Effects of Gibberellic Acid on Primary Terpenoids and Δ9-Tetrahydrocannabinol in Cannabis sativa at Flowering Stage
Abstract
Plants synthesize an astonishing diversity of isoprenoids, some of which play essential roles in photosynthesis, respiration, and the regulation of growth and development. Two independent pathways for the biosynthesis of isoprenoid precursors coexist within the plant cell: the cytosolic mevalonic acid (MVA) pathway and the plastidial methylerythritol phosphate (MEP) pathway. However, little is known about the effects of plant hormones on the regulation of these pathways. In the present study we investigated the effect of gibberellic acid (GA3) on changes in the amounts of many produced terpenoids and the activity of the key enzymes, 1-deoxy-D-xylulose 5-phosphate synthase (DXS) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), in these pathways. Our results showed GA3 caused a decrease in DXS activity in both sexes that it was accompanied by a decrease in chlorophylls, carotenoids and Δ9-tetrahydrocannabinol (THC) contents and an increase in α-tocopherol content. The treated plants with GA3 showed an increase in HMGR activity. This increase in HMGR activity was followed by accumulation of stigmasterol and β-sitosterol in male and female plants and campestrol in male plants. The pattern of the changes in the amounts of sterols was exactly similar to the changes in the HMGR activity. These data suggest that GA3 can probably influence the MEP and MVA pathways oppositely, with stimulatory and inhibitory effects on the produced primary terpenoids in MVA and DXS pathways, respectively.
Terpenoids constitute the largest family of natural plant products, with over 30 000 members (Dewick 2002). Members of this diverse group of natural products are found in all organisms. In higher plants, isoprenoids participate in a wide variety of biological functions such as photosynthesis, respiration, growth, cell cycle control, plant defense, and adaptation to environmental conditions. Specific examples include photosynthetic pigments (chlorophylls and carotenoids), hormones (abscisic acid (ABA), gibberellins (GA), cytokinins, and brassinosteroids), a side chain of the electron transporter (plastiquinone), structural components of membranes (phytosterols), and antimicrobial agents (phytoalexins). Beyond these plant-specific functions, many plant isoprenoids have been shown to have industrial and medical importance. The plant-produced isoprenoids β-carotene (provitamin A) and α-tocopherol (vitamin E) are both basic nutrients required for the maintenance of human health (Shintani and DellaPenna 1998; Hirschberg 1999). Industrial uses of isoprenoids include products such as colorants, fragrances, and flavorings (Lange and Croteau 1999).
All isoprenoids are derived from the ubiquitous C5 building blocks isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). These precursors can be synthesized by two different routes: the classical mevalonate pathway in the cytoplasm or the alternative non-mevalonate pathway in plastids (Arigoni et al. 1997; Rohmer 1999). The plastidial pathway, now known as the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway, has been fully elucidated by a combination of biochemical and genomic approaches (Rodriguez-Concepcion and Boronat 2002). It provides the precursors for monoterpenes, diterpenes, carotenoids, tocopherols, and the prenyl moiety of chlorophyll (Eisenreich et al. 2001). In the first reaction, catalyzed by 1-deoxy-D-xylulose 5-phosphate synthase (DXS), 1-deoxy-D-xylulose 5-phosphate (DXP) is synthesized from glyceraldehydes 3-phosphate and pyruvate in a condensation/decarboxylation reaction. The DXS reaction is also the first step for thiamine and pyridoxol biosynthesis (Kuzuyama et al. 1998; Takahashi et al. 1998). The cytosolic pathway, which starts from acetyl-CoA and proceeds through the intermediate mevalonate (MVA), provides the precursors for sterols and ubiquinone (Laule et al. 2003). Since terpenoids have different functions as primary metabolites (in growth and development) and secondary methabolites (in plant response to environment), then to understand how they interact with plant growth regulators is important.
Gibberellinss form a large family of diterpenoid compounds, some of which are bioactive growth regulators. GA is widely regarded as a growth-promoting compound that positively regulates processes such as seed germination, stem elongation, leaf expansion, pollen-tube growth, trichome, flower and fruit development and floral transition (Olszewski et al. 2002; Razem et al. 2006). Until recently, the isopentenyl diphosphate used for GA biosynthesis was thought to be derived from mevalonic acid. However, it has been established that cytosolic mevalonate is not involved in GA biosynthesis in plants (Estévez et al. 2001). In spite of the economic significance of the terpenoids and their many essential functions, relatively little is known about terpenpoid metabolism and its regulation in plants. To understand the role of giberrellic acid (GA3) in the regulation of two terpenoid biosynthesis pathways, we studied the response of the main end products of these pathways and cannabinoids under GA3 treatment in cannabis plants.
