An Examination of the Function of Male Flowers in an Andromonoecious Shrub Capparis spinosa
Supported by the Projects on the Research and Development of High Technology of Xinjiang Uygur Autonomous Region (200810102), China, the Construction of Scientific and Technological Platforms Project from the Chinese Ministry of Science and Technology (2005DKA21006 and 2005DKA21403) and the National Natural Science Foundation of China (90302004).
Abstract
The pollen donor and pollinator attractor hypotheses are explanations for the functions of the male flowers of andromonoecious plants. We tested these two hypotheses in the andromonoecious shrub Capparis spinosa L. (Capparaceae) and confirmed that pollen production and cumulative volume and sugar concentration of nectar do not differ between male and perfect flowers. However, male flowers produced larger anthers, larger pollen grains and smaller ovaries than perfect flowers. Observations on pollinators indicated that two major pollinators (Xylocopa valga Gerst and Proxylocopa sinensis Wu) did not discriminate between flower morphs and that they transferred pollen grains a similar distance. However, there were more seeds per fruit following hand pollination with pollen from male flowers than from perfect flowers. Individuals of C. spinosa with a larger floral display (i.e. bearing more flowers) received more pollen grains on the stigma of perfect flowers. Female reproductive success probably is not limited by pollen. These results indicate that male flowers of C. spinosa save resources for female function and that they primarily serve to attract pollinators as pollen donors.
Andromonoecy is a breeding system in flowering plants in which individuals produce both perfect flowers and male flowers, and it is widely distributed in 33 families and approximately 4 000 species of angiosperms (Yampolsky and Yampolsky 1922; Bawa and Beach 1981; Cruden and Lloyd 1995; Miller and Diggle 2003; Vallejo-Marín and Rausher 2007a). It has been suggested that andromonoecy evolved from hermaphroditism by loss of the female reproductive structure, which is the first step in the evolution of a plant breeding system towards monoecy, androdioecy and dioecy (Primack and Lloyd 1980; Bertin 1982). Consequently, evolution and maintenance of the male flower in andromonoecious plants have attracted considerable attention (Narbona et al. 2002; Connolly and Anderson 2003; Huang 2003; Cuevas and Polito 2004; Schlessman et al. 2004; Perglová et al. 2006; Tanaka et al. 2006; Miller and Diggle 2007; Vallejo-Marín and Rausher 2007a; 2007b).
Most authors have hypothesized that andromonoecy is a mechanism for optimal resource allocation to male and female function: male flowers reduce resource investment and permit the resources saved to be re-allocated toward other fitness-enhancing traits (Bertin 1982; Solomon 1986; Spalik 1991). Under the frame of the optimal resources allocation model, two hypotheses were proposed to explain the function of the male flower in andromonoecious plants (Vallejo-Marín and Rausher 2007b). The pollen donor hypothesis predicts that production of male flowers reduces resource investment in the female reproductive structure. The selective advantage of the additional male organs is that they serve as a source of outcrossing pollen, thereby increasing the chance for a plant to fertilize more ovules of other individuals and to increase its male reproductive success (Stephenson and Bertin 1983; Solomon 1985; Elle and Meagher 2000). The pollinator attractor hypothesis presumes that male flowers increase floral display and therefore attract more pollinators. As a result, more pollen grains could be dispersed and deposited on the stigmas of perfect flowers, which potentially would increase female reproductive success (Solomon 1987; Podolsky 1992; Harder and Barrett 1996). However, these two hypotheses are not mutually exclusive: male flowers could enhance both donation and reception of pollen (Schlessman et al. 2004; Vallejo-Marín and Rausher 2007b).
