Alternative Splicing and Differential Expression of Two Transcripts of Nicotine Adenine Dinucleotide Phosphate Oxidase B Gene from Zea mays
Supported by the State Key Basic Research and Development Plan of China (2003CB114302) and the National Natural Science Foundation of China (30571122).
Abstract
With the exception of rice, little is known about the existence of respiratory burst oxidase homolog (rboh) gene in cereals. The present study reports the cloning and analysis of a novel rboh gene, termed ZmrbohB, from maize (Zea mays L.). The full-length cDNA of ZmrbohB encodes a 942 amino acid protein containing all of the respiratory burst oxidase homolog catalytically critical motifs. Alternative splicing of ZmrbohB has generated two transcript isoforms, ZmrbohB-α and -β. Spliced transcript ZmrbohB-β retains an unspliced intron 11 that carries a premature termination codon and probably leads to nonsense-mediated mRNA decay. Expression analysis showed that two splice isoforms were differentially expressed in various tissues and at different developmental stages, and the major product was ZmrbohB-α. The transcripts of ZmrbohB-α accumulated markedly when the maize seedlings were subjected to various abiotic stimuli, such as wounding, cold (4 °C), heat (40 °C), UV and salinity stress. In addition, several abiotic stimuli also affected the alternative splicing pattern of ZmrbohB except wounding. These results provide new insight into roles in the expression regulation of plant rboh genes and suggest that ZmrbohB gene may play a role in response to environmental stresses.
Nicotine adenine dinucleotide phosphate oxidase (NOX) is an enzyme that catalyzes the production of superoxide (O2•−) by transferring electrons from nicotine adenine dinucleotide phosphate (NADPH) to molecular oxygen, with secondary generation of H2O2. Reactive oxygen species (ROS) generated by NOX have been shown to play crucial roles in biotic interactions, abiotic stress and development in higher plants (Torres and Dangl 2005; Sagi and Fluhr 2006; Carter et al. 2007). Plant NOXs, termed RBOH (respiratory burst oxidase homolog), are predicted to contain cytosolic flavin adenine dinucleotide- (FAD) and NADPH-binding domains and six conserved transmembrane helices that correspond to those identified in the mammalian NOX catalytic subunit gp91phox (phox for phagocyte oxidase) and to carry an N-terminal extension comprising two Ca2+-binding EF-hand motifs (Keller et al. 1998; Sagi and Fluhr 2006).
Plant rboh genes were first isolated from rice (Oryza sativa) (Groom et al. 1996), and then identified in various plant species, such as Arabidopsis thaliana (Keller et al. 1998; Torres et al. 1998), tobacco (Nicotiana benthamiana) (Yoshioka et al. 2003), and potato (Solanum tuberosum) (Yoshioka et al. 2001; Yamamizo et al. 2007). Recent studies have shown that activation of particular RBOH isoforms is responsible for ROS accumulation during biotic and abiotic stresses. In Arabidopsis, 10 rboh genes are known, and AtrbohD and AtrbohF play a role in ROS production in response to avirulence pathogens (Torres et al. 2002). A recent result demonstrates that the oxidative burst generated by these enzymes suppresses the spread of cell death by antagonizing salicylic acid-dependent pro-death signals (Torres et al. 2005). Guard cell NOXs encoded by AtrbohD and AtrbohF genes are implicated in the intercellular ROS signaling and cell death that arises from ozone exposure (Joo et al. 2005). These two rboh genes are also required for the abscisic acid (ABA)-induced stomatal closure (Kwak et al. 2003). In tobacco, NtrbohD from N. tabacum, and NbrbohA and NbrbohB from N. benthamiana are shown to be required for ROS accumulation following perception of pathogen signals (Simon-Plas et al. 2002; Yoshioka et al. 2003). Likewise, tomato (Lycopersicon esculentum) plants transformed with antisense constructs of rboh show a reduced level of ROS in the leaf, and are compromised in wound-induced gene expression (Sagi et al. 2004). Furthermore, NOX activity also plays a role in regulation of plant development. NOX-mediated H2O2 synthesis is implicated in ABA-induced seed germination and root elongation in Arabidopsis (Kwak et al. 2003). During root hair development, a requirement for ROS is demonstrated in A. thaliana using a knockout mutant in AtrbohC/RHD2 (ROOT HAIR DEFECTIVE 2) (Foreman et al. 2003). Analysis of the AtrbohC/RHD2 mutant reveals that ROS produced by AtRbohC are responsible for localized cell expansion during the root hair growth (Foreman et al. 2003), and a positive feedback mechanism involving AtRbohC, ROS, and Ca2+ can determine cell shape (Takeda et al. 2008). Moreover, suppression of tomato rboh gene expression by the antisense approach induces a wide range of developmental abnormalities, and results in ectopic expression of flower-specific homeotic genes (Sagi et al. 2004). These reports show that plant NOX isoforms have different functions and participate in multiple distinct signaling pathways. Complexity in specific roles and in regulation of these enzymes is supported by different tissue distribution, variation in gene expression and amounts of superoxide produced by NOX isoforms.