Cannabis is a dioecious plant that is a source of fiber, food, oil and medicine. Cannabinoids represent a distinctive class of compounds found only in Cannabis sativa. These C21 compounds belong to the chemical class of natural terpenophenols. Δ9-tetrahycannabinol (THC) and cannabidiol (CBD) are the most important of these compounds. The experiments with labeling patterns show that the cannabinoids are derived entirely or predominantly (98%) from the deoxyxylulos pathway (Fellermeier et al. 2001). They are produced by glandular trichomes that occur on most aerial surfaces of the plant (Hilling 2004). Cannabis is used in modern medicine for the treatment of emesis in chemotherapy. As well as being useful anti-emetics, cannabinoids appear to have therapeutic value as antispasmodics, analgesics, and appetite stimulants and may also have potential in the treatment of epilepsy, glaucoma, and asthma (Agurell and Nilsson 1972; Guzman 2003; Howlett et al. 2004). In the present study, as well as primary terpenoids, we examined the effect of GA3 on THC content.
Result
Effect of GA3 on levels of chlorophyll, carotenoids and α-tocopherol
Effects of different concentrations of GA3 on levels of chlorophyll, carotenoids and α-tocopherol in the leaves of male and female cannabis plants were investigated. Male plants treated with 50 μM GA3 had lower chlorophyll a and total contents compared to the control (Figure 1A). Treatment of female leaves with 50 and 100 μM GA3 significantly decreased chlorophyll a, b and total contents in a dose-dependent pattern (Figure 1B). The carotenoid contents of treated plants were lower compared with the control (Figure 2A). Low concentrations of GA3 (50 μM) were more effective on decreasing carotenoid contents in male plants. Figure 2B shows the effects of different concentrations of GA3 on α-tocopherol content. All concentrations of GA3 sprayed on leaves caused an increase in α-tocopherol content in both sexes. The highest increase was detected with 50 μM GA3 in male plants. Apparently, the pattern of changes in α-tocopherol content was an invert of that in chlorophyll and carotenoid contents.
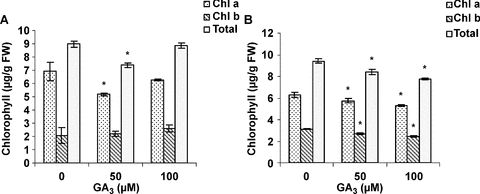
Effects of gibberellic acid (GA3) on chlorophyll a, b and total in leaves of male (A) and female (B) cannabis plants.Values are means of four replications ± standard deviation (SD). Asterisks indicate the significance of difference at P < 0.05 level by Duncan test when compared with controls.
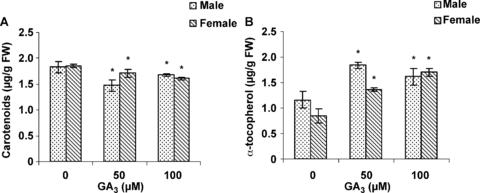
Effects of gibberellic acid (GA3) on (A) carotenoids and (B) α-tocopherol in leaves of female and male cannabis plants.Values are means of four replications ± standard deviation (SD). Asterisks indicate the significance of difference at P < 0.05 level by Duncan test when compared with controls.
Effect of GA3 on DXS and HMGR activity
In the present study, the effects of GA3 on key enzyme activity of terpenoid biosynthetic pathways (DXS and HMGR) were investigated. As shown in Figure 3, male plants had more DXS activity than female plants and a significant decrease was observed over DXS activity in treated plants (male and female) by GA3 which was linear with increasing GA3 concentration. GA3 treatment had a stronger effect on decreasing DXS activity in male plants.
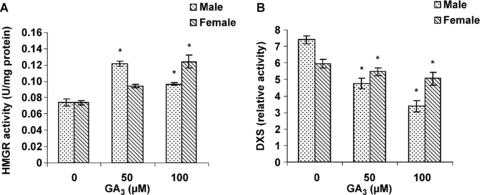
Effects of gibberellic acid (GA3) on 1-deoxy-D-xylulose 5-phosphate synthase (DXS) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) activities in the leaves of female and male cannabis plants.Values are means of four replications ± standard deviation (SD). Asterisks indicate the significance of difference at P < 0.05 level by Duncan test when compared with controls.
