Overexpressing HRS1 Confers Hypersensitivity to Low Phosphate-Elicited Inhibition of Primary Root Growth in Arabidopsis thaliana
Supported by the National Natural Science Foundation of China (30521001) and the Ministry of Science and Technology of China (2005CB120904).
Abstract
Phosphate (Pi) deficiency causes dramatic root system architecture (RSA) changes in higher plants. Here we report that overexpression of HRS1 leads to enhanced sensitivity to low Pi-elicited inhibition of primary root growth in Arabidopsis thaliana seedlings. Bioinformatic investigations uncovered that HRS1 and its six homologs encode putative G2-like transcription factors in Arabidopsis. Analysis of promoter::GUS reporter lines revealed that HRS1 transcripts were present mainly in the root hair region and root hair cells under Pi-sufficient conditions. Pi deprivation increased HRS1 expression level and expanded its expression domain. Although HRS1 knockout mutant did not differ from wild type (WT) control irrespective of Pi status, its overexpression lines were significantly more susceptible to low Pi-elicited primary root shortening. In both WT and HRS1 overexpression seedlings, low Pi-induced primary root shortening was accompanied by enhanced root hair cell differentiation, but this enhancement occurred to a greater extent in the latter genotype. Collectively, our data suggest that HRS1 may be involved in the modulation of primary root and root hair growth in Pi-deprived Arabidopsis seedlings, and provide useful clues for further research into the function of HRS1 and its homologs and the mechanisms behind RSA changes under Pi-deficient conditions.
Phosphorus (P) is a major mineral nutrient needed for plant growth. Because the concentration of inorganic phosphate (Pi), which is the main form of phosphorus taken up by plant roots, is low in alkaline calcareous soils, crop plants often face severe Pi shortages (Theodoru and Plaxton 1993). Pi deficiency causes a variety of adaptive responses in plants (Raghothama 1999; Rausch and Bucher 2002; López-Bucio et al. 2003; Franco-Zorrilla et al. 2004; Ticconi and Abel 2004; Amtmann et al. 2006). These responses include upregulation of the biochemical and physiological processes that enhance the recycling, solubilization, uptake and more efficient use of Pi, and reprogramming of the development of aerial and root organs that results in enlarged root/shoot ratio and extensive root system architecture (RSA) changes. Pi deprivation also causes increased accumulation of anthocyanin pigments in the aerial tissues although the physiological function of this event has not been well investigated at present (Ticconi et al. 2001). The low Pi-induced RSA changes in higher plants consist of inhibition of primary root elongation and enhancement of lateral root and root hair growth (Williamson et al. 2001; Linkohr et al. 2002). These developmental modifications are essential for expanding the root surface area and the use of Pi from a wider soil volume. Because of their pivotal importance to plant adaptation to low Pi growth environment, knowledge on the molecular basis of RSA changes is essential for achieving a more complete understanding of the mechanisms underlying plant responses to Pi deficiency.
Owing to the availability of whole genome sequence information and functional genomics resources, Arabidopsis thaliana has been found to be an efficient model for studying the molecular mechanisms regulating plant responses to low Pi stress. Many Arabidopsis genetic loci and genes involved in Pi deprivation responses have been characterized (Yuan and Liu 2008). For example, the primary root of pdr2 mutant is more severely shortened than that of wild type (WT) control (Ticconi et al. 2004). On the other hand, the primary root of lpi mutants is insensitive to low Pi-elicited inhibition (Sánchez-Calderón et al. 2006). The molecular cloning of PDR2 and LPI genes has not been reported. In contrast, the SIZ1 locus has been molecularly cloned and found to encode a SUMO E3 ligase that regulates several important aspects of Pi deficiency responses (Miura et al. 2005). Compared with WT controls, siz1-1 mutant seedlings display exaggerated RSA changes, higher levels of aerial anthocyanin accumulation, and more complex regulation of phosphate starvation inducible (PSI) genes under Pi-limiting conditions. Three LPR quantitative trait loci (QTLs) (LPR1, LPR2, LPR3), with the null mutants of LPR1 and LPR2 being resistant to low Pi-elicited shortening of primary root, have been identified (Reymond et al. 2006; Svistoonoff et al. 2007). LPR1 and LPR2 encode multicopper oxidases, and are expressed in the root tip region including apical meristem and root cap (Svistoonoff et al. 2007). Several more genes, BHLH32, WRKY75 and ZAT6, have been found to affect both biochemical and root developmental aspects of Pi-starved Arabidopsis seedlings (Chen et al. 2007; Devaiah et al. 2007a,b). Finally, the PRD gene, encoding a putative AINTEGUMENTA-like protein, has been shown to regulate the elongation of both primary and lateral roots under Pi-deficient conditions (Camacho-Cristóbal et al. 2008).
