The Expression of Manganese Superoxide Dismutase Gene from Nelumbo nucifera Responds Strongly to Chilling and Oxidative Stresses
Supported by the National Natural Science Foundation of China (30370912) and the Natural Science Foundation of Guangdong Province (04009773 and 2006B20101010).
Abstract
A manganese superoxide dismutase (Mn-SOD) gene, NnMSD1, was identified from embryonic axes of the sacred lotus (Nelumbo nucifera Gaertn.). The NnMSD1 protein contains all conserved residues of the Mn-SOD protein family, including four consensus metal binding domains and a signal peptide for mitochondrial targeting. Southern blot analysis suggests the existence of two Mn-SOD genes in sacred lotus. NnMSD1 was highly expressed in developing embryonic axes during seed development, but appeared in cotyledons only at the early stage of development and became undetectable in the cotyledons during late embryogenesis. The expression of the NnMSD1 gene in germinating embryonic axes, in response to various stresses such as heat shock, chilling, and exposure to stress-related chemicals, was also studied. Heat shock strongly inhibited the expression of the NnMSD1 gene, whereas the NnMSD1 transcript level increased strongly in chilling stress treatment. An increase in expression was also highly induced by H2O2 in germinating embryonic axes. The results suggest that the expression pattern of the NnMSD1 gene differed between developing axes and cotyledons, and that the NnMSD1 gene expression responds strongly to chilling and oxidative stress.
The sacred lotus (Nelumbo nucifera Gaertn.) is a primitive angiosperm and one of the oldest plants that has survived from the last ice age. Seeds of the sacred lotus hold the world record for seed longevity, with 80% viability observed in 200–1 300-year-old fruits (Shen-Miller et al. 2002). In order to germinate upon imbibition, an aged seed must be able to quickly repair severe cellular damage accrued over long-term storage. The great ability of lotus seeds to survive in extreme stress conditions is also well documented. Lotus seeds maintained 100% viability even after 24 h treatment in an air oven at 100 °C (Huang et al. 2003) and low-dose soil γ-radiation of 3 Gy did not affect viability (Shen-Miller et al. 2002).
Superoxide dismutases catalyze the breakdown of super-oxide radicals, and provide the first line of defense against oxygen toxicity. They are essential for aerobic life and are involved in stress resistance and longevity (Carlioz and Touati 1986; Orr and Sohal 1994). Three groups of superoxide dismutases (SODs) can be distinguished on the basis of the metal co-factor at the active site: Cu/Zn SOD, Mn-SOD, and Fe-SOD (Fridovich 1989). Mn-SOD and Fe-SOD appear to be closely related both in structural and in evolutionary aspects, but have no resemblance to Cu/ZnSOD (Bowler et al. 1994). Cu/Zn SODs occur in the cytoplasm and chloroplasts; Mn-SODs are usually found in the mitochondrial matrix and in peroxisomes; and Fe-SODs are present within the chloroplasts of some plants (Raychaudhuri and Deng 2000). The roles of SODs under environmental stresses have been studied extensively (Yu and Rengel 1999; Raychaudhuri and Deng 2000). It was reported that the Mn-SOD protected Candida albicans against various stresses (Hwang et al. 2003) and the induced overexpressed Mn-SOD extends the lifespan of adult Drosophila melanogaster (Sun et al. 2002).
Unlike most other organisms, plants contain multiple SOD isozymes. Nine SOD isozymes have been described in maize (Baum and Scandalios 1981) and seven in Arabidopsis (Kliebenstein et al. 1999). The first plant SOD gene was cloned from maize (Cannon et al. 1987). Since then, a number of SOD genes have been cloned in other plant species, for example, tobacco (Bowler et al. 1989), tomato (Perl-Treves et al. 1988), rice (Kaminaka et al. 1999), peach (Bagnoli et al. 2002), aspen (Akkapeddi et al. 1994), Arabidopsis (Kliebenstein et al. 1999), camphor tree (Chen et al. 2002), and cassava (Shin et al. 2005). In the present study, we cloned the Mn-SOD gene, NnMSD1 (GenBank Accession No. AY934858), from sacred lotus, and investigated its expression during seed development and germination, as well as its responses to various stresses.