In order to obtain more information on the function and regulatory patterns of rbohs, it is necessary to isolate and characterize more rboh genes from different species. The molecular and biochemical characterization of rboh genes in plants has been studied extensively, but, most studies are carried out in dicot plants such as Arobidopsis, tobacco and potato. Maize (Zea mays) is a model plant and economically important species. The rbohs, however, have not been reported yet. In the present study, an rboh gene named as ZmrbohB from maize was cloned and characterized. Our data showed that there is alternative splicing in the coding region of the rboh gene. The accumulation of ZmrbohB mRNA after several abiotic stress treatments was also investigated. Our results provide new insight into roles in the expression regulation of plant rboh genes.
Results
Cloning and sequence analysis of ZmrbohB cDNA
Based on the conserved regions of plant rboh genes, degenerate primers Pzrb3 and Pzrb4 were designed and synthesized for the amplification of the middle region of ZmrbohB. A 1360 bp fragment with high sequence identity to the maize Unigene CL562_1 was obtained. The Unigene CL562_1 shares high nucleotide sequence similarity with plant rbohs and contains a stop codon (TGA). Then it was identified by polymerase chain reaction (PCR). Several reverse specific primers designed according to the obtained region were used for the amplification of the 5′ upstream cDNA region of ZmrbohB by the 5′ rapid amplification of cDNA ends (RACE) method, and uncovered the 1154 bp sequence containing a translation start codon (ATG). After analysis of the corresponding fragments, which overlapped each other in, a 3423 bp ZmrbohB transcript was deduced and had an open reading frame of 2829 nucleotides, encoding a protein of 942 amino acids with a predicted molecular mass (MW) of approximately 106.3 kDa and a pI of 9.24.
The deduced amino acid sequence of ZmRbohB contained catalytically critical motifs, including two EF-hand motifs, FAD and NADPH binding sites (Figure 1), which are conserved in plant NOXs from many species (Sagi and Fluhr 2006). The topology analysis showed that six transmembrane-spanning domains (TMD1-6), usually identified in human gp91phox and plants NOXs, were also conserved in the ZmRbohB sequence (Figure 1). Likewise, TMD3 and TMD5 contained pairs of histidine residues that are important for heme binding in human gp91phox (Finegold et al. 1996) (Figure 1). Moreover, human gp91phox amino acid residues Pro-415 and Asp-500, which are indispensable for the catalytic activity (Segal et al. 1992), were also conserved in ZmRbohB (Figure 1). In all Nox isoforms, the first NADPH-ribose binding domain (GXGXXP) is followed by a phenylalanine residue, which is typical of NADPH-rather than NADH-specific enzymes (Cheng et al. 2001). In the ZmRbohB sequence, this phenylalanine residue was also found (Figure 1).

Alignment of the predicted amino acid sequences of ZmRbohB, OsRbohA, OsRbohC and AtRbohF.Multiple alignments of the predicted ZmRbohB, OsRbohA, OsRbohC and AtRbohF proteins was made with the CLUSTALW program. A line above the alignment is used to mark strongly conserved positions. Three characters (‘*’, ‘:’ and ‘.’) are used. Dashes indicate gaps in the sequence to allow for maximal alignment. Six potential transmembrane-spanning domains (TMD 1 to TMD 6) are indicated with overlines. Histidine residues involved in heme binding are boxed in gray scale. Solid triangles under the sequences indicate amino acid residues that are required for the human nicotine adenine dinucleotide phosphate (NADPH) oxidase function and conserved between gp91phox and the Rboh proteins (Segal et al. 1992; Torres et al. 1998). EF hand motifs in the N-terminal domain of Rbohs are overlined. Beneath these motifs is the sequence of a canonical helix E-loop-helix F (Kretsinger 1996). n is usually a hydrophobic residue. Dashed indicate variable amino acid residues. X, Y, Z, and -X, contain oxygen within their side chains. Carbonyl oxygen of # serves as a ligand. -Z is usually glutamic acid. The GenBank accession numbers of the rboh genes are DQ890023, NM_001050700, NM_001062650, NM_105079, respectively.
Comparison of the Rboh protein sequences
Figure 2 shows a phylogenetic tree for polypeptide ZmRbohB and for 32 reported plant Rboh proteins. ZmRbohB was most similar to OsRbohC from rice (85.8% identity), followed by rice OsRbohA (74.1%). It also had high sequence identity to tobacco NbRbohA (71.6%), potato StRbohA (70.9%), Arabidopsis AtRbohF (69.5%) and tomato LeRboh1 (51.9%).