From the information described above, it appears highly likely that the regulation of RSA changes in Pi-deprived plants may involve the action of multiple genes. Consequently, the characterization of additional genes participating in this regulation is necessary for more detailed dissection of the molecular basis behind low Pi-induced RSA changes. In the work described in this paper, we found that overexpression of a putative G2-like transcription factor gene (resided in Arabidopsis genomic locus At1g13300) led to hypersensitivity to low Pi-elicited primary root shortening. This gene was therefore named as HRS1. The G2-like transcription factors are characterized by the possession of a DNA binding domain originally defined in the maize Golden2 protein (Rossini et al. 2001). Database searching revealed that, in the Arabidopsis genome, HRS1 has six potential homologs with unknown function. The six genes are tentatively designated as HHO1 (HRS1 Homolog 1) to HHO6 to facilitate their comparisons with HRS1. In the following sections we report bioinformatic analysis of HRS1 and its homologs and genetic and cell biological investigations of HRS1 involvement in the regulation of primary root and root hair growth in Pi-deprived Arabidopsis.
Results
Bioinformatic analysis of HRS1 and homologous genes
In Arabidopsis, there are about 40 genes encoding G2-like transcription factors (Riechmann and Ratcliffe 2000; Riechmann et al. 2000). Except for PHR1 (Rubio et al. 2001), GLKs 1 and 2 (Fitter et al. 2002), APL (Bonke et al. 2003) and KANADIs 1 to 4 (Eshed et al. 2001; Kerstetter et al. 2001; Emery et al. 2003; Eshed et al. 2004; Pekker et al. 2005), there is still no published information on the function of the remaining Arabidopsis G2-like transcription factors. Although the size of the amino acid sequences differs considerably among divergent Arabidopsis G2-like transcription factors, the putative DNA binding domains in these proteins are highly conserved and resemble that of maize G2 protein (Rossini et al. 2001) (Figure 1). In the phylogenetic analysis of Arabidopsis G2-like transcription factors, HRS1 and HHOs 1 to 6 consistently form a distinct clade (Figure 2, http://datf.cbi.pku.edu.cn/family_tree/GARP-G2-like.gif). Interestingly, two closely related protein pairs, one consisting of HRS1 and HHO1 and the other of HHO2 and HHO3, are reproducibly observed in the phylogenetic trees constructed using different tree-building methods (Figure 2, data not shown). The amino acid sequence identities among the seven members are generally above 20%. However, the identity scores are much higher for the closely related pairs: 55% between HRS1 and HHO1 and 52% between HHO2 and HHO3. The genes encoding HRS1 and its six homologs are distributed in four Arabidopsis chromosomes except for chromosome 5. From the microarray data published by Ma et al. (2005) and the data deposited at the AtGenExpress Visualization Tool website (http://jsp.weigelworld.org/), HRS1 and HHO1 appear to be mainly expressed in the roots. HHO2 and HHO3 are constitutively expressed, and their expression profiles seem to be coordinated in both vegetative and reproductive organs. HHO4, HHO5 and HHO6 are also constitutively expressed, with the expression level of HHO5 being relatively higher than that of HHO4 and HHO6.
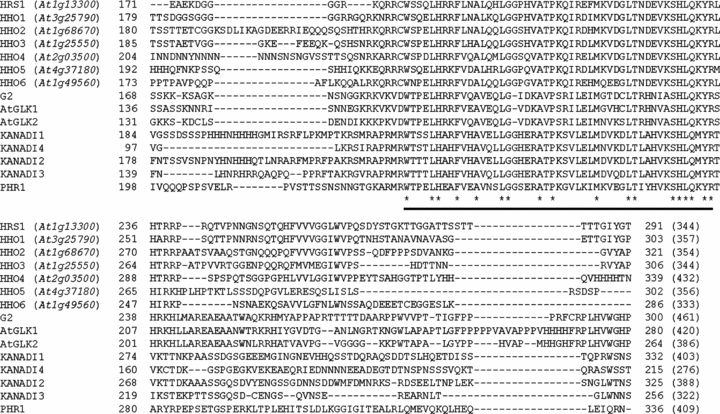
Presence of putative G2-like DNA binding domain in the deduced proteins of HRS1 and homologs (HHOs 1 to 6).The amino acid sequences of HRS1, HHOs 1 to 6, G2 (GenBank accession AF298118), AtGLK1 (GenBank accession AY028367), AtGLK2 (GenBank accession AY028368), KANADI1 (GenBank accession AY048688), KANADI2 (GenBank accession AY048689), KANADI3 (GenBank accession AY048690), KANADI4 (GenBank accession AY048691) and PHR1 (GenBank accession AJ310799) were aligned using the software ClustalW. Due to space constraints, only a portion of the alignment is shown. The numbers immediately before and after the displayed sequences indicate the positions of the aligned elements within their respective proteins. The conserved G2-like DNA binding domain is underlined. The invariant amino acid residues within this domain are marked by asterisks. The Arabidopsis loci encoding HRS1 and homologs are in parentheses. The values in brackets indicate the numbers of residues in the complete proteins of HRS1, HHOs 1 to 6, G2, AtGLKs 1 and 2, KANADIs 1 to 4 and PHR1, respectively.