Capparis spinosa L. (Capparaceae) is a deciduous perennial shrub with a natural distribution from the Mediterranean region to the dry regions in west and central Asia (Heywood 1993; Fici 2001). It flowers during summer, and anthesis of a single flower lasts about 16 h. The flowers open in the early evening and wither the next morning (Petanidou et al. 1996). Capparis spinosa was previously believed to be hermaphroditic (Eisikowitch et al. 1986; Dafni et al. 1987). However, in a preliminary study in the desert regions of the northern part of Xinjiang, China, we found that it is andromonoecious (Zhang and Tan 2008). Here, we report the results of an investigation designed to test the pollen donor hypothesis and the pollinator attractor hypothesis in C. spinosa. Dyed pollen has been used to determine the donor of male flowers in andromonoecious species such as Besleria triflora (Podolsky 1993), Sagittaria guyanensis ssp. lappula (Huang 2003) and Solanum carolinense (Connolly and Anderson 2003), and dyed pollen massulae have been used in some orchid studies (e.g. Peakall 1989). Dyed pollen is suitable for C. spinosa because our previous experiments showed that the flower visitors did not discriminate between the stained and non-stained pollen grains when they visited the flowers. We address the pollen donator hypothesis by asking: (i) whether male flowers develop smaller and non-functional female reproductive structures but larger and functional male reproductive structures than perfect flowers; (ii) whether the different ratios of male and perfect flowers could influence pollen movement; and (iii) whether male flowers have higher siring success than perfect flowers. We evaluate the pollinator attractor hypothesis by determining (i) whether male flowers produce more pollen and larger rewards (i.e. pollen and nectar) to pollinators than perfect flowers and therefore attract more pollinators than perfect flowers; and (ii) whether plants with larger floral displays (more flowers) receive more pollen on each stigma than the plants with fewer flowers.
Results
Flower morphology
The flower of C. spinosa is zygomorphic, with four green sepals and four white petals, white or pink anthers, and an ovary elevated by a gynophore. Two types of flowers were observed: perfect flowers with a developed ovary and an elongated pistil slightly longer than the stamens; and male flowers with an aborted ovary and a pistil shorter than the stamens. They differ significantly in several traits. Male flowers had larger (in width) anthers and a smaller (in both length and diameter) ovary and a shorter gynophore than perfect flowers (Table 1). Male flowers also had less biomass than perfect flowers (Table 2).
| Character | Male flowers (mm) | Perfect flowers (mm) | d.f. | t | P | |
|---|---|---|---|---|---|---|
| Sepals | Length | 16.90 ± 2.36 | 17.48 ± 1.34 | 29 | −1.24 | 0.22 |
| Width | 7.36 ± 1.52 | 8.23 ± 1.84 | 29 | −2.78 | 0.00 | |
| Petals | Length | 29.65 ± 2.53 | 29.03 ± 2.54 | 29 | 0.53 | 0.60 |
| Width | 18.42 ± 2.34 | 18.13 ± 2.76 | 29 | 0.26 | 0.61 | |
| Stamens | Number | 68.11 ± 8.08 | 67.45 ± 9.21 | 29 | −0.08 | 0.94 |
| Length of filaments | 35.67 ± 3.11 | 36.42 ± 3.35 | 99 | −1.84 | 0.07 | |
| Length of anthers | 3.44 ± 0.18 | 3.48 ± 0.15 | 29 | −0.75 | 0.46 | |
| Width of anthers | 1.34 ± 0.09 | 1.28 ± 0.05 | 29 | 3.81 | 0.00 | |
| Ovary | Length | 4.40 ± 0.63 | 6.53 ± 0.66 | 29 | −11.91 | 0.00 |
| Diameter | 1.68 ± 0.23 | 2.47 ± 0.19 | 29 | −15.74 | 0.00 | |
| Gynophore | Length | 7.38 ± 2.01 | 29.99 ± 1.88 | 29 | −57.17 | 0.00 |
| Floral organs | Male flowers (mg) | Perfect flowers (mg) | d.f. | t | P |
|---|---|---|---|---|---|
| Stamens | 79.1 ± 14.8 | 81.1 ± 15.4 | 31 | −0.79 | 0.43 |
| Pistil | 1.5 ± 0.9 | 8.1 ± 1.2 | 31 | 23.34 | 0.00 |
| Perianth and pedicel | 136.9 ± 26.1 | 138.9 ± 27.6 | 31 | −0.37 | 0.72 |
| Total | 217.2 ± 39.0 | 228.1 ± 40.5 | 31 | −1.41 | 0.17 |
Pollen grains of both male and perfect flowers were elliptical in shape. However, male flowers produced larger pollen grains than perfect flowers (311 ± 39 μm2 for male flowers versus 299 ± 38 μm2 for perfect flowers, t= 69.97, degrees of freedom (d.f.) = 299, P < 0.01, Figure 1). There was no significant difference in the mean number of pollen grain between male and perfect flowers (t=−0.66, d.f. = 29, P > 0.05). Male flowers produced (488.27 ± 82.45) × 104 pollen grains and perfect flowers (487.14 ± 83.44) × 104 pollen grains. The pollen to ovule ratio (P/O) of perfect flowers was 1.57 × 104.