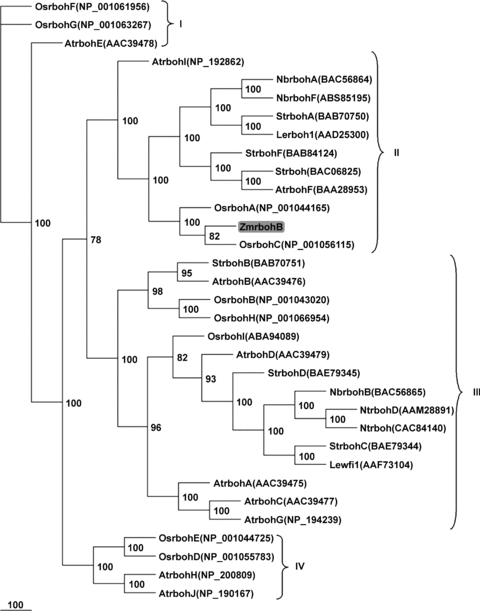
Unrooted phylogenetic tree of various higher plant respiratory burst oxidase homologs.Accession number of each sequence used for the alignment is indicated between brackets. Bootstrap values of 50% or higher are shown on significant nodes. Species names are: At, Arabidopsis thaliana; Le, Lycopersicon esculentum; Nb, Nicotiana benthamiana; Nt, Nicotiana tabacum; Os, Oryza sativa; St, Solanum tuberosum; Zm, Zea mays.
From this tree, four main clusters could be distinguished. The first contains AtRbohE, OsRbohF and OsRbohG. OsrbohF is a defense-related gene (Yoshie et al. 2005), which deposits in the database by Wong et al. (2007) and is identical to the clone OsrbohE obtained by Yoshie et al. (2005). The second major cluster includes NbRbohA and LeRboh1, which are constitutively expressed in tobacco and tomato, respectively (Amicucci et al. 1999; Yoshioka et al. 2003). But this cluster also contains rice OsrbohA (Yoshie et al. 2005), Arabidopsis AtrbohF (Kwak et al. 2003) and potato StrbohA (Kumar et al. 2007), which are inducible genes. Our ZmRbohB was classified into this cluster, too. The third major cluster contains two sub-groups. One sub-group contains StRbohB-D (Yoshioka et al. 2001; Yamamizo et al. 2007), NbRbohB (Yoshioka et al. 2003), NtRbohD (Simon-Plas et al. 2002) and AtRbohD (Deskian et al. 1998; Kwak et al. 2003) proteins whose transcription has been shown to be induced by biotic stress. The second sub-group only includes three Arabidopsis Rboh proteins, AtRbohA, C and G. The fourth major cluster consists of four proteins of unknown function, AtRbohH, AtRbohJ, OsRbohD and OsRbohE (Sagi and Fluhr 2006).
Alternative splicing of the ZmrbohB mRNA
To confirm our deduced cDNA sequence, reverse transcription (RT)-PCR was carried out using the primers PBF1 and PBR1 located at 5′ and 3′ UTR (untranslated region). After sequencing, two transcripts, the shorter ZmrbohB-α and the longer ZmrbohB-β, were identified (GenBank accession nos.: ZmrbohB-α, DQ890023; ZmrbohB-β, EU807966). Compared with ZmrbohB-α, which was identical to the deduced cDNA sequence, ZmrbohB-β retained an excessive 112 bp fragment (Figure 3).

Alternative splicing by intron retention of ZmrbohB transcripts and its effects on predicted translational products.(A) Schematic of ZmrbohB gene organization, showing two splice variants by alternative splicing. Exons are shown as numbered boxes, while introns are represented as thin lines.(B) Comparison of splice variants of ZmrbohB gene and deduced amino acid sequences around locations of alternative splice. An unspliced intron in ZmrbohB-β is boxed in gray scale. The terminated codons are indicated with overlines.
This observation prompted us to study whether the ZmrbohB gene presented alternative splicing by an intron retention event. The ZmrbohB genomic sequence was isolated by nested long distance PCR. Sequence comparison with genomic DNA sequence (GenBank accession no.: EU523147) revealed that two transcripts were synthesized as the result of an alternative splicing of a single precursor mRNA. The coding region of ZmrbohB-α was composed of fourteen exons interrupted by thirteen introns, and all of the sequences at exon-intron junctions followed the GT-AG rule. This transcript was a potential functional isoform. ZmrbohB-β retained an unspliced intron 11 with a TGA termination codon, i.e., presumably contained one premature termination codon (PTC) in the reading frame (Figure 3). The PTC in ZmrbohB-β was farther than 94 nucleotides upstream of the exon11-12 junction, and followed the 50 bp-PTC rule in mammals (Nagy and Maquat 1998). So this PTC-containing transcript would be the target of nonsense-mediated decay (NMD) pathway and regulate ZmrbohB mRNA stability, if that pathway is to operate in plants as in humans (Wang and Brendel 2006; Ali and Reddy 2008). Alternative splicing in the coding region of the plant rboh gene is, to the best of our knowledge, not reported. This finding indicated that ZmRbohB activity may be regulated by mRNA splicing.