Gibberellic acid treatment increased HMGR activity in the treated plants (Figure 3). But some differences are obvious between the two groups of plants of different sex (Figure 3). The male individuals had a greater HMGR activity at lower GA3 concentrations. The male plants treated with 100 μM GA3 showed a lower HMGR activity than that of those treated with 50 μM GA3. However, the activity of HMGR was stimulated by GA3 in a dose-dependent pattern in the leaves of female plants.
Effect of GA3 on phytosterols
The mature leaves of male and female cannabis plants were used to determine the effect of GA3 on squalene (biosynthetic precursor to all steroids), campesterol, stigmasterol, and β-sitosterol (the most representative phytosterols of the MVA-pathway). A significant increase in squalene (Figure 4), stigmasterol and β-sitosterol accumulation occurred in female and male plants treated with 50 and 100 μM GA3 (Figure 5). The changes in the amount of squalene and β-sitosterol were coordinate with the changes in HMGR activity. Also GA3 had a stimulatory effect on Campestrol accumulation in male plants.
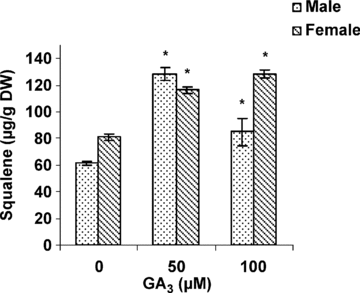
Effects of gibberellic acid (GA3) on squalene content in leaves of female and male cannabis plants.Values are means of four replications ± standard deviation (SD). Asterisks indicate the significance of difference at P < 0.05 level by Duncan test when compared with controls.
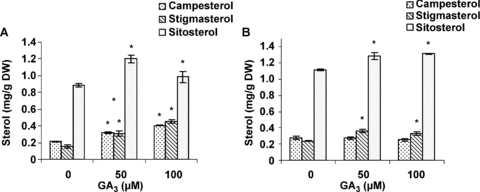
Effects of gibberellic acid (GA3) on campesterol, stigmasterol and sitosterol content in leaves of (A) male and (B) female cannabis plants.Values are means of four replications ± standard deviation (SD). Asterisks indicate the significance of difference at P < 0.05 level by Duncan test when compared with controls.
Effect of GA3 on THC in leaves and flowers
A comparison between male and female plants showed that females had higher amounts of THC; especially in the flowers (Figure 6). THC content of the leaves was slightly lower than that of flowers. The application of GA3 to cannabis plants resulted in a decrease in THC content. GA3 treatment had a stronger effect in decreasing THC content in the flowers of male plants in comparison with that of female plants. However, the leaves of the two sexes indicated similar responses to GA3 treatment.
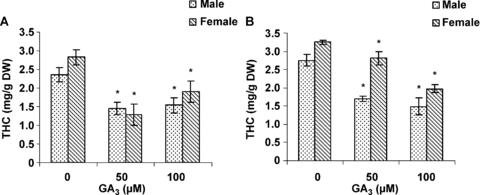
Effect of gibberellic acid (GA3) on Δ9-tetrahydrocannabinol (THC) content in (A) leaves and (B) flowers of the male and female cannabis plants.Values are means of four replications ± standard deviation (SD). Asterisks indicate the significance of difference at P < 0.05 level by Duncan test when compared with controls.
Discussion
The phytyl (C20) conjugates chlorophylls and tocopherols, and carotenoids (C40) are produced by the MEP pathway. GA3 treatment decreased chlorophyll contents in C. sativa plants. Reduction in chlorophyll content after GA3 application has been reported in, wheat (Misra and Biswal 1980), peach trees (Monge et al. 1994), rice seedlings (Yim et al. 1997) and pea (Bora and Sarma 2006). Perez et al. (1974) have shown that a mutant of tomato with increased levels of GA3 contains less chlorophyll. Our results showed that GA3 caused a decrease in carotenoid contents. Apparently carotenoid and chlorophyll accumulation is controlled through a similar mechanism, because both of them are reduced by GA3. The previous studies indicate that GA3 treatments delayed the chloroplast-chromoplast conversion of colored fruit (Goldschmidt 1988; Pfander 1992). The development of chromoplasts is accompanied by the accumulation of carotenoids (Vainstein et al. 1994). Also Rodrigo and Zacarias (2007) reported that GA3 reduced the ethylene-induced expression of early carotenoid biosynthetic genes and the accumulation of phytoene in orange.