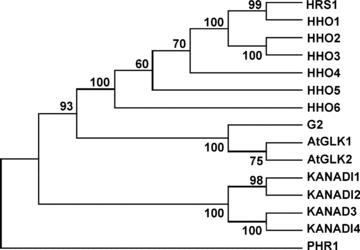
Phylogenetic relationships of HRS1 and homologs (HHOs 1 to 6) to G2 and representative G2-like transcription factors (AtGLKs 1 and 2, KANADIs 1 to 4, PHR1) from Arabidopsis.HRS1 and homologs constitute a distinct group of closely related proteins in the phylogenetic tree shown here, which was constructed using the neighbor-joining method. The bootstrap values displayed were calculated based on 500 replications. Highly similar phylogenetic trees were obtained using other tree-building methods (i.e. Unweighted Pair Group Method with Arithmetic Mean [UPGMA], minimum evolution, maximum parsimony). The tight clustering of HRS1 and homologs is also observed in the phylogenetic analysis involving all Arabidopsis G2-like transcription factors (http://datf.cbi.pku.edu.cn/family_tree/GARP-G2-like.gif).
Analysis of HRS1 expression pattern using promoter::GUS reporter lines
Three independent homozygous promoter::GUS reporter (pHRS1::GUS) lines were prepared to analyze the expression pattern of HRS1 by histochemical GUS staining. Examinations of GUS signals in these lines confirmed that HRS1 was predominantly transcribed in the roots of Arabidopsis plants when grown with sufficient Pi (500 μM Pi, Figure 3A), although a low level of HRS1 expression was also found in the petioles of senescent leaves (Figure 3A, arrowed). To study the expression pattern of HRS1 in more detail, seedlings of the three reporter lines, cultured with 500 and 5 μM Pi, respectively, were examined. The GUS signals were detected mainly in the seedling roots irrespective of Pi supply levels (data not shown). Closer inspections revealed that, under 500 μM Pi, HRS1 transcription occurred in the root hair region of primary root and root hair cells, but was not detected in either the elongation or cell division zones of primary root (Figure 3B). At low Pi concentration (5 μM), HRS1 transcription was strongly upregulated in both the differentiated primary root tissues and root hair cells (Figure 3C), and was induced de novo in the elongation zone (Figure 3C). The primary root grown with 5 μM Pi was cross-sectioned in order to view the distribution of GUS signals in different cell types. In the section derived from the root hair zone, strong GUS signals were present in all epidermal and cortex cells but absent from the inner cell types (Figure 3D). In the section derived from the elongation zone, GUS signals were mainly found in the H cells (Figure 3E), which are the progenitors of root hair cells (Ishida et al. 2008).
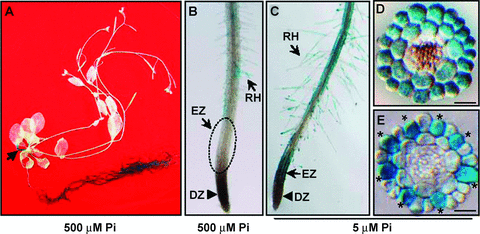
Analysis of HRS1 expression patterns using promoter::GUS reporter transgenic lines.HRS1 expression is indicated by the blue GUS signals. The results shown are representative of four separate experiments involving three independent homozygous promoter::GUS transgenic lines.(A)HRS1 expression pattern in a 6-week-old Arabidopsis plant grown with 500 μM Pi. GUS signals were mainly located in the root tissues, although a small amount of them was also found in the petioles of senescent leaves (arrowed). Similar GUS staining pattern was obtained for 2-week-old seedlings (data not shown).(B) GUS signals in the primary root of a 10-d-old seedling grown with high (500 μM) Pi supply. GUS signals were mainly found in the differentiated region of primary root and root hair cells (RH). No GUS signals were found in the elongation zone (EZ, marked with a circle), or the cell division zone (DZ).(C) GUS signals in the primary root of a 10-d-old seedling grown with low (5 μM) Pi supply. Strong GUS signals were found in the root hair zone, EZ, and RH cells.(D) A representative cross section derived from the root hair zone of a 10-d-old seedling grown with low (5 μM) Pi supply. GUS signals were found in both the epidermal (first layer) and cortex (second layer) cells.(E) A representative cross section derived from the elongation zone of a 10-d-old seedling grown with low (5 μM) Pi supply. GUS signals were mainly found in the H cells (marked by asterisks) of the epidermal layer. Scale bars in D and E: 50 μM.
Responses of HRS1 overexpression and knockout lines to Pi deprivation
Four independent and homozygous overexpression lines (p35S::HRS1 lines L3, L11, L27 and L46) and one homozygous knockout line (hrs1-1) were developed for HRS1 (Figure 4, top panel). Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) experiments showed that the overexpression lines contained much higher levels of HRS1 transcripts than the WT controls (Figure 4A). By contrast, HRS1 transcripts were absent from the knockout line hrs1-1 (Figure 4B, right panel). The seeds of WT and HRS1 overexpression and knockout lines were germinated directly on the Murashige and Skoog (MS) media with different Pi concentrations. Low Pi supplies (≤ 20 μM) inhibited primary root elongation in all three genotypes (Figure 4C). However, the inhibition of primary root elongation was consistently and significantly greater for p35S::HRS1 than for WT or hrs1-1 seedlings at all three low Pi concentrations (Figure 4C). Inhibition of primary root elongation was also observed at 100 μM Pi in p35S::HRS1 seedlings, by which concentration the primary root elongation in WT and hrs1-1 seedlings was no longer affected (Figure 4C). The hrs1-1 mutant behaved similarly to the WT controls in primary root growth in the range of Pi concentrations examined in this work (Figure 4C). Except for Pi deficiency, p35S::HRS1 seedlings and WT controls did not differ significantly in primary root growth on the media deficient in potassium, nitrogen, sulfur or iron (Figure 4D).