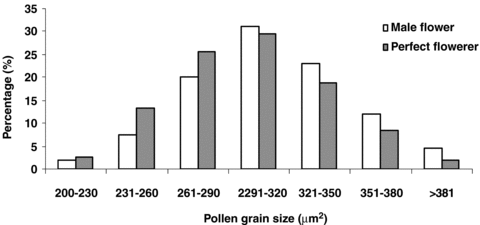
Pollen grains size of male and perfect flowers of Capparis spinosa (n= 300).
Nectar production and sugar concentration
In both male and perfect flowers, nectar was secreted from the surface of a nectary disc at the beginning of anthesis, and then it accumulated as a single drop at the base of the sepal. In both flower types, nectar was visible from 19.00 hours (GMT+8, and hereafter), when the flower had just opened. Rate of nectar accumulation increased from 06.00 hours (Figure 2A) and then declined gradually from 11.00 hours on the following day. There was no significant difference in nectar production or in cumulative nectar production between male and perfect flowers at the same time points (same time after flower opened) (t=−1.15, d.f. = 199, P > 0.05). The variation of sugar concentration in nectar showed similar patterns in both flowers types (Figure 2B).
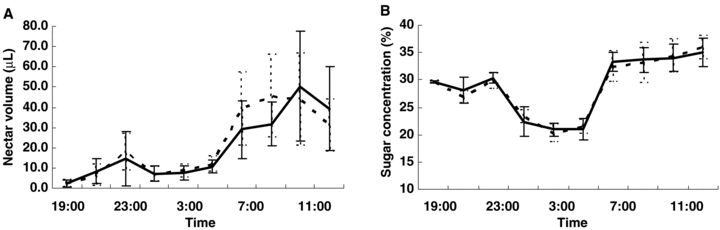
Nectar production and sugar concentration (mean ±SD) in male (——) and perfect (- - -) flowers of Capparis spinosa.
Observations on pollination
Visitors to the flower
Six species of insects were recorded as flower visitors. Two species, Xylocopa valga Gerst and Proxylocopa sinensis Wu, were observed contacting floral reproductive structures during each visit and pollinating the flowers (Figure 3). They visited C. spinosa in two discrete periods during the day (20.00–22.00 hours and 07.00–11.00 hours). Both species visited almost every flower of a plant. Four other species of insects (Herse convolvuli L., Halictus sp., Apis mellifera, and Myrmica sp.) were found to be nectar robbers and potential pollinators, with minor contributions towards pollination since they did not contact the stigma during their visits to the flower (Figure 3).
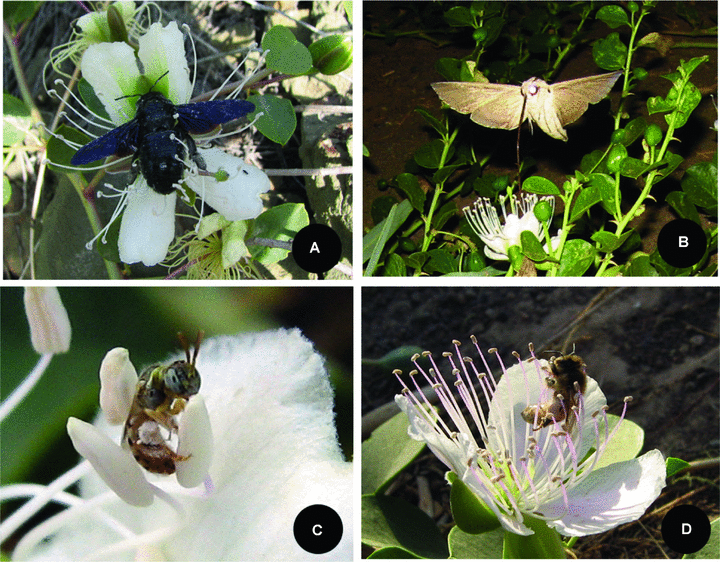
Insect visitors to Capparis spinosa.(A)Xylocopa valga Gerst take nectar while body parts (abdomen and wings) touch anthers.(B)Herse convolvuli L. during a flower visit; note that no contact was made with the sexual structures.(C)Halictus sp. collecting pollen with their forelegs.(D)Apis mellifera L. collecting pollen with their forelegs.