Tocopherols (α-, β-, γ-, and δ-tocopherol) are lipophilic antioxidants that collectively constitute vitamin E (Crowell et al. 2008). Tocopherols are synthesized by photosynthetic organisms, occurring mainly in leaves and seeds. Literature sources indicate that the major tocopherol form in leaf tissues is α-tocopherol (Szymanska and Kruk 2008). Therefore, we measured the changes in the amounts of α-tocopherol in response to GA3 treatment. Our results showed that GA3 increased α-tocopherol content in cannabis plants. A stimulatory effect of applied GA3 on α-tocopherol content was also observed in Catharanthus roseus (Abdul Jaleel et al. 2007). On the other hand, we found a reversed relationship between photosynthetic pigments (chlorophyll and carotenoids) and α-tocopherol contents in treated plants with GA3. Both α-tocopherol and chlorophyll contain a phytol moiety as part of their molecule. The substrate used for their biosynthesis may be derived from a common pool. It is possible that the decrease in chlorophyll and carotenoid biosynthesis caused an increase in the substrate accumulation and therefore α-tocopherol biosynthesis. Also Rise et al. (1989) reported a transient increase in α-tocopherol accompanied with a decrease in chlorophyll during the course of senescence in several plant species. Their results indicated that the phytol released by chlorophylase during the initial stages of chlorophyll breakdown, may be used for the biosynthesis of α-tocopherol during senescence.
In the present study, GA3 treatment caused a decrease in the DXS activity in male and female plants. This is the first report about the effect of GA3 on DXS activity. Many reports support a regulatory role of DXS for the production of MEP-derived isoprenoids in plants (Rodriguez-Concepcion 2006). Estévez et al. (2001) reported that transgene-mediated upregulation or downregulation of DXS levels in Arabidopsis were correlated with concomitant changes in the levels of MEP-derived isoprenoid end-products. However, in this study the decrease in the DXS activity did not correlate with a decrease in the entire MEP pathway end products. The results showed an increase in α-tocopherol content and a decrease in chlorophyll and carotenoids. These data support this hypothesis that several enzymes share control over the metabolic flux through the MEP pathway (not only DXS). The MEP pathway might be regulated at several control points to compensate for fluctuations in precursor/product equilibrium and redistribute the balance of control within the pathway (Enfissi et al. 2005). Another possibility for this uncoordinated activity between the DXS and the plastidic isoperenoids is the exchange of precursors between the cytosol and the plastid. Strong biochemical evidence for such exchange of precursors was reported by Kasahara et al. (2002), Nagata et al. (2002) and Hemmerlin et al. (2003).
Analysis of HMGR activity in cannabis plants demonstrated that HMGR activity was greater in plants treated with GA3. Consistent with our result, Russell and Davidson (1982) reported that GA3 increased HMGR activity in pea seedlings. It was shown that higher levels of HMGR activity were usually associated with the rapidly growing parts of plants (Brooker and Russell 1975). On the other hand, GA is widely regarded as a growth-promoting compound. Therefore, it can be a candidate for stimulating of the HMGR activity. The lower HMGR activity was seen in the male plants treated with 100 μM GA3. The decrease observed in the HMGR activity could reflect the GA3 interaction in high concentration with other plant hormones. As it is has been shown that exogenous application of GA3 caused a clear increase in ACC content, ACC oxidase activity and ethylene biosynthesis occur during the breaking of dormancy and onset of germination in Fagus sylvatica L. seeds (Calvo et al. 2004). Furthermore, it is possible that ethylene caused the decrease observed in HMGR activity.
Sterol levels and metabolism have been reported to vary with factors such as light intensity, light quality, day length and temperature (Westerman and Roddick 1981). The data in this study show that GA3 treatment increased squalene and phytostrol contents in a pattern similar to the changes in the HMGR activity. Since HMGR is an important control point for the MVA pathway in plants (Kato-Emori et al. 2001), the increase in HMGR activity should result in increasing the supply of phytostrols. Free sterols are found predominantly in cell membranes and are thought to contribute to the proper functioning of membranes by controlling the fluidity characteristics of the membrane (Devarenne et al. 2002). Douglas and Paleg (1974) also demonstrated that the inhibitors of GAs biosynthesis caused a decrease in sterol accumulation. Huttly and Phillips (1995) suggested that GA3 causes an increase in cell number and size to produce a significant effect. Since these processes need the production of cell membrane, it can be assumed that GA3 should induce the phytosterol biosynthesis to influence its effects on growth in plants.