The expression of these alternative-spliced transcripts in various maize tissues was investigated by RT-PCR using the primers Prb1 and 2. The primer Prb1 bridged the exon 9-10 junction and the Prb2 located at exon 12. The results showed that the ZmrbohB transcripts were detected throughout the plant (Figure 4). The transcripts of ZmrbohB-α accumulated more abundantly than those of ZmrbohB-β in all of the tissues examined (Figure 4). Furthermore, ZmrbohB was differentially expressed in various tissues and at different developmental stages (Figure 4). In seedlings, the expression levels of ZmrbohB-α and -β in leaves and stems were higher than those in roots. In adult plants, the expression of both ZmrbohB-α and -β was found to be higher in stems than in leaves, roots and male flowers, and was faint in ears (Figure 4).

Expression of ZmrbohB gene in various tissues.Total RNA extracted from seedlings (roots, stems, and leaves) and adult plants (roots, stems, leaves, ears and male flowers). Total RNAs were reverse-transcribed to cDNA and amplified by polymerase chain reaction (PCR) using primers (Pbr1 and Pbr2). The actin gene served as an internal control.
Changes in mRNA level of ZmrbohB in response to abiotic stresses (salt stress, low temperature, heat, ultraviolet and wounding)
Growing evidence indicates that plant NOXs are involved in several signal transduction pathways in plants (Torres and Dangl 2005). Thus, we first examined the effects of some abiotic stresses on the expression of ZmrbohB-α in maize seedling leaves. One set of E-E-jn (exon-exon junction) primers Prb1 and Prb3, which spanned intron 9 and intron 11 respectively, was designed, and contaminating ZmrbohB-β will not be amplified by these primers. It was found that the response of ZmrbohB-α to 200 mM NaCl started 1 h after treatment and kept a high level within 4 h, then declined (Figure 5A). The accumulation of ZmrbohB-α transcript was markedly induced by 4 °C treatment. The transcripts increased within 30 min, reached the top level at 1 h, and returned to the control level at 4 h (Figure 5B). A similar change in the expression of ZmrbohB-α was observed in the leaves of maize seedlings exposed to UV treatment, when compared with that of cold treatment, although the response of ZmrbohB-α to UV was weaker (Figure 5C). ZmrbohB-α expression was also upregulated by heat treatment, which increased within 2 h after initiation of the stimuli and reduced after 4 h (Figure 5D). After wounding, transcript levels of ZmrbohB-α increased transiently and rapidly. The mRNA accumulation increased markedly within 30 min, the peak appeared at 45 min, and then decreased to the base level after 1 h (Figure 6A). Moreover, we examined the effects of several abiotic stresses on the alternative splicing pattern of ZmrbohB in maize seedling leaves. Compared with the control samples (no stress treatment), the ratio of ZmrbohB-β/ZmrbohB-α was reduced under salt stress conditions (Figure 7). Similar results were observed under cold, heat and UV stress conditions (Figure 7). Nevertheless, the alternative splicing pattern of ZmrbohB was not changed by wounding (Figure 7). In addition, the transcription pattern of ZmrbohB was further analyzed by real-time quantitative PCR. Results indicated that the transcription levels of ZmrbohB were upregulated under different treatments (Figure 8).
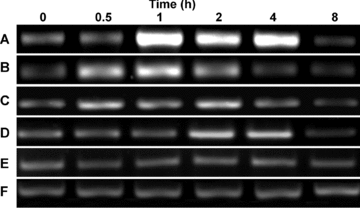
Expression analysis of ZmrbohB-α under NaCl, cold, UV and heat treatment using semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR).(A) 200 mM NaCl treatment. (B) Cold treatment at 4 °C. (C) UV treatment. (D) Heat treatment at 40 °C. (E) Controls (no treatment). (F) The actin gene was used as the internal control for normalization of RNA loading.

Expression analysis of ZmrbohB-α under wounding treatment using semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR).(A) Wounding treatment. (B) Controls (no treatment). (C) The actin gene was used as the internal control for normalization of RNA loading.
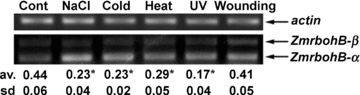
The alternative splicing pattern of ZmrbohB under various stress conditions.The maize seedlings treated by NaCl (200 mM, 2 h), cold (4 °C, 2 h), heat (40 °C, 2 h), UV (2 h) and wounding (45 min) were used for reverse transcription-polymerase chain reaction (RT-PCR) analysis.Total RNAs were reverse-transcribed to cDNA and amplified by PCR using primers (Pbr1 and Pbr2). The band density was quantified by Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA). Numbers below each lane indicate band density of ZmrbohB-β relative to ZmrbohB-α. Values represent the averages (av) and SD of three independent replicates. The actin gene served as an internal control. *Significantly different (P < 0.05, one way anova) compared with values for cont. (control, 0 h).