Results
Structure of the sacred lotus NnMSD1 gene
The full length cDNA of NnMSD1 contains an open reading frame (ORF) of 699 nucleotides, flanked by 35 bp of a non-coding sequence at the 5′ end and 251 bp of a non-coding sequence with a 21 bp poly (A) tail at the 3′ end (Figure 1). Kozak consensus sequence was identified in the 5′-non-coding sequence, which may facilitate the initial binding of mRNA to the small submit of the ribosome. A comparison with the sequence of the NnMSD1 gene and its cDNA sequence indicated that six exons and five introns were present in the gene. The introns have a high AT content (>60%). The Intron 2 and Intron 3 are 102 bp and 142 bp, respectively, which are much shorter than the other three Introns (Table 1). The cDNA of NnMSD1 encodes a protein of 233 amino acid residues with a predicted molecular mass of 25.9 kDa and an isoelectric point of 6.6.
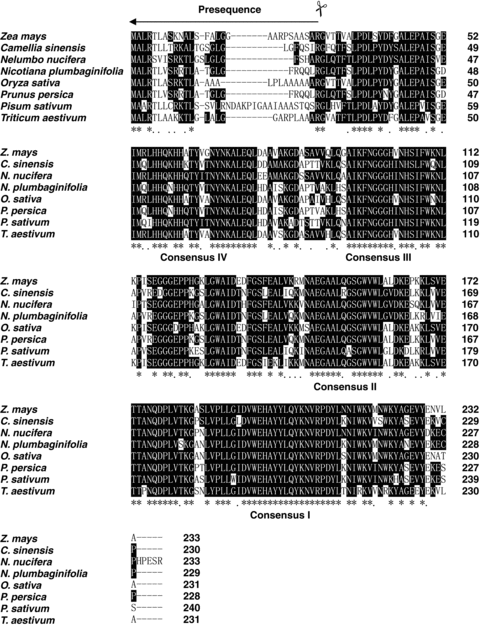
Alignment of the NnMSD1 deduced polypeptide sequence with other manganese superoxide dismutases (Mn-SODs) from various plant species. The amino acid residues conserved in sacred lotus and the other plants are marked by white letters in a black box. The listed Mn-SODs are from Triticum aestivum (AAX68501), Pisum sativum (CAA42737), Zea mays (AAA72020), Oryza sativa (Q43008), Camellia sinensis (AAT68778), Prunus persica (CAB56851), Nelumbo nucifera (AAX22235) and Nicotiana plumbaginifolia (CAA32643).
| Intron | Intron/exon boundaries | Size (bp) | A + T (%) | Nucleotide position |
|---|---|---|---|---|
| 1 | AG/gtaatt…tgcag/GG | 772 | 65.2 | 322–1 094 |
| 2 | AGT/gtaagt…tgcag/GA | 104 | 72.1 | 1 141–1 244 |
| 3 | TG/gtaagt…ggcag/TG | 142 | 66.9 | 1 371–1 512 |
| 4 | AG/gtgaagt…tacag/GA | 924 | 66.3 | 1 570–2 493 |
| 5 | CAG/gtgag…tgcag/TA | 723 | 62.8 | 2 572–3 294 |
Comparison of the amino acid sequences of Mn-SOD genes from different species and analysis of conserved residues
Alignment of the deduced amino acid sequence of Mn-SOD from sacred lotus (AAX22235) with those from the other plants indicates that the N-terminal regions are less conserved (Figure 2). The sequences of Mn-SOD from Triticum aestivum (AAX68501), Pisum sativum (CAA42737), Zea mays (AAA72020), Oryza sativa (Q43008), Camellia sinensis (AAT68778), Prunus persica (CAB56851), Arabidopsis thaliana (AAC24832), Nicotiana plumbaginifolia (CAA32643) and sacred lotus share similarities that range from 73% to 78%. In addition, the sequences of Fe-SOD from P. sativum (CAD42655), Z. mays (BAD89495), A. thaliana (NP849441), O. sativa (XP550626), Lycopersicon esculentum (AAQ18699), Glycine max (AAQ13492), Vigna unguiculata (AAF28773) and Mn-SOD from sacred lotus share similarities that range from 25% to 30%.

