Protecting Cell Walls from Binding Aluminum by Organic Acids Contributes to Aluminum Resistance
Supported by the National Natural Science Foundation of China (30830076) and China Postdoctoral Science Foundation Funded Project (20070420234).
Abstract
Aluminum-induced secretion of organic acids from the root apex has been demonstrated to be one major Al resistance mechanism in plants. However, whether the organic acid concentration is high enough to detoxify Al in the growth medium is frequently questioned. The genotypes of Al-resistant wheat, Cassia tora L. and buckwheat secrete malate, citrate and oxalate, respectively. In the present study we found that at a 35% inhibition of root elongation, the Al activities in the solution were 10, 20, and 50 μM with the corresponding malate, citrate, and oxalate exudation at the rates of 15, 20 and 21 nmol/cm2 per 12 h, respectively, for the above three plant species. When exogenous organic acids were added to ameliorate Al toxicity, twofold and eightfold higher oxalate and malate concentrations were required to produce the equal effect by citrate. After the root apical cell walls were isolated and preincubated in 1 mM malate, oxalate or citrate solution overnight, the total amount of Al adsorbed to the cell walls all decreased significantly to a similar level, implying that these organic acids own an equal ability to protect the cell walls from binding Al. These findings suggest that protection of cell walls from binding Al by organic acids may contribute significantly to Al resistance.
Aluminum toxicity is a global problem that limits crop production on acid soils, and is characterized by a rapid inhibition of root elongation (Kochian 1995). Despite the agricultural importance of this problem, little is known about the fundamental mechanisms of Al toxicity and resistance (Ma 2007; Poschenrieder et al. 2008). On the other hand, many plant species or cultivars within the same species exhibit significant genetic variability in their ability to resist Al toxicity. Two strategies for the plant Al resistance have been suggested (Taylor 1991; Kochian 1995). One is based on exclusion of Al from the root symplasm, while the other mechanism relies on the ability to truly tolerate Al ions once they enter the cytosol. Among the likely exclusion mechanisms, a role for organic acid efflux has been well documented in a number of plant species (Ma 2000; Ryan et al. 2001; Kochian et al. 2004), and genes responsible for Al-activated malate and citrate efflux have also been cloned from a number of plant species (Sasaki et al. 2004; Hoekenga et al. 2006; Ligaba et al. 2006; Furukawa et al. 2007; Magalhaes et al. 2007).
Depending on plant species, Al-activated exudation of malate, oxalate, or citrate has been shown to be correlated with differential Al resistance (Ryan et al. 2001). As a number of different organic acids are present in the plant cytoplasm, the mechanisms regulating the Al-activated root exudation of a certain organic acid in a particular plant species still remain unknown (Kochian et al. 2004). Furthermore, it is significant that a large number of plant species exhibit Al-activated citrate efflux because citrate3−, as a tricarboxylic acid anion, chelates Al3+ much more strongly than does the dicarboxylic anions oxalate2− and malate2−. Hence, citrate is much more effective in detoxifying Al than oxalate and malate when applied exogenously to the roots of Al sensitive genotypes exposed to Al. Additionally, it is still a matter of debate whether the amount of secreted organic acids measured in Al resistant genotypes is sufficient to detoxify most of the Al present in the thin layer around the sensitive regions of the roots (Parker and Pedler 1998; Kinraide et al. 2005). Therefore, it seems that attempts at estimating the detoxifying ability of organic acids, through their chelating of Al in solutions outside the root, have not adequately explained this Al resistance mechanism.
On the other hand, accumulating evidence supports the hypothesis that the root apoplast plays an important role in the expression of Al toxicity and Al resistance (Horst 1995; Zheng and Yang 2005). Fixation of Al in the cell wall materials is one possibly important aspect of an Al exclusion mechanism, as proposed by Taylor (1991). Al strongly binds to plant cell walls (Delhaize et al. 1993a; Eticha et al. 2005b). In giant algal cells of Chara corallina, up to 99.9% of the total cellular Al was shown to be accumulated in the cell wall (Taylor et al. 2000). In our previous study, root cell walls were extracted from an Al-resistant wheat (Triticum aestivum L.) cultivar, Atlas 66, to investigate the effect of cell wall properties on the kinetics of Al adsorption and desorption. We demonstrated that root cell walls treated with pectinase or malate decreased Al adsorption in root cell walls isolated from the entire root system (Zheng et al. 2004). According to the model proposed by Horst (1995), Al bound to cell wall materials increases the rigidity of the cell wall, affects cell wall loosening and thus ultimately inhibits root elongation. Thus, any substances that can protect the active sites in the cell wall from Al binding should result in increased Al resistance.