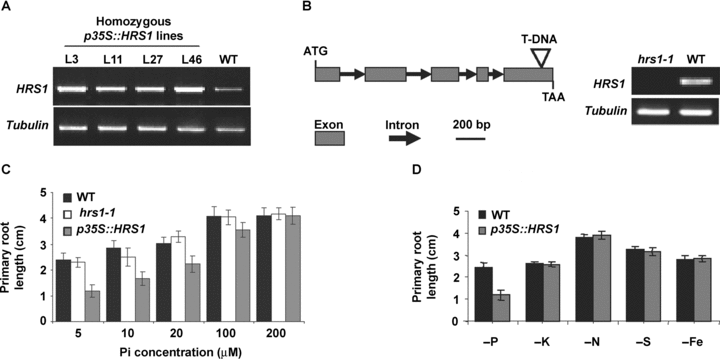
Responses of HRS1 mutants to Pi deprivation.(A) Comparisons of HRS1 transcript levels in four homozygous and independent p35S::HRS1 overexpression lines (L3, L11, L27, L46) to that of wild type (WT) control by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR)analysis. The amplification of tubulin transcripts served as an internal control. The results shown are representative of three independent evaluations.(B) Identification of HRS1 knockout mutant hrs1-1. A T-DNA insertion was found in the last exon of the genomic open reading frame of HRS1 in the Salk_067074 line from which hrs1-1 was identified. Semiquantitative RT-PCR analysis using HRS1 specific primers showed the absence of HRS1 transcripts in hrs1-1 but not WT control. Tubulin transcripts were amplified as an internal control.(C) Comparisons of the primary root length of WT, HRS1 knockout (hrs1-1) and p35S::HRS1 (line L3) seedlings on the media with different Pi levels. Primary root length was measured using 8-d-old seedlings germinated directly on the media with the indicated Pi concentrations. The mean values and error bars displayed were calculated using the data from three independent analyses. The other three p35S::HRS1 lines (L11, L27, L46) behaved similarly to line L3 in showing enhanced sensitivity to the low Pi-induced primary shortening (data not shown).(D) The HRS1 overexpression (p35S::HRS1, line L3) seedlings exhibited greater inhibition of primary root elongation under the deficiency of phosphorus (P) but not that of potassium (K), nitrogen (N), sulfur (S) or iron (Fe). The mean values and error bars were calculated using the data from three independent growth trails. Highly similar results were obtained when other p35S::HRS1 lines (L11, L27, L46) were used for the analysis (data not shown).
The hypersensitive phenotype of p35S::HRS1 lines to Pi deprivation-induced primary root shortening was further tested by transferring the seedlings grown with sufficient Pi onto the low Pi media. Three days after germination on the medium with 500 μM Pi, WT and p35S::HRS1 seedlings were transferred onto the MS media containing 5, 50 and 500 μM Pi, respectively. After a further growth for 9 d, dramatic differences were observed between WT and p35S::HSR1 seedling roots on low (5 or 50 μM), but not high (500 μM), Pi media (Supplemental material). Quantitative analysis showed that for WT seedlings the average length of primary roots under 5 or 50 μM Pi was approximately 46% or 61% of that attained in the presence of 500 μM Pi (Supplemental material). By contrast, for p35S::HRS1 seedlings, the average length of primary roots under 5 or 50 μM Pi was only about 20% or 31% of that in the presence of 500 μM Pi (Supplemental material). However, other Pi deficiency responses, including enhanced lateral root growth and anthocyanin accumulation in the aerial tissues, were not compromised in the p35S::HRS1 seedlings grown on low Pi media (Supplemental material). These observations, plus the data depicted in Figure 4C, demonstrated clearly that the overexpression mutants of HRS1, but not its knockout line, were more susceptible to the low Pi-induced inhibition of primary root growth than the WT Arabidopsis control.
