oral | Liver | − | 200 | Usual dose (contraindicated for congestive heart failure) | |
| Ticlopidine Hydrochloride | Oral | Liver | − | 200–600 | Usual dose | |
| Limaprost Alfadex | Oral | Lung | − | 30 µg | Usual dose | |
| Drugs for peripheral occlusive arterial disease (prostaglandin preparations) | Alprostadil Alfadex | Injection | Lung | − | 40–120 µg | Usual dose |
| Alprostadil | Injection | Lung | − | 5–10 µg | Usual dose | |
| Beraprost | Oral | Liver | − | 120 µg | Usual dose | |
| Anticoagulant | Argatroban Hydrate | Injection | Liver | − | See the package insert | Usual dose |
| Recomodulinthrombomodulin alfa | Drip infusion | Kidney | − | 380 U/kg per 30 min once a day | 130 U/kg | |
| Human anti–thrombin III,freeze–dried concentrated | Injection | Unknown | − | 1000–3000 U/day (20–60 U/kg) DIC: 1500 U/day (30 U/kg) | Usual dose | |
| Human activated protein C, freeze–dried concentrated | Injection | Unknown | − | See the package insert | Usual dose | |
| Danaparoid Sodium | Injection | Kidney | − | 2500 anti–factor Xa units | Contraindication | |
| Dabigatran Etexilate Methanesulfonate | Oral | Kidney | + | 110–150 mg/administration two times a day | Contraindication | |
| Fondaparinux Sodium | Subcutaneous injection | Kidney | − | 2.5 | Contraindication | |
| Warfarin Potassium† | Oral | Liver | − | Appropriate dose | Usual dose (contraindicated for severe kidney disorder) | |
| Cerebroprotective drugs | Edaravone | Oral | Liver (kidney <1%) | Unknown | 60 | Usual dose (contraindicated for severe kidney disorder) |
| Vasopressor | Etilefrine Hydrochloride | Oral | Liver (kidney 7%) | − | 15–30 | Usual dose |
| Injection | 2–10/administration | Usual dose | ||||
| Droxidopa | Oral | Liver (kidney 15–20%) | + | 100–900 | Usual dose | |
| Phenylephrine Hydrochloride | Injection | Unknown | Unknown | 2–10/administration | Not determined | |
| Midodrine Hydrochloride | Oral | Liver | + | 4–8 | Usual dose | |
| Amezinium Metilsulfate | Oral | Kidney 77% | − | 20 | 10 mg at the initiation of dialysis | |
- Heparin preparations, anti–heparin preparations, and anti–pulmonary hypertension drugs were excluded from this table. †The risk of gastrointestinal bleeding due to warfarin is increased by the gastrotoxic and antiplatelet activities of non–steroidal anti–inflammatory drugs (NSAIDs). NSAIDs such as meloxicam, lornoxicam, celecoxib, ibuprofen, indomethacin, mefenamic acid, bulocome, piroxicam, and tenoxicam may further intensify the action of warfarin by inhibiting CYP2C9, a primary warfarin–metabolizing enzyme.(1) Lornoxicam reportedly increased the serum warfarin concentration (AUC) 1.58 times,(2) and its concomitant use with celecoxib increased the prothrombin time–international normalized ratio (PT–INR), which had been controlled in a normal range, to 10, causing severe anemia in some patients,(3) but information concerning other NSAIDs is scarce. Caution is urged against the concomitant use of all other NSAIDs including bucolome and tramadol. The concomitant use of pyrimidine fluoride anticancer drugs (TS–1, capecitabine, tegafur, 5–FU, etc.), the antibacterial agent sulfamethoxazole (primary component of ST compound preparations), and the antiarrhythmic drug amiodarone should also be avoided, because they elevate the blood level of warfarin and markedly increase the PT–INR. On the other hand, the antituberculous agent rifampicin and the antiepileptics phenobarbital, carbamazepine, and primidone occasionally attenuate the effect of warfarin by inducing CYP2C9. Since there are many other drugs that interact with warfarin, see the package insert of warfarin for details. (1) Lacy CF, Armstrong LL, Goldman MP, Lance LL: Lexi Comp's Drug Information Handbook 18 ed, 2009–2010. Hudson, Ohio, 2009. (2). Malhi H, Atac B, Daly AK, Gupta S: Warfarin and celecoxib interaction in the setting of cytochrome P450 (CYP2C9) polymorphism with bleeding complication. Postgrad Med J 80: 107–109, 2004. (3) Kohl C, Steinkellner M: Prediction of pharmacokinetic drug/drug interactions from In vitro data: interactions of the nonsteroidal anti–inflammatory drug lornoxicam with oral anticoagulants. Drug Metab Dispos 28: 161–168,2000.
- * This table presents information obtained from package inserts and by a review of the literature available at the time of editing in a simplified form. Therefore, we ask the readers to understand that the data may not be universal and to check the latest package inserts and literature for details.