In order to investigate the ability of different organic acids to detoxify Al under the same experimental conditions, we have selected in this study three well documented Al-resistant genotypes of the plant species buckwheat (Fagopyrum esculentem Moench), wheat, and Cassia tora L. which exhibit Al-activated root oxalate, malate, and citrate efflux, respectively (Ryan et al. 1995; Zheng et al. 2005; Yang et al. 2006). We found that the amount of secreted malate in wheat was less than that of oxalate in buckwheat and citrate in C. tora under Al exposures that resulted in the same inhibition of root elongation. However, the ability of these three organic acids to decrease the capability of Al binding in cell walls was very similar.
Results
A progressive inhibition of root elongation was observed when roots of all three plant species were exposed to increasing Al concentrations. To elicit a 35% inhibition of root elongation, the Al concentration in the growth medium was 10, 20 and 50 μM Al respectively, for wheat, C. tora and buckwheat (Figure 1). Also, under these Al exposure conditions that elicited the same degree of root inhibition, rates of Al-activated root of malate, citrate and oxalate were 15.0, 20.5 and 21.5 nmol/cm2per 12 h for wheat, C. tora and buckwheat, respectively (Figure 2).
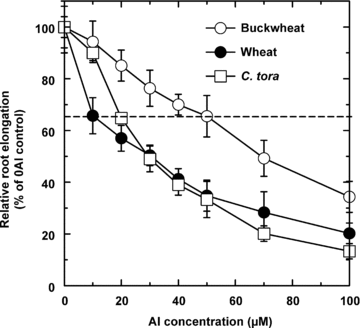
Effect of Al on root growth in the buckwheat, wheat and Cassia tora plants. Three-day-old seedlings were exposed to varying concentrations of Al for 24 h. Relative root elongation was defined as the percentage of root growth of the Al treatment to that of no-Al control. Values are means ±SD (n= 10).
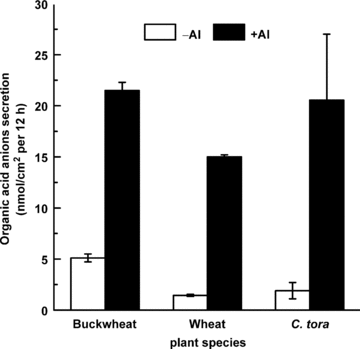
Oxalate, malate, and citrate secretion from buckwheat, wheat and Cassia tora roots in the presence of Al at which the inhibition of root elongation was all about 35%. Three-day-old seedlings of buckwheat, wheat, or C. tora were exposed to 0.5 mM CaCl2 (pH 4.5) solution containing 50, 10, 20 μM Al, respectively. Root exudates were collected at 12 h after initiation of Al treatment. Organic acids were analyzed by the enzymatic method. Data are means ±SD (n= 3).
In order to determine the ability of the three different organic acids to detoxify Al externally, a bioassay experiment was conducted to determine the effect on root growth of the Al-organic acid complexes at different ratios of Al to organic acid. The results clearly showed that citrate at a 1:1 ratio with Al can completely detoxify Al, but the ratio of organic acid to Al that resulted in the same degree of Al detoxification for oxalate and malate was 2:1 and 8:1, respectively (Figure 3). That is, under the conditions of Al exposure used for these experiments, the theoretical amount of malate, citrate and oxalate required to detoxify the different Al concentrations used here should be 80, 20, and 100 μM respectively.
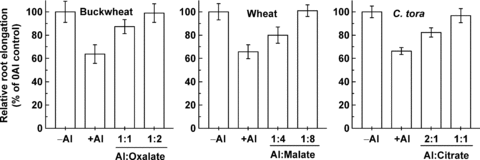
Amelioration of Al toxicity by oxalate, malate, and citrate in buckwheat, wheat, and Cassia tora Seedlings, respectively. Three-day-old seedlings were exposed to 0.5 mM CaCl2 solution (pH 4.5) containing 50, 10, or 20 μM Al and various concentrations of added organic acids. The seedlings grown without Al or organic acids were used as the controls (–Al). Data are means ±SD (n= 10).