Comparisons of root hair cell development and root tip length in WT and p35S::HRS1 seedlings
Previous investigation has shown that the inhibition of primary root elongation by Pi deprivation is associated with diminished mitotic activity in the cell division zone, which is preceded by the reduction of both cell size and number in the elongation zone (Sánchez-Calderón et al. 2005). In this work, we confirmed the previous findings. Furthermore, we observed that, as early as 2 d after germination (dag) on the low Pi medium, there were already more root hair cells developed towards the root tip region (including the elongation zone, cell division zone and root cap) in p35S::HRS1 seedlings than in the WT controls (Figure 5A), and as a result, the length of the root tip region in the p35S::HRS1 seedlings was generally much shorter than that in the WT controls (Figure 5A). Interestingly, at this time, the shortening of primary root and other RSA change (i.e. increased lateral root growth) were still not apparent in either genotype (Figure 5A). Consistent with the above observation, more systematic quantitative comparisons revealed that, on the medium containing 500 μM Pi, the length of the root tip increased significantly from 4 to 8 dag for both WT and p35S::HRS1 seedlings (Figure 5B). By contrast, under low Pi levels (5 or 50 μM Pi), root tip length decreased substantially for both genotypes during the same observation period (Figure 5B). However, the decrease exhibited by p35S::HRS1 seedlings was much larger than that of WT controls, which was especially evident on the medium with 5 μM Pi (Figure 5B).
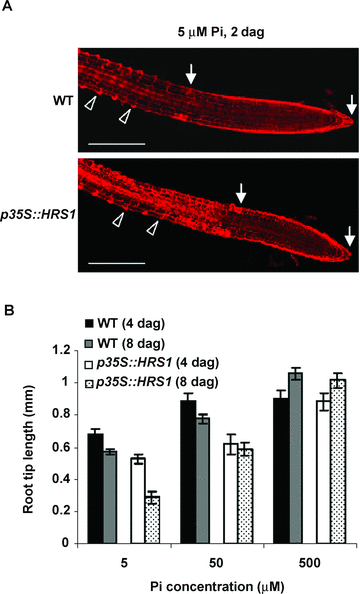
Root hair cell development in wild type (WT) and HRS1 overexpression (p35S::HRS1, line L3) seedlings under Pi-limiting conditions.(A) WT and p35S::HRS1 seeds were germinated directly on the medium containing 5 μM Pi. The seedling roots were examined using confocal microscopy at 2 d after germination (dag). Arrowheads indicate developing root hair cells, which characteristically contain localized bulges in the cell wall. The root tip region (consisting of the elongation zone, cell division zone and root cap) is marked by two arrows. The p35S::HRS1 seedlings exhibited more extensive root hair cell development towards the elongation zone than the WT controls, which was associated with a much smaller root tip length in the former genotype. The results are representative of three independent experiments. Scale bars: 200 μm. (B) Changes of root tip length in WT and p35S::HRS1 seedlings from 4 to 8 dag on low (5 or 50 μM) or high (500 μM) Pi media. The mean values and error bars were determined using the data from three independent growth trials. Results, highly similar to those depicted in (A) and (B), were obtained in repeated growth assays using the additional p35S::HRS1 lines (L11, L27) (data not shown).
Discussion
Pioneering studies have shown that G2-like transcription factor genes play important roles in the growth and development of higher plants and their responses to adverse environmental conditions. For example, the GLK gene pairs are required for normal chloroplast development in both dicot and monocot plant species (Rossini et al. 2001; Fitter et al. 2002). The four KANADI genes are involved in the establishment of polarity in lateral organs (Eshed et al. 2001, 2004; Kerstetter et al. 2001; Emery et al. 2003; Pekker et al. 2005). APL is essential for the control of phloem identity (Bonke et al. 2003), and PHR1, an important regulator of plant Pi deficiency responses (Rubio et al. 2001), is indispensable for the expression of several Pi starvation inducible genes. In contrast, functional studies of HRS1 and its six homologs, representing a distinct group of G2-like transcription factor genes, have not been reported previously. The seven members may play active roles in Arabidopsis growth and development, because they are expressed in one or more vegetative and reproductive organs (Ma et al. 2005, Figure 3). As the first step towards studying the function of the seven genes, the expression pattern of HRS1 and its involvement in regulating Arabidopsis primary root growth under Pi-limiting conditions were investigated in this work.
Based on the analysis of multiple promoter::GUS reporter lines in this work, it is clear that HRS1 is mainly expressed in the root tissues under either normal or low Pi growth conditions, which is consistent with the data published previously (Ma et al. 2005; http://jsp.weigelworld.org/). However, following Pi deprivation treatment, HRS1 expression levels in the root hair zone and root hair cells were upregulated (Figure 3). Moreover, HRS1 expression domain was also expanded into the elongation zone by Pi deprivation (Figure 3). These findings suggest potential involvement of HRS1 in regulating the growth and/or function of roots and root hairs in Pi-deprived Arabidopsis. However, HRS1 itself is unlikely to be a major controller because its knockout mutant did not differ from WT control in root growth under either normal or Pi-deficient conditions (Figure 4). One possibility is that HRS1 may act redundantly with its homologs in the regulation of root growth under low Pi conditions. Functional cooperation and redundancy have frequently been found in the studies of closely related G2-like transcription factor genes. For instance, the single gene knockout mutants of the GLK gene pair do not differ significantly from WT controls in growth and development, whereas the double mutant of this gene pair displays severe defects in chloroplast function and leaf development (Fitter et al. 2002). Similarly, the single gene knockout mutants of four KANADI genes do not obviously affect the establishment of polarity in lateral organs, but the double or triple mutants of these genes exhibit developmental abnormalities in all lateral organs (Kerstetter et al. 2001; Eshed et al. 2001, 2004). In view of these precedents, it will be essential in the future to develop mutants lacking HRS1 and one or more of its homologs and to examine their root growth under both normal and Pi-deprived conditions. Of particular interest will be the double mutants that are defective in the expression of HRS1 and HHO1 or HHO2 and HHO3, because there are higher similarities in the expression patterns and amino acid sequences in between the members of the two gene pairs.