Phylogenetic relationship of the deduced amino acid sequences of manganese superoxide dismutase (Mn-SOD) and Fe-SOD families from different plant species. Cluster analysis was carried out using the DNAMAN Alignment Program. The species and corresponding accession numbers are as follows. Mn-SOD: Triticum aestivum (AAX68501), Pisum sativum (CAA42737), Zea mays (AAA72020), Oryza sativa (Q43008), Camellia sinensis (AAT68778), Capsicum annuum (AAB88870), Avicennia marina (AAN15216), Euphorbia esula (AF242310), Zantedeschia aethiopica (AF094832), Prunus persica (CAB56851), Nelumbo nucifera (AAX22235), N. plumbaginifolia (CAA32643) and A. thaliana (AAC24832); Fe-SOD: P. sativum (CAD42655), Z. mays (BAD89495), A. thaliana (NP-849441), O. sativa (XP-550626), Lycopersicon esculentum (AAQ18699), Glycine max (AAQ13492), and Vigna unguiculata (AAF28773).
Although plant Mn-SOD N-terminal regions are poorly conserved, they have the general features of mitochondrial targeting sequences, which direct Mn-SOD proteins to the mitochondrion. The N-terminal sequence of NnMSD1 shares common characteristics with mitochondrial transit peptides, such as an abundance of basic residues and the complete absence of acidic residues. Based on signal PV2.0 (Nielsen et al. 1997) and TARGET PV1.0 (Emanuelsson et al. 2000) prediction to N-terminal peptides, the deduced sacred lotus peptide has an N-terminal signal peptide of 24 amino acids (MALRSVISRKTLGSLGLGFSHARG) for mitochondrial subcellular localization. The Mn-SOD sequences contain four perfectly conserved regions in all plant species, namely consensus I to IV (Figure 1). Homology is most conserved in the domains corresponding to α-helices one (consensus V) and two (consensus III). In addition, the amino acids involved in the coordination of the metal ions are highly conserved (Borgstahl et al. 1992). After removing the 24 amino acid signal peptides, the predicted mature sacred lotus Mn-SOD and the other plant Mn-SODs share sequence similarities ranging from 77% to 81%. The predicted mature NnMSD1 showed highest homology (81%) to the Mn-SODs from C. sinensis, P. persica, and N. plumbaginifolia. The mature sacred lotus Mn-SOD protein has a calculated molecular mass of approximately 23.4 kDa and a pI of 5.9.
Phylogenetic analysis of Mn-SODs from different plant species revealed two subgroups (Figure 2). It should be noted that all Mn-SODs from cereals are grouped in the same subgroup. Subgroup 1 consists of the sequences from P. sativum, C. sinensis, A. thaliana, P. persica, N. plumbaginifolia, N. nucifera, Avicennia marina, Capsicum annuum, Euphorbia esula, and Zantedeschia aethiopica.
Genomic organization of NnMSD1 gene
In order to determine the copy number of the NnMSD1 gene, the genomic DNA (5 μg) of sacred lotus leaves was digested with EcoRI, HindIII, or BglII and hybridized with a 423 bp 32P-labeled probe. Two strongly hybridizing bands were detected for each restriction enzyme (Figure 3). These data indicate that there are two NnMSD1 genes in the sacred lotus genome.