The kinetics of Al adsorption to isolated cell walls from root tips of the three plant species was determined either in the absence of organic acid, or after the cell wall extracts were preincubated in 1 mM malate, citrate or oxalate (Figure 4). It was found that in the absence of organic acid pretreatment, C. tora cell walls adsorbed the most Al, followed by buckwheat, and then wheat. After the cell wall materials were preincubated with 1 mM malate, oxalate, and citrate, the total amount of Al adsorbed was decreased by 35%, 28% and 33% in wheat (Figure 4B); 26%, 27% and 29% in buckwheat (Figure 4A); and 27%, 23% and 33% in C. tora (Figure 4C) respectively. Interestingly, as shown in Figure 4D–F, Al desorption from the cell wall materials was influenced very little by the presence or absence of any of the organic acids in the desorption solution.
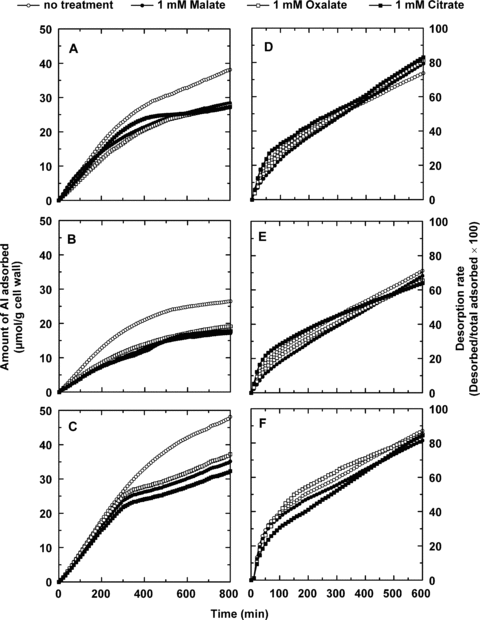
Effect of different organic acids pre-incubation on kinetics of Al adsorption (A–C) and desorption (D–F) in the root apical cell wall (0–1 cm) of buckwheat (A, D), wheat (B, E) and Cassia tora (C, F). Cell walls (0.01 g) placed in a 2 mL column were incubated with 1 mM malate, oxalate or citrate for 12 h at room temperature. The organic acid solution was then sipped away, and the residue was washed with 0.5 mM CaCl2 solution at pH 4.5 at a speed of 6 mL/min for 2 h to remove the free malate not adsorbed. The kinetic studies were conducted as described in adsorption and desorption kinetics. At least two independent replicates were conducted and one set of results is presented.
Discussion
Chelation of Al outside root with organic acids cannot fully explain organic acid-mediated Al resistance
Measurement of root elongation of seedlings in simple salt solution in the presence or absence of Al is a widely used method to estimate Al resistance as well as a good system to study Al-activated root organic acid exudation. In recent years, there has been considerable evidence presented in published reports showing that organic acids play an important role in both internal and external Al detoxification (Ma et al. 2001; Kochian et al. 2004; Ma 2007). Depending on the plant species, citrate, malate, and oxalate have been identified as the major Al-induced organic acids in root exudates. However, it is difficult to directly compare the amount of organic acids secreted in different studies, as the data have often been obtained under different experimental conditions including different Al concentrations, Al exposure times, plant ages and other experimental conditions. Furthermore, since different plant species or genotypes within species often have widely different levels of Al resistance (Barceló and Poschenrieder 2002), comparing the amount of organic acids secreted by exposure to the same Al concentration is meaningless. Therefore, in the present study Al toxicity was normalized by using the level of Al that induced the same degree of root growth inhibition in the three species.
Here we found that Al concentrations at approximately 10, 20, and 50 μM, respectively, resulted in a similar degree of inhibition of root elongation of 35% for wheat, C. tora and buckwheat (Figure 1). In experiments where external organic acid was added to the Al containing solutions to ameliorate Al toxicity, twofold higher oxalate concentrations and eightfold higher malate levels were required to provide the same amelioration of toxicity as citrate (Figure 3), based on these bioassays, it is reasonable to speculate that the ratio of malate : citrate : oxalate exudation in the three species should be approximately 4:1:5 to lead to the same level of Al resistance in wheat, C. tora, and buckwheat. However, the measured rates of Al-activated malate, citrate and oxalate secretion were about 15, 20, and 20 nmol/cm2 per 12 h for wheat, C. tora and buckwheat (Figure 2), indicating that the amount of malate released into the solution bathing the roots was about four times less than what would be predicted from these experiments. Although the root exudates were collected from the whole root system, the exudation of organic acids had been confirmed to be restricted to the apical 5 mm zone (Ryan et al. 1995; Ma et al. 1997; Zheng et al. 2005). Furthermore, the detoxification of Al in solution by organic acid anions also depends on their relative affinities for binding Al. For 1:1 molar ratios of Al to organic acid, the log association constants for citrate, oxalate and malate with Al are 12.3, 6.53 and 6.0 (Hue et al. 1986), which means that citrate is a much more effective Al chelator than oxalate, and oxalate is slightly better than malate. So, if the organic acids secreted under Al stress really protect the root apex from Al toxicity by chelating the Al outside the root in the solution around the root apex, the amounts of organic acids secreted should be in the order: malate > oxalate > citrate. Given that the organic acids secreted were confined to the first few millimeters of the root tip and that the concentrations would be much higher than those in the bulk solution, the estimated organic acids concentrations at the root surface would still be too small to adequately reduce the root-surface activity of Al (Kinraide et al. 2005). Therefore, we can not satisfactorily explain why wheat secreted much less malate than predicted compared with the oxalate exudation in buckwheat, and citrate release in C. tora.