Δ9-tetrahydrocannabinol is the cannabinoid responsible for the main psychoactive effects of most Cannabis drug preparations (Mechoulam 1970). Factors that control biosynthesis and distribution of cannabinoids within the plant are unknown. We investigated the impact of altered GA3 levels on this secondary metabolite in C. sativa. The results indicated that apart from the influence of GA3, female plants had more THC than male plants in leaves and flowers. The treated plants with GA3 had lower THC content in comparison with that in control plants. It is demonstrated that cannabinoids are synthesized from the DXP pathway (Fellermeier et al. 2001). Our results detected GA3 can decrease DXS activity. Furthermore, our results show that GA3 decreased THC content by decreasing of the DXS activity and necessary precursors for THC biosynthesis.
In conclusion, the pattern of changes in the amounts of primary terpenoids (chlorophyll, carotenoids and phytosterols) in C. sativa suggests that GA3 have opposite effects on the primary terpenoid biosynthesis of the MEP and MVA pathways. Also, the appearance of the direct relationship between DXS and HMGR activity, and their main end products confirm an important role for these enzymes in the MEP and MVA pathways regulation. However, to understand the role of GA3 in terpenoid biosynthesis regulation we need further investigation.
Materials and Methods
Plant material
The seeds of Cannabis sativa L. (with geographical source from Iran) were sown in pots (15 cm, i.d. soil-leaf mold-perlit = 2:1:1) and cultivated in a phytotron (25°C 14:10 h light : dark (LD) cycle). The plants were fertilized regularly with a Hoglan?s nutrient solution every week.
GA3 treatment of plants
Female flowering plants were treated when glandular trichomes on bracts were globose and resinous and male flowering plants were treated when flower blooms and pollen grains were visible. Similar sized male and female plants were subjected to GA3 (Merck, Germany) treatment by spraying the whole plants with 50 and 100 μM GA3 solutions and tap water as a control, until the solution started dripping. The treatment took place with three sprays at 24 h intervals. The plants were harvested 24 h after the final treatment.
Chlorophyll and carotenoid determination
Chlorophyll and carotenoids were extracted from leaves with 95% ethanol and quantified by measuring the absorbances at 664, 648 and 470 nm as described by Lichtenthaler (1987).
Quantitative analysis of squalene and phytosterols by GC
Quantitative analysis of squalene and phytosterols (b-sitosterol and stigmasterol) was carried out by gas chromatography (GC). Freeze-dried leaves (100 mg) were extracted with ethyl acetate at 100 rpm on a gyratory shaker (20 mL, twice, 25°C for 6 h). The acidic compounds were removed with aqueous 5% KOH (10 mL, thrice) followed by the removal of the basic compounds with aqueous 5% HCl (10 mL, twice). The organic fraction, which contains the neutral compounds, was washed with water (10 mL, twice) and then dried with anhydrous sodium sulfate. The solvent was evaporated and the residue was dissolved in hexane (2 mL) and then centrifuged for 10 min at 6000 rpm to remove the suspended particles.
Chromatography was carried out with Agilent technologies (Wilmington, DE, USA) equipment including a 7683 automated sample-injection system, a split/split less injector, a 30 m × 320 μm I. D., 0.25 μm film thickness HP-5 fuse-silica capillary column coated with 5% phenymethyl siloxane (J&W Scientific, Folsom, CA, USA) and a flame ionization detection (FID) controlled by the Agilent Chemstation software. The oven temperature was held at 240°C for 10 min, then raised to 260°C at 2°C/min and then held at that temperature for 30 min; injection port 270°C; detector 300°C; split ratio 15:1, injection volume 1.0 μL; nitrogen carrier gas 1.0 mL/min, hydrogen 30 mL/min and detector flow of make-up gas (nitrogen) 400 mL/min in the constant make-up flow mode.