A total of 380 visits of X. valga and P. sinensis to 765 flowers were recorded. The mean visitation rate to male flowers was 11–20 visits per flower/h, which was not significantly different from 13–19 visits per flower/h of perfect flowers (U= 63.50, d.f. = 189, P > 0.05). The average time that a pollinator spent on each flower did not differ between the two types (3.74 ± 0.51 seconds for male flower and 3.69 ± 0.49 seconds for perfect flower, t= 0.54, d.f. = 230, P > 0.05).
Pollen flow pattern
A total of 158 perfect flowers on 14 individuals was investigated. Except for three individuals that were about 25 m from the pollen source, dyed pollen grains were found on the stigmas of perfect flowers of 11 individuals. The number of pollen grains on these stigmas from dyed male and perfect flowers with different floral ratios is similar, and the distance of dispersal was no more than 25 m. The chances are the same that an individual near the pollen source plant will receive pollen grain from male and perfect flowers and that the floral ratios of the pollen source plant have no influence on pollen dispersal or stigma pollen load in the individual (Table 3). The number of pollen grains deposited on the stigma was correlated to the size of floral display in one individual (Figure 4). Individuals with larger floral displays (more flowers) tended to receive more pollen grains on the stigma of perfect flowers (P < 0.01).
| Individual | Floral sex ratio (male: perfect) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 17:6 | 10:10 | 6:15 | |||||||
| Dyed pollen from male flowers | Dyed pollen from perfect flowers | Total | Dyed pollen from male flowers | Dyed pollen from perfect flowers | Total | Dyed pollen from male flowers | Dyed pollen from perfect flowers | Total | |
| 1 | 4 | 9 | 13 | 2 | 2 | 4 | 2 | 0 | 2 |
| 2 | 12 | 3 | 15 | 0 | 0 | 0 | 0 | 1 | 1 |
| 3 | 11 | 1 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 2 | 1 | 3 | 0 | 5 | 5 |
| 5 | 20 | 29 | 49 | 1 | 3 | 4 | 1 | 2 | 3 |
| 6 | 8 | 1 | 9 | 16 | 2 | 18 | 0 | 0 | 0 |
| 7 | 12 | 0 | 12 | 0 | 0 | 0 | 17 | 7 | 24 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 8 |
| 9 | 3 | 1 | 4 | 2 | 26 | 28 | 18 | 13 | 31 |
| 10 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 2 |
| 11 | 2 | 1 | 3 | 2 | 0 | 2 | 3 | 6 | 9 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
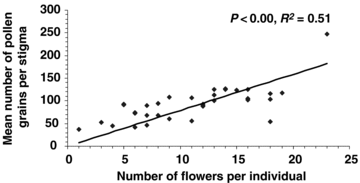
Relationship between floral display and mean number of pollen grains per stigma.