Different organic acids have a similar capacity to protect the root cell wall from binding Al
Externally applied Al rapidly binds to root cell walls (Zhang and Taylor 1990), and it is likely that this is involved in the inhibition of root growth (Eticha et al. 2005a; Liu et al. 2008; Yang et al. 2008). To better understand the physiological and biochemical basis of Al toxicity and tolerance in plants, kinetic analysis of Al uptake into roots has been conducted to estimate the rate of movement of Al into the root apoplasm and symplasm (Zhang and Taylor 1990; Tice et al. 1992). To more clearly investigate cell wall binding of Al in a previous study, cell wall materials isolated from the entire root system of the Al-resistant wheat cultivar, Atlas 66 were examined for their ability to bind Al (Zheng et al. 2004). However, as it is now well known that the root apex is the critical site for Al toxicity and resistance (Ryan et al. 1993; Sivaguru and Horst 1998; Rangel et al. 2007), in the current study the cell wall fractions of apical root sections (0–10 mm) of Al-resistant buckwheat, wheat and C. tora were isolated and used to compare the kinetics of Al adsorption and desorption. The amount of adsorbed Al in C. tora and buckwheat cell walls was greater than that in wheat (Figure 4), indicating that the properties of the cell wall were different from different plant species.
As organic acids secreted from the root symplasm must first pass through the cell wall before reaching the root surface or entering the growth medium, it is reasonable to assume that interactions occur between organic acids and the cell wall, and that this could influence Al interactions with the cell wall. A recent study with custom-made cells demonstrated that organic acid can influence cell wall pectate properties, which resulted in decrease of Al adsorption and increase of Al desorption in root cell walls (Mimmo et al. 2009). In the previous report on cell wall fractions from the entire root system, when malate at 1 mM was preincubated with cell wall materials extracted from Atlas 66, the amount of Al adsorbed decreased significantly (Zheng et al. 2004). As citrate and oxalate are more efficient in chelating Al in solution (the stability constants decrease in the order: Al-citrate > Al-oxalate > Al-malate), we further compared the effect of these three frequently reported organic acids on Al binding in root apical cell walls. Preincubation of cell wall materials with 1 mM malate, oxalate or citrate all significantly decreased the amount of Al adsorbed in apical cell wall sections in wheat (Figure 4B), buckwheat (Figure 4A) and C. tora (Figure 4C). However, it was interesting to note that there were no significant differences among these three organic acids in their ability to reduce the amount of Al adsorbed (Figure 4D–F). Moreover, it is also important to note that malate was equally as effective as citrate in decreasing Al adsorption (Figure 4A–C), indicating that malate has the same ability to protect cell wall materials from binding Al as does the much more effective Al chelator, citrate. In other words, all three organic acids can protect sites in the cell wall from Al binding to the same degree. Another interesting observation is that the total amount of Al adsorbed in cell walls from the Al-tolerant wheat line ET8 was similar to that adsorbed in the Al-sensitive wheat line ES8 (ET8 and ES8 are near-isogenic lines differing at the Al resistance locus), although ES8 accumulates several times more Al in root tips than does ET8 (data not shown). The efflux of malate from the ET8 root apex under Al stress, thereby protecting sensitive sites in cell walls from binding Al, may fully explain why ET8 accumulates less Al than ES8, which shows no Al-dependent malate efflux.