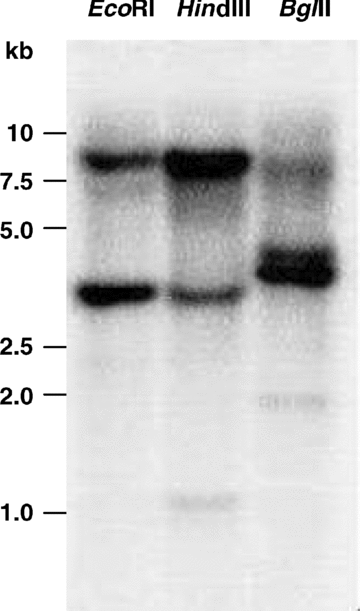
Southern blot of genomic DNA from sacred lotus leaves. Five micrograms of genomic DNA was digested with the restriction endonucleases (EcoRI, HindIII, and BglII), separated by agarose gel and transferred onto a piece of nylon membrane (Hybond-N+, Amersham Biosciences). The blotted membrane was hybridized with a [32P] -labeled probe from NnMSD1.
Expression of NnMSD1 gene in developing and germinating seeds
The development pattern of sacred lotus seeds is similar to that of most dicot seeds. There are three stages in the sacred lotus seed development: tissue differentiation (0–13 d after pollination, DAP), cell expansion (14–20 DAP) and desiccation-maturation stage (21–30 DAP). The expression patterns of the NnMSD1 gene were investigated during seed development by reverse transcriptase-polymerase chain reaction (RT-PCR) (Figure 4A). Strong and constant levels of NnMSD1 expression were detected in sacred lotus embryonic axes during seed development. The cotyledons showed low NnMSD1 gene expression on the 15 DAP, whereas there was no detectable NnMSD1 transcript in the cotyledons of 20 DAP and 25 DAP (Figure 4A).
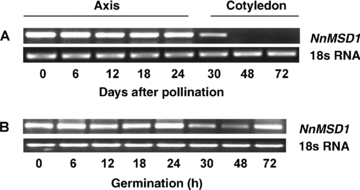
Expression of NnMSD1 gene in developing and germinating embryonic axes and cotyledons of sacred lotus by reverse transcription-polymerase chain reaction (RT-PCR). (A) Expression pattern of NnMSD1 gene in developing sacred lotus axes and cotyledons. (B) Expression profile of the NnMSD1 gene in germinating sacred lotus axes. 18s RNA was used as a control for equal loading.
The expression profile of the NnMSD1 gene during sacred lotus seed germination was examined using gene-specific primers (Figure 4B). NnMSD1 transcript was detected in mature dry embryonic axes. Within 24 h after imbibition, the transcript levels of the NnMSD1 gene in sacred lotus embryonic axes were the same as in the dry embryonic axes. However, its expression decreased obviously in the 30 h and 48 h after imbibition, and was restored in the 72 h after imbibition (Figure 4B).
Expression patterns of the NnMSD1 gene in germinated embryonic axes under various stress conditions
The expression patterns of the NnMSD1 gene in germinated sacred lotus embryonic axes under various stresses, such as heat shock (42 °C), chilling (4 °C), and exposure to stress-related chemicals, were investigated by RT-PCR. The stress-related chemicals include polyethylene glycol (PEG)-6000 (20% w/v), H2O2 (1.5%), NaCl (300 mmol/L), and abscisic acid (ABA) (100 μmol/L). The results of RT-PCR analysis show that NnMSD1 in the germinated embryonic axes responded strongly to chilling and oxidative stresses. However, NnMSD1 gene expression in germinated embryonic axes was inhibited by heat-shock and NaCl. No obvious effects of ABA and PEG on the NnMSD1 gene expression were observed (data not shown).
To get more information about the expression of NnMSD1 under chilling and oxidative stresses, real-time PCR was used to study the transcription of NnMSD1 after chilling and 1.5% of H2O2 treatments, respectively. For studying the effects of chilling on the NnMSD1 mRNA levels, 3-d-old germinated sacred lotus seeds were treated with chilling (4 °C) for 2, 6, 12, 24 and 48 h, respectively. NnMSD1 was strongly expressed after 2–48 h chilling treatment (Figure 5A).