α-Tocopherol extraction and measurement
Tocopherols were extracted basically as described for cereal seeds by Panfili et al. (2003), by grinding and homogenizing 25 mg of freeze-dried leaf material in 100 μL 100% methanol. After 20 min of incubation at 30°C, the samples were centrifuged at 24 000 g for 5 min, the supernatant was transferred to new tubes, and the pellet was re-extracted twice with 250 μL 100% methanol at 30°C for 30 min, pooling all supernatants.
Chromatographic conditions
Ultra-performance liquid chromatography (UPLC) analysis was carried out with a Waters Acquity ultraperformance liquid chromatograph equipped with a PDA detector. An Acquity UPLC BEH C18 column (2.1 mm × 150 mm, 1.7 μm particles) was used for separation. Methanol (100%) was used as mobile phase; the flow rate was 0.48 mL/min. PDA detector was accomplished at 290 nm for α-tocopherol. Injection volume was 10 μL.
Sample collection for cannabinoids measurement
Female and male samples were collected as flowering tops and leaves separately. Flowering top samples included bracts and small leaves (small, palmately compound leaves-surrounding the flowers) in females and flowers and pollen grains in males. Mature leaves (∼7 cm length) from male and female plants were used for cannabinoid measurement. All samples were dried at room temperature in darkness.
THC extraction and measurement
Sample material (50 mg) was placed in a test tube with 1 mL chloroform. Sonication was applied for 15 min. After filtration, the solvent was evaporated to dryness and the residue was dissolved in 0.5 mL methanol.
Chromatographic conditions
Apparatus and column condition was the same as for α-tocopherol analysis. The mobile phase was an acetonitrile-water gradient from 70:30 to 100:0 in 5 min, remaining at 100:0 in 1 min, and returning to 30:70 in 1 min (flow 0.4 mL/min). Buffer (0.05% trifluoroacetic acid (TFA) resulting in a pH of 3.0) was added to both solvents to eliminate the tailing of phenolic compounds. Injection volume was 7 μL. PDA detectore was accomplished at 230 nm for THC.
Enzyme assays
All enzymes assayed in this work were extracted from fresh leaves with an extraction buffer of 50 mM Tris-HCl, 10 mM b-mercaptoethanol, 1% (w/v) polyvinylpyrrolidone (PVP), and pH 7.5. The leaves were ground in the extraction buffer (1 g FW/mL) for 5 min with a pestle and mortar on ice, followed by centrifugation at 24 000 g and 4°C for 30 min to obtain a solids-free extract. The protein content was determined by the Bradford method (1976) using bovine serum albumin as a standard.
The activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) was determined by the method of Toroser and Huber (1998). The enzyme extract was added (∼50 mg protein per mL) to 50 mM of Tris-HCl assay buffer (pH 7.0) containing 0.3 mM of HMG-CoA (Sigma, St. Louis, MO, USA, Cat. H6132), 0.2 mM of nicotinamide adenine dinucleoside phosphate hydrogen (NADPH) and 4 mM of dithiothreitol. NADPH oxidation in the reaction solution was monitored at 25°C by the decreasing absorbance at 340 nm, against the solution free of HMG-CoA as a blank. One HMGR enzyme unit is equivalent to the oxidation of 1 mM of NADPH per min.
The activity of 1-deoxy-D-xylulose 5-phosphate synthase was determined by the fluorometric method of Querol et al. (2001), which is based on the reaction of 1-deoxy-D-xylulose 5-phosphate with 3, 5-diaminobenzoic acid in an acidic medium to form a highly fluorescing quinaldine derivative. The reaction mixture contained 40 mM Tris-HCl (pH 7.5), 2.5 mM MgCl2, 5 mM b-mercaptoethanol, 1 mM thiamin diphosphate, 10 mM sodium pyruvate, and 20 mM DL-glyceraldehyde 3-phosphate, and the enzyme extract (∼50 mg protein per mL reaction solution). The reaction solution was maintained at 37°C for 1 h, stopped for heating at 80°C for 5 min, and then spun down at 21 000 g for 5 min to remove the denatured proteins. The supernatant was mixed with 1 mL of 10 mM 3, 5-diaminobenzoic acid in 5 M phosphoric acid, and then heated in a boiling water bath for 15 min. The fluorescence intensity of the reaction product, which is proportional to DXS activity, was determined with a fluorescence spectrophotometer at 396 nm excitation and 510 nm emission.
(Handling editor: Xiaoya Chen)
Acknowledgements
We would like to express our sincere gratitude to Professor Jan Szopa for THC standard and his help with UPLC analysis.