Real-time polymerase chain reaction (PCR) analysis of the ZmrbohB transcripts under various stress conditions.The maize seedlings treated by NaCl (200 mM, 2 h), cold (4 °C, 2 h), heat (40 °C, 2 h), UV (2 h) and wounding (45 min) were used for real-time PCR analysis. Columns represent the relative expression levels of ZmrbohB transcripts. The mRNA amount in control seedlings (control, 0 h) served as a calibrator for the calculation of relative expression levels in all cases (relative expression arbitrarily set to one). The data are presented as means and SE from three independent replicates.
Discussion
The NOXs play pivotal roles in plant stress responses, growth and development (Torres and Dangl 2005), and several rboh genes have been identified in different plant genomes, including 10 members in genome of Arabidopsis (Foreman et al. 2003), and nine members in rice (Wong et al. 2007). However, most studies in plants are carried out in dicot plants such as Arabidopsis, tobacco and potato. Moreover, the phylogenetic analysis revealed that AtRbohB, E and F, formed monophyletic subgroups with two rice proteins, respectively (Figure 2). But there was one clade that only consisted of three Arabidopsis Rbohs, AtRbohA, C and G, without rice isoforms (Figure 2) (Yamamizo et al. 2007). These suggest that sequence-based phylogenetic clustering of Rboh orthologs among different species could not reliably predict their functions, and they must be established experimentally on a case-by-case basis.
In maize seedlings, increasing pharmacological studies have also showed that NOX-mediated ROS play an important role in signaling and development (Jiang and Zhang 2003; Rodriguez et al. 2007). For example, NOXs may implicate in the ABA signal transduction pathway leading to the induction of antioxidant defense systems (Jiang and Zhang 2003; Zhang et al. 2006; Hu et al. 2007). However, maize DNA sequences that encode NOXs have not been reported, the detailed molecular mechanisms remain to be determined.
In this study, we isolated a novel rboh gene from maize, namely ZmrbohB, and the amino acid sequence comparison revealed that ZmRbohB was most similar to rice OsRbohC (85.8% identity). It also had high sequence identity to rice OsRbohA (74.1% identity), which was induced in response to pathogen attack (Yoshie et al. 2005). Moreover, this comparison showed that the maize sequence contained several features common to NOX and contributing to the catalytic activity of these enzymes, such as EF hand motifs, six transmenbrane-spanning domains, and binding sites for the FAD, the NADPH-ribose and the NADPH-adenine (Figure 1). Recently using the maize expressed sequence tag (EST) and genomic sequence databases, we have identified five full length and four partial rboh sequences, bringing the total number for presently known maize rboh genes to at least nine (Y Zhang, MY Jiang, unpubl. data, 2008). It will be interesting to see further studies on the maize rboh gene family and molecular evolution of rboh genes in plants.
In the present study, we found that the ZmrbohB gene presented alternative splicing by intron retention events (3, 4, 7). Two ZmrbohB splice variants were differentially expressed in various tissues and at different developmental stages, and the major product was ZmrbohB-α (Figure 4). Alternative splicing is an important posttranscriptional regulatory mechanism that can increase protein diversity and affect mRNA stability. Considerable attention has been paid to its function in plants. In recent bioinformatics studies, alignment of cDNA/EST with genomic sequences has revealed that the frequency of alternatively spliced genes in plants is high and intron retention is the predominant mode (Wang and Brendel 2006; Ner-Gaon et al. 2007). For example, 20%–30% of expressed genes are alternatively spliced in A. thaliana and O. sativa (Wang and Brendel 2006). However, there are a few reports regarding the experimental analysis of individual genes in maize. Our results revealed that there were at least two ZmrbohB splice variants, ZmrbohB-α and -β, in maize. Compared with ZmrbohB-α, ZmrbohB-β retained an unspliced intron 11, i.e., intron retention (Figure 3). In human, NOX5, a homolog to gp91phox, has been described with four splice variants, and they contribute to ROS signaling in the vasculature (BelAiba et al. 2007). Even though the several biological roles of plant rboh genes have been extensively studied, no information about the alternative splicing of plant rboh genes is available. Here, it is clear that the regulation of alternative splicing occurs at the coding region of ZmrbohB (3, 4), but its biological roles remain to be elucidated.