Considering the equal effect of malate compared with citrate in decreasing Al adsorption in root apical cell walls, we propose that the protection of sensitive sites in root cell walls from Al binding by organic acids secreted from the root symplasm may also play an important role in this Al resistance mechanism as the chelation of Al by the same organic acids in the solution bathing the root apex. In a study looking at Al interactions with pectins in root cell walls, it was reported that certain organic acids (citrate, malate) could remove Al bound to cell wall pectins and this possibly could alleviate toxicity, and thus be involved in Al resistance (Wehr et al. 2003). The functions of cell walls are numerous, and include providing tensile strength and limited plasticity for keeping cells from rupturing from turgor pressure, providing mechanical protection from insects and pathogens, and physiological and biochemical activities in the wall that contribute to cell–cell communication. Although attention has been paid to the role of cell wall in response of the cell to changes in its environment (Kinraide 2004), little is known about this role. Here, we present a new theory that the interaction between root exudates and cell wall components plays an important role in plant stress responses, and this seems to be true at least in the response of red clover plants to Fe deficiency (Jin et al. 2007). The theory may open up a new field for molecular/physiological research of plants in response to environmental stresses.
In conclusion, as organic acids (malate, oxalate, citrate) all caused a similar decreasing in Al adsorption to root cell walls in wheat, buckwheat, and C. tora, we hypothesize that the interactions between organic acids secreted and the cell walls play an important role in Al resistance in plants.
Materials and Methods
Plant material and seedling growth
The Al-resistant plant species buckwheat (Fagopyrum esculentum Moench cv. Jiangxi), wheat (Triticum aestivum L. cv. ET8), and Cassia tora L. were used in this study. Seeds were surface-sterilized for 20 min in a 1% (v/v) sodium hypochlorite solution, washed three times with de-ionized water and soaked in de-ionized water overnight. They were then transferred to an incubator at 25 °C for germination. The germinated seeds were transferred to a net tray floating in a container filled with 5 L of 0.5 mM CaCl2 solution at pH 4.5. The solution was renewed daily. The seedlings were grown for an additional 3 d in a growth chamber with a 14 h/26 °C day and a 10 h/23 °C night regime, a light intensity of 250 μmol photon/m2 per s and humidity of 70%. Root tips of 3-d-old seedlings (10 mm long) were excised into mortars containing 75% ethanol solution and immediately placed in an ice-water bath for cell wall materials extraction.
Root growth experiment
Three-day-old seedlings were exposed to 0.5 mM CaCl2 solution (pH 4.5) containing 0, 10, 20, 30, 40, 50, 70, or 100 μM AlCl3. Root length was measured before and after treatment (24 h). The relative root elongation was defined as the percentage of root growth of the Al treatment to that of the control without Al.
To determine the differential amelioration ability of different organic acids to Al toxicity, 3-d-old seedlings were transplanted to 0.5 mM CaCl2 solution (pH 4.5) containing 10, 20, or 50 μM AlCl3 for wheat, C. tora, or buckwheat respectively. The organic acids were added at a different molar ratio to Al. Root length was measured before and after treatment (24 h). The seedlings grown without Al or organic acids were used as the controls.
Collection of root exudates and organic acid assays
Three-day-old seedlings were transplanted into 1.1 L plastic pots (20 wheat seedlings per pot and 40 seedlings for buckwheat and C. tora) filled with 1 L of 0.5 mM CaCl2 solution (pH 4.5) containing 0, 10, 20, or 50 μM AlCl3. Root exudates were collected after 12 h of treatment. Organic acids in root exudates were purified according to Zheng et al. (1998). Malic, citric and oxalic acids in root exudates were assayed according to Delhaize et al. (1993b).
Calculation of root surface area
After collection of root exudates, the roots were subjected to a scanner connected to a computer. The 0–5 mm root apices surface area was analyzed by root image analysis software (WinRHIZO, Régent Instruments, Québec, Canada).
Cell wall preparation
The cell wall was extracted according to Zhong and Läuchli (1993).
Modification of cell wall properties
Cell wall powder (10 mg) was weighted into a 2 mL column equipped with a filter at the bottom and was submerged in 1.5 mL of 0.5 mM CaCl2 solution at pH 4.5 for 2 h. Then the CaCl2 solution was sipped by the peristaltic pump attached, and the cell wall material subjected to further treatments. For the organic acid pre-incubation, 1.5 mL of 0.5 mM CaCl2 (pH 4.5) containing 1 mM malate, oxalate, or citrate was added and incubated at room temperature for 12 h. Then the organic acid solution was sipped away, and the residue was washed with 0.5 mM CaCl2 solution at pH 4.5 at a speed of 6 mL/min for 2 h.
Adsorption and desorption kinetics
The adsorption and desorption kinetics was conducted according to Zheng et al. (2004).
(Handling editor: Biao Ding)
Acknowledgements
The authors would like to thank Professor Leon V. Kochian (US Plant, Soil, and Nutrition Laboratory, USDA-ARS, Cornell University, Ithaca, New York) for his critical reading of the manuscript and giving valuable suggestions.