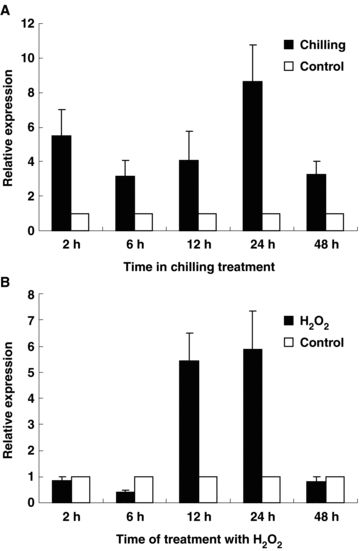
Real-time polymerase chain reaction (PCR) analysis of NnMSD1 expression in germinated sacred lotus embryonic axes under chilling and H2O2 stress. (A) Chilling. (B) H2O2 treatment.
To study NnMSD1 gene response to H2O2, the 3-d-old germinated seeds were incubated separately in aqueous solutions containing 1.5% of H2O2 for 2, 6, 12, 24 and 48 h, respectively. The results suggested that the expression of NnMSD1 in the germinated embryonic axes was inhibited after 2, 6 and 48 h treatment, but stimulated significantly after 12 and 24 h treatment (Figure 5B).
Discussion
We have isolated and characterized the NnMSD1 gene encoding a Mn-SOD from sacred lotus embryonic axes. NnMSD1 has six exons and five introns, which is consistent with the MnSOD genes identified from other plant species (Fink and Scandalios 2002). The deduced amino acid sequence of NnMSD1 revealed a high degree of homology with Mn-SODs from other plants (Figure 1). Sacred lotus mature Mn-SOD protein displays high homology (81%) to the Mn-SOD proteins from C. sinensis, P. persica, and N. plumbaginifolia. The 24-amino acid stretch located upstream of the immature Mn-SOD protein could be the leader sequence for translocation into the mitochondrial matrix (Schatz and Krebs 1987). Two copies of the MnSOD gene are present in the sacred lotus genome, which is similar to the peach MnSOD gene (Bagnoli et al. 2002), whereas in maize and wheat the corresponding genes have been reported to belong to small multigene families (Zhu and Scandalios 1994; Wu et al. 1999). The existence of two sacred lotus MnSOD copies could provide an explanation for the variation in the number of Mn-SOD isoforms found in the cotyledons (Huang et al. 2000) and the other different tissues analyzed (data not shown). In contrast with our results, Ushimaru et al. (2001) reported that seven electrophoretically distinct SOD isozymes, two Fe-SOD and five Cu/Zn-SOD isozymes, were detectable in sacred lotus seedlings. No Mn-SOD isozyme was detected in their experimental system.
The roles of SODs in plants under environmental stresses have been studied extensively. It was reported that the Mn-SOD protected Candida albicans against various stresses (Raychaudhuri and Deng 2000). Plants often experience temperature stress during their life cycle (Stone 2001). The expressions of Mn-SOD genes under temperature stress were studied extensively in plants. The Mn-SOD gene was induced in both winter and spring cereals that were subjected to cold-acclimating conditions (Wu et al. 1999). However, Tsang et al. (1991) found that the expression of Mn-SOD gene in tobacco was unaffected by chilling but that SOD gene expression increased rapidly after the plants were returned to their normal growth temperature. It was reported that transgenic alfalfa overexpressing Mn-SOD had enhanced freezing-stress tolerance (McKersie et al. 1993). The presence of transgenic Mn-SOD had clear effects on maize foliar tolerance to chilling (Breusegem et al. 1999). Our results indicated that the chilling caused an increase in the NnMSD gene expression in 48 h treated period (Figure 5A). However, heat shock always induces the expression of the Mn-SOD gene (Privalle and Fridovich 1987). In the germinating sacred lotus seeds, heat shock significantly inhibited NnMSD1 gene expression. Considering that the breakdown of superoxide radicals may produce excessive H2O2, which will do harm to the cell, the inhibition can be protective to the germinating seeds.