In the present study, we also analyzed the transcriptional patterns of ZmrbohB against a variety of abiotic stresses. In recent years, more and more studies have shown that transcriptional activation of some plant rboh genes is common, especially under pathogenic attack. For instance, the mRNA of NtrbohD may accumulate when Nicotiana tabacum leaves and cells are treated with the fungal elicitor cryptogein (Simon-Plas et al. 2002), and NbrbohB is induced specifically by the protein elicitor INF1 from the potato pathogen Phytophthora infestans as well (Yoshioka et al. 2003; Asai et al. 2008). OsrbohA and OsrbohC transcripts, which play a role in rice immune response, are induced after inoculation with an incompatible pathogen (Yoshie et al. 2005; Wong et al. 2007). In A. thaliana suspension cultures, treatments with harpin elicitor or hydrogen peroxide induce the expression of the AtrbohD gene (Deskian et al. 1998). Likewise, expression of both AtrbohD and AtrbohF mRNAs are upregulated by ABA in Arabidopsis guard cells (Kwak et al. 2003). Moreover, a series of studies also demonstrate that Solanum tuberosum at least contains five Strboh genes, StrbohA-D and F (Yoshioka et al. 2001; Kumar et al. 2007; Yamamizo et al. 2007). Of the five NOXs in potato, StrbohB-D genes are induced by pathogen signals, and the mitogen-activated protein kinases (MAPKs) seem to play a central role in the regulation of pathogen-responsive NOX at the transcriptional level (Yoshioka et al. 2001; Yoshioka et al. 2003; Yamamizo et al. 2007). Here, we provide evidence that the full-length functional isoform, ZmrbohB-α, becomes activated by transcriptional control under several abiotic stress stimuli (5, 6). For example, the level of transcript encoding ZmrbohB-α was enhanced transiently and rapidly after wounding. Similar results are found with transcripts encoding StrbohA in potato (Kumar et al. 2007). Under heat treatment, ZmrbohB-α expression was upregulated. Arabidopsis rboh mutants were also shown to be more sensitive to heat stress (Larkindale et al. 2005). A continued increase was also observed for cold treated samples until 2 h. That is similar to Lerboh1, whose expression is induced by treatment at 2–4 °C (Amicucci et al. 1999). Furthermore, the results of RT-PCR also indicated that the expression of the ZmrbohB-α gene was induced by salt stress. On the other hand, ZmrbohB also underwent alternative splicing in response to a variety of abiotic stresses. Our results revealed that the changes of alternative splicing pattern were similar in maize seedlings treated with salt, cold, heat and UV. The relative ratio of transcripts that retained alternative introns was reduced by these external stimuli. These results indicate that some stresses regulate the splicing of ZmrbohB through a common mechanism. The relative ratio of these two transcripts was, however, not changed by wounding, suggesting that the constitutive presence of the two isoforms may be needed for wound response. That is similar to the tomato prosystemin, whose alternative splicing pattern is not changed by wounding (Li and Howe 2001). Taken together these results indicate that there is not one single signaling pathway that relays stress conditions to ZmrbohB splicing machinery and alternative splicing leads to a net increase in the functional isoform under various abiotic stresses. In plants, serine/arginine-rich (SR) proteins are widely believed be the major regulators of alternative splicing and a majority of SR genes display alternative splicing specific to a given stress (Ali and Reddy 2008). Thus, different stresses may regulate ZmrbohB sometimes through different signaling components, likely through different SR proteins or their isoforms. Since expression of ZmrbohB was changed by wounding, cold, heat, UV and salt-stress, it is a reasonable hypothesis that a putative ZmRbohB-mediated ROS is a common signaling event that is shared by various signaling pathways leading to activation of abiotic-stress responses in maize, just like the hypothesis that NOXs act as a major source of ROS production in many different signals (Torres and Dangl 2005). However, whether induction of ZmrbohB gene expression is required for its activity regulation will be needed to be studied further. The identification of the cis-acting elements presented in this rboh gene promoter and the cognate trans-acting factors may be the important step, and transgenic analysis may also be helpful in addressing whether the ZmRbohB is a regulator of abiotic-stress tolerance.
Materials and Methods
Plant materials and treatments
Seeds of maize (Zea mays L. cv Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a light chamber at a temperature of 25–28 °C, with a photosynthetic active radiation (PAR) of 200 μmol/m2 per s with a 14:10 h light: dark (LD) cycle, and watered daily.
For the tissue-specific experiments, seedlings and adult plants were grown for 10 or 60 d after germination, respectively, and various tissues of seedlings (roots, stems and leaves) and adult plants (roots, stems, leaves, ears and male flowers) were harvested, frozen immediately in liquid nitrogen, and stored at −80 °C until use.