Table 4 provides information about lipid-lowering agents and Table 5 about antidiabetic drugs.
| Classification | Drug | Major elimination route | Dialyzability | Dose for normal renal function (mg/day) | Adjustment for dialysis patients (mg/day) | |
|---|---|---|---|---|---|---|
| Generic name | Administration route | |||||
| Statins (hydroxymethylglutaryl CoA reductase inhibitors) | Atorvastatin Calcium Hydrate | Oral | Liver | − | 10–40 | Usual dose |
| Simvastatin | Oral | Liver | − | 5–20 | Usual dose | |
| Pitavastatin Calcium | Oral | Liver | − | 1–4 | Usual dose | |
| Pravastatin Sodium | Oral | Liver | ± | 10–20 | Usual dose | |
| Fluvastatin Sodium | Oral | Fecal | − | 20–60 | Usual dose | |
| Rosuvastatin Calcium | Oral | Liver (kidney ≤10%–33%) | − | 2.5–20 | 2.5–5.0 | |
| Cholesterol absorption inhibitors | Ezetimibe | Oral | Fecal | − | 10 | Usual dose |
| Fibrate | Clinofibrate | Oral | Fecal (kidney ≤1%) | − | 600 | Usual dose |
| Clofibrate | Oral | Kidney? | − | 750–1500 | Caution needed for administration (careful administration?) | |
| Bezafibrate | Oral, sustained release | Kidney 50–70% | − | 400 | Contraindication | |
| Fenofibrate | Oral | Liver | − | 134–201 | Contraindication | |
| Nicotinic acid agents | Nicomol | Oral | Non–kidney? | Irrelevant (not applicant?) | 600–1200 | Usual dose |
| Niceritrol | Oral | Fecal | Irrelevant (not applicant?) | 750 | 250 | |
| Bile acid sequestrants | Colestimide | Oral | Fecal | Irrelevant (not applicant?) | 3–4g | Usual dose |
| Colestyramine | Oral | Fecal | Irrelevant (not applicant?) | 18–27 g | Usual dose | |
| Probucol | Probucol | Oral | Liver | − | 500–1000 | Usual dose |
| Others, miscellaneous? | Ethyl icosapentate | Oral | Unknown | − | 1800–2700 | Usual dose |
- * This table presents information obtained from package inserts and by a review of the literature available at the time of editing in a simplified form. Therefore, we ask the readers to understand that the data may not be universal and to check the latest package inserts and literature for details.
| Classification | Drug | Major elimination route | Dialyzability | Dose for normal renal function (mg/day) | Adjustment for dialysis patients (mg/day) | |
|---|---|---|---|---|---|---|
| Generic name | Brand name | |||||
| Insulin | Various insulin preparations | Liver kidney muscle | − | Necessary dose | Necessary dose† | |
| Sulfonyl ureas | Acetohexamide | Oral | Liver | − | 250–1000 | Contraindication |
| Gliclazide | Oral | Liver | − | 40–160 | Contraindication | |
| Glyclopyramide | Oral | Kidney (rat) | − | 125–500 | Contraindication | |
| Glibenclamide | Oral | Liver | + | 1.25–10 | Contraindication | |
| Glimepiride | Oral | Liver | − | 1–6 | Contraindication | |
| Chlorpropamide | Oral | Liver (kidney 20%) | − | 100–500 | Contraindication | |
| Tolbutamide | Oral | Liver | − | 0.5–2.0 | Contraindication | |
| Nonsulfonylurea secretagogues | Nateglinide | Oral | Liver (kidney 5–16%) | − | 270–360 | Contraindication |
| Mitiglinide Calcium Hydrate | Oral | Liver | − | 30 | Caution needed for administration (careful administration?) | |
| Biguanides | Buformin Hydrochloride | Oral | Kidney 84.5% | + | 100–150 | Contraindication |
| Metformin Hydrochloride | Oral | Kidney 80–100% | + | 500–750 | Contraindication | |
| Oral | Kidney 80–100% | + | 500–2250 | Contraindication | ||
| Thiazolidinediones | Pioglitazone Hydrochloride | Oral | Liver | − | 15–45 | Contraindication |
| Preparations containing pioglitazone/metformin | Oral | Pioglitazone: liver Metformin: kidney | ± | Pioglitazone 15–30 / metformin 500 | Contraindication | |
| α–glucosidase inhibitors | Acarbose | Oral | Fecal | Irrelevant | 150–300 | Usual dose |
| Voglibose | Oral | Fecal | Irrelevant | 0.6–0.9 | Usual dose | |
| Miglitol | Oral | Kidney 30% | + | 150–225 | Caution needed for administration (careful administration?) | |
| Dipeptidyl peptidase–4 (DPP–4) inhibitors | Alogliptin Benzoate | Oral | Kidney | − | 25 | 6.25 |
| Sitagliptin Phosphate Hydrate | Oral | Kidney 79∼88% | − | 50–100 | Contraindication | |
| Vildagliptin | Oral | Liver (kidney 33%) | − | 50–100 | 50 | |
| Glucagon–like peptide (GLP) agonists | Liraglutide (Genetical Recombination) | Subcutaneous injection | Non–kidney | − | 0.3–0.9 | Usual dose |
| Exenatide | Subcutaneous injection | Degraded by kidney | − | 10–20µg | Contraindication | |
- † Since insulin is partly metabolized in the kidney, a dose reduction is necessary in dialysis patients, but the dose should be adjusted basically in consideration of the state of blood sugar control.
- * This table presents information obtained from package inserts and by a review of the literature available at the time of editing in a simplified form. Therefore, we ask the readers to understand that the data may not be universal and to check the latest package inserts and literature for details.
Acknowledgments:
We apologize for the long time it took us to prepare and publish the guideline. This was mainly because the field of medical treatment is humongous. The specialization of this guideline is very high to such an extent that each chapter could be considered by itself a separate guideline. To obtain the consensus of as many members of the JSDT as possible, a consensus conference was held over three consecutive years. We want to thank the efforts of all 23 members of the committee who were involved in the preparation of the guideline, especially Dr Kazuaki Shimamoto, the President of Sapporo Medical University School of Medicine, and Dr Kiyotaka Kugiyama, the Professor of Yamanashi University School of Medicine, who represented the Japanese Society of Cardiology. We also want to mention Dr Jun Minakuchi, the Chairperson of the 53rd Annual Meeting of the JSDT, Dr Tsutomu Sanaka, the Chairperson of the 54th Annual Meeting of JSDT, and Hideki Kawanishi, the Chairperson of the 55th Annual Meeting of JSDT.