When seedlings were treated with NaCl, the content of H2O2 was elevated. At the same time, the expression of Mn-SOD was preferentially elevated by salt stress in rice and mangrove (Fadzilla et al. 1997; Lee et al. 2001; Parida et al. 2004). Furthermore, with long term NaCl treatments, the transcript level for mitochondrial Mn-SOD was strongly induced in the NaCl-tolerant variety of pea, but not in the NaCl-sensitive variety (Hernández et al. 2000). It strongly suggested that oxidative stress was induced by salt stress and Mn-SOD play an important role in the response of plants to salt stress. This was proved by the fact that transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance (Wang et al. 2004). Our results showed that the oxidative stress (H2O2) treatment could increase the expression of the NnMSD1 gene in sacred lotus germinating axes (Figure 5B). However, the expression of the NnMSD1 gene was inhibited completely by 300 mmol/L NaCl treatment. Therefore, the variety of early germinating sacred lotus seeds was argued to be sensitive to strong salt stress. Since maize Sod3.1 does not respond to ABA, whereas the expression maize Sod3.2, Sod3.3 and Sod3.4 are induced by ABA (Zhu and Scandalios 1994), it was possible that different transcripts of Mn-SOD genes showed various responses to ABA.
Since overexpressing Mn-SOD extends the lifespan of adult Drosophila melanogaster (Sun et al. 2002), it was intriguing to study the function of the NnMSD1 gene on sacred lotus seed longevity. In our previous study, we demonstrated that the sacred lotus embryonic axes contained more than four isoforms of Fe-SOD and one Mn-SOD (Huang et al. 2000). In addition, the total activity of SOD in sacred lotus developing embryonic axes slightly increased from 6 DAP to 20 DAP, then increased sharply and reached its maximum at 25 DAP, and this high activity of SOD was retained to complete maturation of the seed (Huang et al. 2003). The results in this paper showed that strong and constant expression of the NnMSD1 gene was detected in sacred lotus embryonic axes during seed development and maturation. Furthermore, the abundant NnMSD1 transcripts also existed in the axes of mature and dry seeds (Figure 4A). Our studies suggest a possible role of Mn-SOD in sacred lotus seed tolerance against environmental stresses. The function of sacred lotus NnMSD1 gene in seed thermotolerance will be studied further by developing transgenic plant lines.
Materials and Methods
Plant materials and treatments with exogenous factors
Seeds of sacred lotus (Nelumbo nucifera cv. Hongtoujin) were collected from plants grown in ponds in Sanshui Lotus World, Guangdong Province, China. Developing embryonic axes were used for gene cloning and RNA preparation in the study of gene expression during seed development. Mature seeds were used in germination test. Seeds were germinated in distilled water at 25 °C for 0, 1, 2, and 3 d. Seeds that germinated for 3 d were subjected to various stress treatments by submerging in aqueous solutions containing PEG 6000 (20% w/v), H2O2 (1.5%), NaCl (300 mmol/L), and ABA (100 μmol/L) for 24 h or incubating at different temperatures (heat shock at 42 °C and low temperature at 4 °C) for up to 48 h. All treated materials were immediately frozen in liquid nitrogen and stored at −70 °C until use.
RNA preparation and RACE
Total RNA was isolated from developing embryonic axes by using Trizol Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. Adapter ligated cDNA was synthesized using the SMART rapid amplification of cDNA ends (RACE) cDNA amplification kit (BD Biosciences, San Jose, CA, USA) according to the product manual. 5′-RACE was carried out using 5′ primer and the degenerate primer (DP: 5′-AGGTAGTAA/CGCATGCTCCCA-3′) designed from the conserved region of the plant SODs. 3′-RACE was carried out using CDSIII /3′ PCR primer and the gene specific primer (GSP1: 5′-TTGAAGCATTGGTGAAAAAGG-3′) designed according to sequence of the 680 bp fragment. PCR products were purified and sequenced. As the first strand cDNA was synthesized with the SMART cDNA synthesis kit, only one gene-specific primer was used for 5′ RACE based on the conserved domain WEHAYY of Mn-SOD. The 5′ end RACE product was recovered and sequenced directly. A gene-specific primer was designed for 3′ RACE according to the acquired 5′ fragment of the NnMSD1 gene from sacred lotus cDNA. After the two sequences were assembled, the full length cDNA of NnMSD1 was obtained.