Hand pollination experiments
Results of hand pollination showed that C. spinosa has a mixed mating system and is capable of spontaneous self-pollination (Table 4). Male flowers did not produce fruits in the pollination treatments, which suggest that they are functionally male. Perfect flowers that were hand-pollinated (by either self or cross pollination) with pollen from male flowers produced significantly more seeds per fruit than those with pollen from perfect flowers or with mixed pollen from perfect and male flowers. Fruit set of perfect flowers under natural conditions was lower than that in some hand-manipulated pollinations. However, fruits of perfect flowers produced by natural pollination had significantly more seeds than those produced by hand-pollination (F= 6.08, d.f. = 5, P < 0.01), implying that seed production in nature was not limited by pollen in C. spinosa (Table 4).
| Collection and mode of pollination | Flowers (n) | Fruit-set (%) | Number of seeds per fruit (mean ±SD) | |
|---|---|---|---|---|
| Perfect flower | Spontaneous self-pollination | 32 | 9.4 | 79.7 ± 33.0c |
| Natural pollination | 30 | 56.7 | 161.2 ± 65.3a* | |
| Selfed with self pollen | 33 | 33.3 | 75.6 ± 54.9c | |
| Selfed with perfect flowers pollen | 34 | 55.9 | 83.1 ± 65.6c | |
| Selfed with male flowers pollen | 32 | 50.0 | 135.2 ± 50.5ab | |
| Selfed with perfect and male flowers pollen | 35 | 68.6 | 88.4 ± 25.0c | |
| Crossed with perfect flowers pollen | 30 | 60.0 | 90.7 ± 25.3c | |
| Crossed with male flowers pollen | 30 | 36.7 | 142.8 ± 84.7ab | |
| Crossed with perfect and male flowers pollen | 30 | 40.0 | 115.5 ± 33.9bc | |
| Male flower | Natural pollination | 30 | 0 | 0 |
| Selfed with self pollen | 30 | 0 | 0 | |
| Selfed with perfect and male flowers pollen | 30 | 0 | 0 | |
| Crossed with perfect and male flowers pollen | 30 | 0 | 0 |
- *Letter following number of seeds per fruit indicates no significant difference among means (P > 0.05).
Discussion
Selection of male flowers in C. spinosa
Our study showed that C. spinosa L. has an andromonoecious breeding system, producing male flowers with an undeveloped ovary that never develops into fruit and perfect flowers with an elongated pistil and viable pollen. Significant differences in resource investment were found between male and perfect flowers. Compared with perfect flowers, male flowers increase investment in male traits, in terms of size of anther and pollen grains. Consequently, male flowers in the individual reduce resource investment for developing the female reproductive structure, and thus the individual has the potential for a larger male function.
Male flowers as pollen donors and reinforced male traits have been reported in some andromonoecious plants (Traveset 1995). Greater pollen productivity/viability of male flowers has been observed (Huang et al. 2000; Huang 2003). This represents an advantage to andromonoecy since male reproductive success may increase through pollen donation by male flowers (Stephenson and Bertin 1983). Our results show that male flowers produce larger pollen grains than perfect flowers. Larger pollen grains potentially have faster pollen tube growth and/or more success in fertilizing ovules and therefore higher siring success (Skogsmyr and Lankinen 2002). In habitats with extremely hot summers, anthesis is nocturnal, and fast pollen tube growth ensures completeness of fertilization before extreme temperatures are reached.
Spontaneous self-pollination was almost 10% in C. spinosa, and the number of seeds produced per fruit through self pollen grains was 79.7 ± 33.0 (spontaneous pollination) and 75.6 ± 54.9 (hand pollination). In the extremely hot and dry environment where C. spinosa occurs, and considering the short time of anthesis, pollination may not be guaranteed, and both seed production and male reproductive opportunities may be decreased. Spontaneous self-pollination could serve as a mechanism of reproductive assurance (Fausto et al. 2001).
Increased female reproductive success
Female reproductive success could be reduced by the production of male flowers in the case that fruit set was limited by the number of fertile ovaries (Traveset 1995). However, this does not seem to occur in C. spinosa since only 56.7% of the perfect flowers produced fruits and this ratio was nearly identical to that of most hand pollinations. Fruiting success probably was limited by resources and not by the number of fertile ovaries. The hand pollination experiment showed that seed production also was not limited by pollen, although this may vary among sites and among years depending mainly on insect pollinator abundance (Liao et al. 2006).