In agreement with previous studies (Raghothama 1999; Rausch and Bucher 2002; Amtmann et al. 2006), this work found that Pi limitation has multiple effects on root development, including stimulation of root hair cell differentiation and lateral root development, reduction of root tip length, and inhibition of primary root elongation (Figure 4, supplemental material). However, compared with WT controls, the reduction of root tip length occurred significantly more strongly in Pi-deprived p35S::HRS1 seedlings (Figure 5). Importantly, the more severe reduction of root tip length in Pi-deprived p35S::HRS1 seedlings was accompanied by a more pronounced stimulation of root hair cell development, which preceded primary root shortening (Figure 5). Collectively, these observations lead us to hypothesize that HRS1 may be involved in the control of root hair cell development and that, at least in p35S::HRS1 seedlings, enhanced root hair cell development may contribute to the shortening of root tip region and primary root under Pi-deficiency conditions. This suggestion is consistent with the finding of abundant HRS1 expression in the H cells, which give rise to root hairs after further development (Ishida et al. 2008), under Pi-limiting conditions (Figure 3E). HRS1 may also be involved in more broad aspects of root growth and/or function than just the stimulation of root hair cell development, because it is also expressed in the differentiated root tissues and root hairs under Pi-sufficient conditions and its expression level is substantially increased in the roots and root hairs upon Pi deprivation treatment. As discussed above, HRS1 may interact with one or more of its homologs during its action. Consequently, further comparative analysis of WT and p35S::HRS1 seedlings under low Pi conditions may provide useful clues on the function of this unique group of G2-like transcription factor genes.
Compared with previously characterized Arabidopsis mutants, there is some similarity between the phenotype of HRS1 overexpression lines and that of the knockout mutant (siz1-1) of SIZ1 under Pi-deficient conditions. However, SIZ1 may be a more global regulator, because Pi-deprived siz1-1 seedlings display additional phenotypes (i.e. greater anthocyanin accumulation and more distorted regulation of the expression of several Pi starvation inducible genes) except for stronger reduction of primary root growth (Miura et al. 2005). Interestingly, PHR1 is a sumoylation target of the SIZ1 enzyme reaction, which is necessary for the regulation of AtIPS1 and AtRNS1 expression by PHR1 in Pi-deprived Arabidopsis (Miura et al. 2005). Considering that the products of HRS1 and its homologs resemble that of PHR1 in possessing the putative G2-like DNA binding domain, it will be interesting to test if HRS1 and its homologous proteins may also be targets of SIZ1-catalyzed sumoylation reaction in further investigations. The phenotype of pdr2 also shows resemblance to that of HRS1 overexpression lines. However, pdr2 is unlikely to be caused by mutations in HRS1 or its homologs, because pdr2 locus has been mapped to chromosome 5 (Ticconi et al. 2004), and HRS1 and its homologs are not located on this chromosome. Nevertheless, it is important to investigate whether HRS1 and PDR2 may act in the same genetic pathway to affect primary root growth in Pi-deprived Arabidopsis in future research. Contrary to siz1-1 and pdr2, the lpi mutants and the loss-of-function alleles of LPR1 show resistance to low Pi-elicited inhibition of primary root shortening (Reymond et al. 2006; Sánchez-Calderón et al. 2006). At present, it is not clear whether there are physical correspondences among LPI genes, HRS1 and HRS1 homologs, because the chromosomal locations and molecular characterization of lpi mutations have not been reported. Svistoonoff et al. (2007) report that strong LPR1 expression in the root cap tissue is required for the control of primary root length in low Pi-grown Arabidopsis seedlings by LPR1. From this finding, we deduce that LPR1 and HRS1 are unlikely to interact directly in the modulation of primary root growth in Pi-deprived Arabidopsis, because HRS1 expression was not detected in the root cap (Figure 3). In addition to the Arabidopsis loci and genes discussed above, it may also be worthwhile to investigate whether there are functional interactions between HRS1 and BHLH32, WRKY75, ZAT6 or PRD in future studies, as the latter four genes have also been found to affect root growth in Arabidopsis under low Pi conditions (Chen et al. 2007; Devaiah et al. 2007a,b; Camacho-Cristóbal et al. 2008). Finally, because Ward et al. (2008) have recently demonstrated that iron toxicity could be responsible for low Pi-elicited primary root shortening in Arabidopsis, it becomes necessary to analyze the effect of iron status on the phenotype of p35S::HRS1 seedlings on Pi-limiting growth medium in further research. This may yield useful information on the physiological basis behind the enhanced inhibition of primary root growth in p35S::HRS1 seedlings under Pi-deficient conditions.