DNA and PCR amplification
Genomic DNA was extracted from sacred lotus leaves according to the protocol described by Pich and Schubert (1993). Two gene-specific primers GSP2 (5′-GGCGCTGATCAAATTGGAAG-3′) and GSP3 (5′-GAAGACTGCCTGCCCAACTC-3′) were designed based on the sequence of the acquired NnMSD1 cDNA to amplify the corresponding genomic DNA sequence. The PCR product of 3469 bp was sequenced directly.
Analysis of the DNA sequence
The homologous sequences of the gene NnMSD1 were searched using BLAST (Altschul et al. 1990) and the sequence alignments were carried out using the Cluster W program (Thompson et al. 1994). The intron position of NnMSD1 gene was determined by comparison of the genomic DNA sequence with the corresponding cDNA sequence.
Southern blot analysis
Five micrograms of genomic DNA was digested with the restriction endonucleases EcoRI, HindIII, and BglII at 37 °C overnight, and the resulting fragments were resolved in a 0.8% agarose gel and transferred onto a piece of nylon membrane (Hybond-N+, Amersham Biosciences, Uppsala, Sweden). The blotted membrane was UV cross-linked and hybridized with a [32P]-labeled probe from NnMSD1 (position 1 281–1 703). In the high stringency condition, the membrane was hybridized at 42 °C for 14 h and washed twice with 0.1% sodium dodecyl sulfate (SDS) in 2 × standard saline citrate (SSC) (150 mmol/L NaCl, 15 mmol/L sodium citrate) solution for 15 min, and then twice with 0.1% SDS in 0.2 × SSC solution for 15 min at 42 °C. Signals were quantified using a Molecular Imager FX (Bio-Rad, Hercules, CA, USA).
RT-PCR and real-time PCR analysis
Total RNA was exacted from the embryonic axes and cotyledons by using Trizol Reagent (Invitrogen), according to the manufacturer's instructions. First strand cDNA was synthesized with total RNA using the TaKaRa (Shiga, Japan) RNA PCR Kit (AMV) Ver. 3.0. The relative levels of Mn-SOD were measured by RT-PCR using N. nucifera 18s rRNA as an internal standard. Second-strand cDNA synthesized and PCR amplification with Mn SOD gene-specific primers (forward primer: 5′-CCATCACCAGAAGCACCATC-3′; reverse primer: 5′-TTTAACCACGCCTAGCCACA-3′) were carried out using the following conditions: 28 cycles of denaturaton at 94 °C for 30 s, annealing at 58 °C for 1 min and extension at 68 °C for 1.5 min. The amplification of the internal standard 18s rRNA cDNA was carried out in a similar manner, but with 22 cycles using two 18s rRNA-specific primers (forward primer: 5′-CCATAAACGATGCCGAC-3′, reverse primer: 5′-CACCACCCATAGAATCAAGA-3′). RT-PCR product was separated on a 2% agarose gel and visualized by ethidium bromide staining. The expected size of RT-PCR product of the NnMSD1 gene was 325 bp. Real-time PCR was carried out and analyzed with an IQ5 Multicolor Real time PCR Detection System (Bio-Rad) following the manufacturer's instructions with another NnMSD1 gene-specific primer (forward primer: 5′-TTATGCGGCTCCATCACCA-3′; reverse primer: 5′-TAACCACTGCGGACGAATC-3′). The program was 95 °C denaturation for 30 s, 40 cycles of 95 °C for 10 s, 56 °C for 15 s, and 72 °C for 20 s.
(Handling editor: Lizhe An)
Acknowledgements
The authors thank Tara Rintoul for editorial reading of the manuscript.