Male flowers have been viewed as pollinator attractors (Solomon 1987; Podolsky 1992; Harder and Barrett 1996). They could improve the female reproductive success in two ways: one, by enhancing the efficiency of pollen grains dispersed between male and perfect flowers; and two, by producing more male flowers that attract pollinators, thus increasing the stigmatic pollen load of perfect flowers (Podolsky 1992; Spalik and Woodell 1994). In C. spinosa, we found no difference in the number of pollen grains and rewards between male and perfect flowers, no evidence of pollinators preferentially visiting male or perfect flowers, and no clear pattern of pollen grains dispersed from male and perfect flowers. Further, male flowers as pollinator attractors do not seem to increase female reproductive success. However, every day the total number of male flowers was higher than that of perfect flowers within the population. Individuals with large floral display are more attractive to flower visitors, and the stigmas of perfect flowers can receive more pollen grains and have a higher stigmatic pollen load, than those with small floral displays (Zhang and Tan 2008). Under the same resource status, individuals of C. spinosa with more male flowers can improve female reproductive success.
Compared with perfect flowers, male flowers can produce more fertile pollen than perfect flowers and thus contribute more to seed production. This implies that male flowers have a larger potential male function than perfect flowers (Elle and Meagher 2000). However, in Solanum carolinense, Vallejo-Marín and Rausher (2007a) found no difference in siring success between male and perfect flowers, especially in species for which the morphology between male flower and perfect flower did not differ significantly (e.g. Vallejo-Marín and Rausher 2007b). In C. spinosa, male flowers have larger anthers and pollen grains than perfect flowers, and seeds per fruit following hand-pollination using pollen from male flowers were significantly higher than that from perfect flowers or mixed pollen from male and perfect flowers. These results differ from those of Vallejo-Marín and Rausher (2007a,b), and they demonstrate that the ultimate selective advantages of male flowers as pollen donors have higher potential siring success than perfect flowers in C. spinosa.
Materials and Methods
Study site
Field studies were conducted in an open field near the Turpan Eremophytes Botanic Garden, Xinjiang Uygur Autonomous Region in northwestern China (42°51′52.5″N, 89°11′06.8″E, 84 m below sea level). The study area is characterized by an extremely dry and hot climate. Annual temperature in Turpan Eremophytes Botanic Garden is 13.9 °C. The highest air temperature in summer is 47.6 °C. Surface temperature of the sand dunes can reach 82 °C. Average annual rainfall is 16 mm, and potential annual evaporation is 2 300 mm (Yin 2004).
Flower morphology
Flower morphology was observed in the field to characterize the floral dimorphism. There was no clear pattern for proportion of male and perfect flowers within individual plants, because the number and position of male and perfect flowers in bloom were random (Zhang and Tan 2008). Soon after flowering began, 30 male and 30 perfect flowers were randomly collected from 26 plants. One or two male and perfect flowers each were collected from each plant. Ten different measurements of the size of flower organs were made on each flower: length and width of sepal, length and width of petal, filament length, length and width of anther, length and diameter of ovary, and gynophore length. Measurements 1–7 were taken from a single sepal, petal or anther chosen at random. In addition, a subset of 64 randomly chosen flowers of each morph was subsequently used to determine biomass of stamens, pistil, and perianth plus pedicel.
The number of pollen grains per flower was determined from a total of 30 flowers of each floral morph in six individuals, using the method in Dafni et al. (2005). Pollen grains of C. spinosa are trizonocolporate, circular in polar view, and elliptical in equatorial view, presenting a polar axis (P) and an equatorial axis (E) clearly distinct with P/E > 1. To get a more accurate estimate of the size of these pollen grains, both polar and equatorial axes of each pollen grain were measured in equatorial view. The polar axis and equatorial axis of pollen grains were measured on 60 flowers of each sex; flowers were collected from 12 individuals. Measurements were made under a Motic BA400 microscope with a color digital camera (Moticam 2006) and Motic digital image analysis system (Advanced 3.2; Motic, China). The area of 300 pollen grains each was measured for male and perfect flowers under a microscope, and pollen grain size (S) was calculated as S = (π× P × E)/4. The number of ovules per ovary in perfect flowers was counted under a stereomicroscope (SMZ 1000; Nikon, Japan), and the pollen to ovule ratio (P/O) was calculated based on the number of pollen grains and ovules in perfect flowers.