In summary, this work has generated new information on the expression patterns of HRS1 under normal or Pi-limiting conditions and on the responses of HRS1 knockout and overexpression mutants to Pi deprivation treatment. The upregulation of HRS1 expression (particularly the de novo induction of HRS1 in root hair progenitor cells) by Pi limitation, plus the enhanced primary root shortening phenotype of p35S::HRS1 seedlings on low Pi medium, suggest that HRS1 may be involved in the reprogramming of root development under Pi-deficient conditions. This provides a useful direction for further investigations on HRS1 and its homologs, which comprise a group of poorly-investigated G2-like transcription factor genes in Arabidopsis. Continued studies of HRS1 and its homologs, together with those on SIZ1, PDR2, LPI, LPR, BHLH32, WRKY75, ZAT6 and PRD genes (see above), will yield a more complete understanding of the molecular genetic and cell biological basis governing low Pi-induced RSA changes.
Materials and Methods
Plant growth conditions and general molecular methods
The Col-0 ecotype of Arabidopsis thaliana was used in this work. Arabidopsis was grown in a controlled environment (22 °C, 100–130 μEm−2 s−1 light intensity, 16 h light/8 h dark photoperiod). Seeds were surface-sterilized and cold-stratified as described previously (Weigel and Glazebrook 2001). MS medium (supplemented with 1% sucrose and solidified with 0.8% agar) was used routinely for Arabidopsis growth. The Pi concentration in the medium was carefully adjusted according to the requirements of the experiments. Pi was provided by the addition of KH2PO4, with KCl amount altered accordingly to maintain a constant K+ concentration in the modified medium. Based on previous investigations (Ticconi et al. 2004) and our own observations, the presence of 500 μM Pi in the MS medium was sufficient for supporting the vegetative growth of Arabidopsis seedlings. However, Arabidopsis seedlings exhibited clear Pi starvation responses (i.e. reduced shoot growth, accumulation of anthocyanin pigments in the aerial organs, inhibition of primary root elongation, increased lateral root growth) on the media containing 50 μM or less Pi. Consequently, in this work, Pi deprivation treatments were augmented by growing WT and mutant Arabidopsis lines on the media with 50 μM or less Pi. Two approaches were used to investigate the growth behavior of WT and mutant Arabidopsis lines, one by direct germination of the seeds on the media containing different Pi concentrations and the other involving the transfer of the seedlings grown from Pi-sufficient medium to the plates with low Pi levels. For comparing the primary root elongation of WT and mutant Arabidopsis materials on the media lacking potassium (K), nitrogen (N), sulfur (S) or iron (Fe), the methods detailed by Sánchez-Calderón et al. (2006) were followed.
The basic molecular methods used in this work were described previously (Sambrook and Russell 2001). The accuracy of DNA cloning experiments was always checked by restriction enzyme digestion analysis and automatic DNA sequencing. High fidelity Taq DNA polymerases (LA-Taq or Pfu, TaKaRa, Japan) were used in all PCR experiments. The gene-specific oligonucleotide primers used in this work are all listed in Table 1.
| Primer | Primer sequence† | Use |
|---|---|---|
| HRS1-F | 5′-TTA CTCGAG ATG ATT AAA AAG TTC AGC-3′ | Amplification of HRS1 coding region cDNA |
| HRS1-R | 5′-TTA GGATCC TTA ATT ATT CTT GAC GTA-3′ | |
| HRS1 promoter-F | 5′-AAT GAGCTC AGC TAG TTT CGA GTT CAA GCT AG-3′ | Amplification of HRS1 promoter |
| HRS1 promoter-R | 5′-ATT AAGCTT GAT GAT ACT TTA GGG ACT TAA TTT-3′ | |
| Salk_067074-LP | 5′-GAC GCT TCT TGA ACG CTC TTC-3′ | Identification of HRS1 knockout mutant hrs1-1 |
| Salk_067074-RP | 5′-GTT GAG ATA GTT AGA TCC GTT-3 | |
| RT-HRS1-F | 5′-GAC GCT TCT TGA ACG CTC TTC-3′ | Semiquantitative RT-PCR |
| RT-HRS1-R | 5′-TTA ATTA TTC TTG ACG TAA TG-3′ | |
| Tubulin-F | 5′-AGA ACA CTG TTG TAA GGC TAA AC-3′ | |
| Tubulin-R | 5′-GAG CTT TAC TGT CTC GAA CAT GG-3′ |
- †The underlined nucleotides form BamHI (GGATCC), HindIII (AAGCTT), SacI (GAGCTC) or XhoI (CTCGAG) restriction sites. RT-PCR, reverse transcription-polymerase chain reaction.