To detect responses of the gene to some abiotic stresses, the plants were excised at the base of the stem and collected when the second leaves were fully expanded. Detached plants were then placed in the distilled water with a continuous light intensity of 200 μmol/m2 per s for 1 h to eliminate wound stress. For salt (NaCl) treatment, plants were transferred into solutions containing 200 mM NaCl and cultivated at 28 °C. Heat stress treatment was imposed incubating at 40 °C. In the low temperature experiment, plants were exposed to 4 °C. For ultraviolet (UV) treatment, plants were exposed to UV light (254 nm, 16 W) at a 50 cm distance from the lamp (CAMAG, Muttenz, Switzerland). At 0, 0.5, 1, 2, 4 and 8 h after every treatment, the second leaves were sampled and immediately frozen in liquid nitrogen for further analysis. For wounding treatment, plants were wounded by crushing their second leaves four times with forceps. At time 0, 15, 30, 45, 60 and 120 min after wounding treatment, the second leaves were sampled and immediately frozen in liquid nitrogen. Detached plants were treated with distilled water for the whole period and served as controls.
Primers
The primers used in this study are presented in Table 1.
| Abbreviation | Sequence (5′-3′) | Description |
|---|---|---|
| Pzrb3 | GCWGARACWMTIAARYTCAACAT | Degenerate primer, Forward |
| Pzrb4 | CCAATTWGGYYTAGCRAARTG | Degenerate primer, Reverse |
| Pzrb11 | AGCTGTCCAGGCTTAAGGAG | Gene specific primer, Forward |
| Pzrb12 | GTCCAGAACTCCAGATGAACATACC | Gene specific primer, Reverse |
| AAP | GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG | Abridged anchor primer |
| AUAP | GGCCACGCGTCGACTAGTAC | Abridged universal amplification primer |
| ZmGSP1-1 | AGTCCAGCCATTATTC | Reverse primer for 5′ RACE |
| ZmGSP2-1 | GGTCTCCAACTGCCACAACTCAATG | Reverse primer for 5′ RACE |
| ZmGSP3-1 | CCATAATCAGGGCGGCATACTC | Reverse primer for 5′ RACE |
| ZmGSP5-1 | CGTTCTTATCCACCAT | Reverse primer for 5′ RACE |
| ZmGSP6-3 | GCACCTGACGGCGGCGGCTC | Reverse primer for 5′ RACE |
| ZmGSP7-2 | GAAGTCGGAGCGGGAGAGGAAAC | Reverse primer for 5′ RACE |
| ZmGSP9-2 | GACTCGATGCCTGAC | Reverse primer for 5′ RACE |
| ZmGSP10-1 | GGTGAGGTCCTGCGAGAAC | Reverse primer for 5′ RACE |
| ZmGSP11-3 | GCCTGATCGACGGCGACAAGG | Reverse primer for 5′ RACE |
| PBF1 | GCCCACCGTACTCGCCTTCTCATCCTCAT | Gene specific primer, Forward |
| PBR1 | GCTACCGACAATCCCAACTACTCTTTATCTTCAT | Gene specific primer, Reverse |
| PBF2 | ATTGCTGTCTCGTTGCGCTTCCTCTTTC | Nested gene specific primer, Forward |
| PBR2 | CGTCCAGAACTCCAGATGAACATACCAAG | Nested gene specific primer, Reverse |
| Prb1 | CAAGAAAGCCTTACCAAAAC | ZmrbohB gene specific primer for RT-PCR analysis, Forward |
| Prb2 | TCCTTCCTCATAAACACTCG | ZmrbohB gene specific primer for RT-PCR analysis, Reverse |
| Prb3 | CAATAATATTCCTTTGGTCCAG | ZmrbohB-α gene specific primer for RT-PCR analysis, Reverse |
| Actin1 | GCGAACAACTGGTATTGTG | Actin gene specific primer for RT-PCR analysis, Forward |
| Actin2 | CATCTGCTGCTGAAAAGTG | Actin gene specific primer for RT-PCR analysis, Reverse |
| Prb5 | ACCCTTTGAATGGCATCCG | ZmrbohB gene specific primer for real-time RT-PCR analysis, Forward |
| Prb6 | AAGGAGTTGCACCAATCCCTAAT | ZmrbohB gene specific primer for real-time RT-PCR analysis, Reverse |
| Actin3 | GATTCCTGGGATTGCCGAT | Actin gene specific primer for real-time RT-PCR analysis, Forward |
| Actin4 | TCTGCTGCTGAAAAGTGCTGAG | Actin gene specific primer for real-time RT-PCR analysis, Reverse |
- RACE, rapid amplification of cDNA ends; RT-PCR, reverse transcription-polymerase chain reaction.
RNA preparation, cDNA synthesis and DNA extraction
Total RNA was isolated from leaves using Plant RNA Purification Reagent (Tiangen Biotech Co., Ltd. Beijing) according to the manufacturer's instructions. DNase treatment was included in the isolation step using the RNase-free DNase (TaKaRa, Dalian, China). Approximately 2 μg of total RNA were reverse transcribed using oligo d(T)16 primer and M-MLV reverse transcriptase (Promega, Madison, WI, USA) at 42 °C for 60 min.