Nectar volume and sugar concentration
Nectar production was measured in bagged male and perfect flowers at 2 h intervals from 19.00 hours of one day to 13.00 hours of the next day from different individuals (i.e. each flower was sampled only once). Flowers in the late bud stage were selected randomly, numbered, and bagged. Nectar was collected in 20 flowers using 25 μL microcapillaries (Duran Ringcaps; Hirschmann Laborgeräte, Eberstadt, Germany). Nectar volume was determined by measuring height of the nectar column in the common-bore tube. Sugar concentration in the nectar was determined using a hand-held refractometer (Rank 0 to 62%; Bellingham & Stanley, UK).
Observations on flower visitors
Insect visits to a flower were recorded for 15 min each hour from 19.30 hours of one day to 12.30 hours of the next day on three consecutive days. Visitors entering the array were recorded. Meanwhile, the number of visited flowers, type of flower visited, duration of each visit, and sequence of the visits were also noted. Only visits lasting ≥1 s were likely to result in pollen removal. Photographs were taken of floral visitors for taxonomic identification.
Pollen flow pattern
The pollen attraction capacity and pollen dispersal distance from male and perfect flowers were investigated by tracking stained (dyed) pollen from male and perfect flowers. Anthesis of C. spinosa is synchronous, and there is no overlapping of flowering between days. Thus, the pollen source plant was artificially set with a different floral sex ratio (male: perfect) on each of three continuous clear days by removing some of the flowers. The plant was set as male flower dominant (17 male and six perfect) on the first day, followed by equal male and perfect (10:10) on the second day, and perfect flower dominant (five male and 16 perfect) on the third day. During late afternoon, when the flowers had just opened, pollen grains from freshly dehiscing anthers on the pollen source plant were stained with dye following the method described in Huang (2003). Pollen grains in male flowers were stained red with 1% safranin solution, and pollen grains in perfect flowers were dyed green using 1% methyl green solution. Before midday of the following day, perfect flowers from 14 individuals near the pollen source in an area of 35 × 35 m were collected, and the number and color of pollen grains on each stigma of perfect flowers were recorded under the microscope.
Hand pollination experiments
Thirty to thirty-five flowers from 20 healthy individual plants (i e. those with intact apices) were hand-pollinated in each treatment. For spontaneous self-pollination, perfect flowers were bagged without hand-pollination. For self-pollination, perfect flowers were hand-pollinated with the pollen grains from the same flower (perfect flowers) or from different flowers (male or perfect flowers) on the same plant using a cotton swab, and then bagged. For cross-pollination, perfect flowers were emasculated before the anthers dehisced and pollinated with the pollen grains from male or perfect flowers of other plants at least 20 m away. The hand-pollinated flowers were subsequently bagged.
All bags were removed after the flowering season. Fruit set and number of seeds in each fruit were counted when fruits matured.
Data analyses
Data were checked for normality before they were subjected to further analysis. Mann-Whitney U-test was used to compare pollinator visiting rate to male and perfect flowers. Paired samples T tests were used for all pairwise comparisons between two floral types. anova was used to determine whether number of seeds per fruit among treatments differed statistically. Bivariate correlation and general linear regression were carried out to assess the relationships between the number of flowers per individual and the mean number of pollen grains per stigma. All data analyses were carried out with the software SPSS 13.0 (SPSS Inc, Chicago, IL, USA).
(Handling editor: Shuangquan Huang)
Acknowledgements
We thank Dr Tian-Hua He, Department of Environmental Biology, Curtin University of Technology, Australia; Dr Carol C. Baskin and Dr Jerry M. Baskin, Department of Biology, University of Kentucky, Lexington, USA, for correcting the English and providing valuable comments on earlier drafts of this manuscript. The field work was supported and assisted by Professor Bo-Rong Pan, Lin-Ke Yin, Hai-Bo Zhang, and other workers at the Turpan Eremophytes Botanic Garden of the Chinese Academy of Sciences. We thank Ren-Xing Huang, College of Life Science and Technology, Xinjiang University, China, for identifying the insect species.