Bioinformatic investigations of HRS1 and homologs
The genomic positions, gene structures, promoter regions, genomic and cDNA sequences, and derived amino acid sequences of HRS1 and homologous genes (HHO1 to HHO6) were retrieved from The Arabidopsis Information Resource (TAIR) website (http://www.arabidopsis.org/). The information on the classification of HRS1 and homologs as G2-like transcription factor genes was obtained from several Arabidopsis transcription factor databases (e.g. Arabidopsis Transcription Regulators [AraTRs] at http://genetics.mgh.harvard.edu/sheenweb/AraTRs.html, Arabidopsis Transcription Factor Database [ArabTFDB] at http://arabtfdb.bio.uni-potsdam.de/v1.1/, Arabidopsis Gene Regulatory Information Server [AGRIS] at http://arabidopsis.med.ohio-state.edu/AtTFDB, Database of Arabidopsis Transcription Factors [DATF] at http://datf.cbi.pku.edu.cn/, and RIKEN Arabidopsis Transcription Factor database [RARTF] at http://rarge.gsc.riken.jp/rartf/). To delineate the putative G2-like DNA binding domain in the deduced proteins of HRS1 and homologous members, multiple amino acid sequence comparisons were conducted. For this purpose, the amino acid sequences of G2 and the representative Arabidopsis G2-like transcriptional factors (AtGLK1, AtGLK2, KANADI1, KANADI2, KANADI3, KANADI4, PHR1) were retrieved from GenBank. The recognition of G2-like DNA binding domain in the aligned sequences was carried out as described previously (Rossini et al. 2001; Fitter et al. 2002; http://arabtfdb.bio.uni-potsdam.de/v1.1/). For investigating the phylogenetic relationships of HRS1 and homologs with G2 and representative Arabidopsis G2-like transcription factor genes, their deduced amino acid sequences were aligned and used to construct phylogenetic trees at the Molecular Evolutionary Genetics Analysis (MEGA) website (http://www.megasoftware.net/) by four different tree building methods (Unweighted Pair Group Method with Arithmetic Mean [UPGMA], neighbor-joining, minimum evolution, maximum parsimony). The evolutionary relationships between the aligned sequences were estimated by calculating both P and PC distances. The bootstrap values were obtained based on 500 replications.
Preparation of promoter::GUS reporter, overexpression and knockout lines
The 5′ flanking sequence of HRS1 (1617 bp) was amplified by genomic PCR and used to replace the 35S promoter in the pJIT166 vector (http://www.pgreen.ac.uk/) that contains the coding region for the bacterial β-glucuronidase (GUS). The resulted HRS1::GUS cassette was transferred into the T-DNA vector pBinPlus (van Engelen et al. 1995), creating the transformation construct pBinHRS1::GUS. This construct was subsequently used to transform WT Arabidopsis plants using the floral dip method (Clough and Bent 1998). Three homozygous HRS1 promoter::GUS reporter transgenic lines were selected in the T3 generation and used in the subsequent experiments.
The cDNA coding sequence of HRS1 (GenBank accession AY124865) was amplified by RT-PCR and inserted downstream of the 35S promoter in the plasmid vector pBPFO7. The resulted 35S expression cassette was further cloned into pBinPlus, resulting in the transformation construct pBin35S::HRS1, which was then used for developing HRS1 overexpression lines as described above. Four homozygous overexpression lines, designated as p35S::HRS1 lines L3, L11, L27 and L46, respectively, were used in the subsequent experiments.
A homozygous HRS1 knockout mutant (hrs1-1) was identified from the T-DNA insertion line Salk_067074 (supplied by ABRC, http://www.biosci.ohio-state.edu/) using HRS1-specific primers (Table 1) and the methods deposited in the SIGnAL website (http://signal.salk.edu/).
HRS1 transcript levels in the homozygous overexpression and knockout lines were evaluated using semiquantitative RT-PCR experiments. Briefly, Total RNA samples were prepared from the roots of 3-week-old plants and reversely transcribed into cDNAs following published methods (Li et al. 2003; Liu et al. 2006). The cDNA contents of the different reverse transcription reactions were normalized by amplifying tubulin transcripts. The evaluation of HRS1 transcript levels in the different mutant lines were conducted using the normalized cDNA samples with HRS1 specific primers (Table 1).
Histochemical staining of GUS
Histochemical GUS staining was conducted as detailed previously (Jefferson 1987). To check the reproducibility of the GUS localization results, multiple staining experiments were executed using three independent homozygous HRS1 promoter::GUS reporter lines. After GUS staining, the root segments derived from p35S::HRS1 seedlings grown with 5 μM Pi were fixed with 1% glutaraldehyde and 4% formaldehyde, followed by embedding in plastic resins (Mission et al. 2004). Cross sections were prepared from defined root regions, and examined for GUS signals as reported in previous publications (Scheres et al. 1994; Mission et al. 2004).
Microscopy
Light microscopy was conducted under a Leica dissecting microscope (MZ16 FA, Germany) equipped with a digital camera. Fluorescent microscopy of propidium iodide-stained Arabidopsis roots was carried out with an Olympus confocal microscope (FV500, Japan) according to previously published methods (Wysocka-Diller et al. 2000).
(Handling editor: Kang Chong)
Acknowledgements
The authors wish to thank Professor Dong Liu (Tsinghua University, China) for constructive comments on the manuscript.