Genomic DNA was isolated from seedling leaves using a hexadecyltrimethylammonium bromide (CTAB) method (Arechaga-Ocampo et al. 2001). The quality and concentration of RNA and DNA samples were examined by ethidium bromide-stained agarose gel electrophoresis and spectrophotometer analysis.
Cloning of the cDNA encoding ZmrbohB
To get the internal conservative fragment, degenerate oligonucleotides Pzrb3 and Pzrb4 (Table 1) were designed and synthesized based on the conserved amino acid and nucleotide sequences of plant rboh genes. RT-PCR was programmed as below: pre-denatured at 94 °C for 3 min, followed by 35 cycles of amplification (94 °C for 40 s, 52 °C for 40 s, 72 °C for 2 min), and then followed by extension for 5 min at 72 °C. The resulting major fragment was cloned into pMD18-T vector (TaKaRa) and three clones were sequenced in both directions. This sequence (1 360 bp) was compared with sequences deposited in the GenBank database using the BLAST program at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov), and a matching sequence (maize Unigene CL562_1, AY109999.1) was found. Gene-specific primers Pzrb11 and Pzrb12 (Table 1) from this Unigene were designed for PCR to identify.
A 5′ RACE approach (Invitrogen, Carlsbad, CA, USA) was used to isolate the unknown 5′ region of the ZmrbohB according to the manufacturer's recommendations. In detail, first-strand synthesis for the ZmrbohB cDNA was carried out with the gene-specific primer (ZmGSP1-1, Table 1). The cDNA was amplified with a gene-specific primer (ZmGSP2-1, Table 1) and the kit 5′ primer (AAP, Table 1) followed by nested PCR with a gene-specific primer (ZmGSP3-1, Table 1) and the kit nested 5′ primer (AUAP, Table 1). The 428 bp PCR product was ligated into pMD18-T vector, cloned and sequenced. Based on informative sequence data, new gene-specific primers (Table 1) were designed and applied in additional 5′ RACE experiments as one walks toward the 5′-end.
By aligning the sequences of 5′ RACE and the partial region products, the full-length cDNA sequence of ZmrbohB was deduced and then amplified using gene-specific primers PBF1 and PBR1 (Table 1). After being sequenced, the full-length cDNA of ZmrbohB was subsequently analyzed for molecular characterization.
Full-length genomic sequence amplification of ZmrbohB
The genomic sequence was amplified with gene-specific primers PBF1 and PBR1 followed by nested PCR with gene-specific primer PBF2 and PBR2 (Table 1). The major PCR product was cloned and sequenced.
Phylogenetic analysis
Protein data matrices were aligned using ClustalX multiple sequence alignment program (version 1.83) with default gap penalties and further manual adjustments to minimize gap insertions. Phylogenetic tree was inferred by the neighbor-joining (NJ) algorithm as implemented in the program PAUP 4.0b10 (Sinauer Associates, Sunderland, MA, USA) and 1 000 bootstrap replicates were carried out. Graphical output was produced by Treeview, and figures were prepared in Illustrator 10.0 (Adobe Systems Incorporated, San Jose, CA, USA).
RT-PCR analysis of ZmrbohB mRNA accumulation
Total RNA was isolated from a given tissue and cDNA was reverse transcribed as described above. To identify the alternative-splicing patterns by semi-quantitative RT-PCR, two primers Prb1 and Prb2 were used (Table 1). The primer Prb1 bridged the exon 9-exon 10 junction and the Prb2 corresponded to exon 12. Another reverse E-E-jn primer Prb3 was designed to span the intron 11 (Table 1) to differentiate between ZmrbohB-α and -β. cDNA was amplified by PCR using Prb1 and Prb3 to detect responses of the ZmrbohB-α to abiotic stresses. To standardize the results, the maize actin (GenBank accession no. J01238) was used as an internal control for RT-PCR with primers Actin1 and Actin2 (Table 1). The cycle number of the PCR reactions was adjusted for each gene to obtain barely visible bands in agarose gels. Aliquots of the PCR reactions were loaded on agarose gels and stained with ethidium bromide.
To confirm some of the semi-quantitative RT-PCR results, real-time RT-PCR was carried out in a DNA Engine Opticon 2 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using the SYBR Premix Ex Taq (TaKaRa) according to the manufacturer's instructions. Each PCR reaction (20 μL) contained 10 μL 2× real-time PCR Mix (containing SYBR Green I), 0.2 μM of each primer (Table 1) and appropriate diluted cDNA. The thermal cycling conditions were 95 °C for 10 s followed by 40 cycles of 94 °C for 5 s, 62 °C for 10 s and 72 °C for 15 s. The expression of ZmrbohB was normalized to actin. The relative changes in ZmrbohB transcripts were calculated as χ-fold changes relative to the control samples (0 h). Each treatment was repeated three times independently.
(Handling editor: Jianhua Zhang)




