Fragment Propagation and Colonization Ability Enhanced and Varied at Node Level after Escaping from Apical Dominance in Submerged Macrophytes
Supported by the Hi-Tech Research and Development (863) Program of China (2003AA06011000-04 and 2002AA601012-06).
Abstract
Aquatic plants develop strong fragment propagation and colonization ability to endure the natural disturbances. However, detailed research of ability to endure the natural disturbances has been lacking to date. Therefore, reproduction (shoot) and colonization (root) of shoot fragments of Potamogeton crispus L. with or without apices were investigated for the effect of apical dominance, and the growth of decapitated shoot fragments at three lengths (2, 4, 6 cm) was compared. Meanwhile, fragment propagation at levels of bud position was studied for bud position effect after escaping from apical dominance. The results showed significant increases occurred in the outgrowth of lateral branches on fragments decapitated compared with the fragments with apices, implying that apical dominance exists. Different lengths of fragments showed little difference in biomass allocations, but significant differences were noted in their propagation. Meanwhile, the effect of bud position was verified, due to the significant difference of average reproduction per node among the three length groups. Thus, the present study has made progress in the current understanding of aquatic plant dispersion among natural systems and contributes to improve methods of in vitro propagation for re-implantation purposes.
Natural physical and biological disturbances, such as current velocity, floods, and fish grazing play an important role in vegetation structures and distribution in aquatic ecosystems (Nilsson 1987; Trémolières et al. 1994; Bornette and Amoros 1996). Correspondingly, aquatic plants develop various strategies to endure these disturbances and survive. In these strategies, enhanced means of dispersal and ability to propagate play an important part (Santamaría 2002). It is widely known that most aquatic plants exhibit asexual reproduction by forming specialized propagules, like stolons, rhizomes and turions, or unspecialized fragments (Van et al. 1978; Titus and Hoover 1991; Barrat-Segretain 1996). Some early reports found that the scouring caused by flooding favored regeneration through unspecialized fragments in aquatic plants and these fragments contributed to species maintenance (Combroux and Bornette 2004). Then, if an unpredictable disturbance like flood or grazing occurs, these plants, particularly the fragile submerged macrophytes, may be fragmented and the fragments may be then washed away by running water and dispersed to other habitats (Johansson and Nilsson 1993; Barrat-Segretain et al. 1998). There they develop root systems and establish rapidly in sediments (colonization) (Barrat-Segretain and Bornette 2000). In such events, different types (with or without apices) and different lengths of fragments (e.g. shoot fragments) would create, survive and colonize a new habitat (Barrat-Segretain et al. 1998). Therefore, all of these fragments formed were believed to contribute to distant dispersal and colonization according to the early findings (Kimbel 1982; Sabol 1987; Campbell 2003). Some reports believed that plant recovery could occur once the fragments were recruited (Hemminga and Duarte 2000).
Wu et al. (2007) found that fragments of Myriophyllum spicatum with apices sank more rapidly than the ones without apices. It can then be deduced that fragments without apices have longer dispersal distances. It was also found that mid-stem fragments had stronger capability of regenerating lateral shoots than fragments with apices (Wu et al. 2007). This may be caused by apical dominance, which has been widely studied in terrestrial plants (Cline 1991; Irwin and Aarssen 1996; Salemaa and Sievänen 2002). Most of these studies confirmed enhanced growth of lateral branches after escaping from apical dominance by decapitation (Cline 1991, 1994, 1996; Bangerth 1994). Although apical dominance was first reported to be found in an aquatic species a decade ago (Terrados et al. 1997), little notice has since been taken in the studies that followed.
Early reports found that longer fragments sank slower, which would ensure longer dispersal distances and then affect the plant dispersal patterns (Wu et al. 2007). It was also found that longer fragments had stronger reproductive capability (Wu et al. 2007). This may be a result of more nodes (resting lateral buds) on longer fragments and/or different reproduction ability among nodes from different positions. Some experts reported that buds at different positions on the same single shoot had different re-growth abilities after decapitation (De Vries and Dubois 1992). However, no consistent conclusion has been made. Bressan et al. (1982) concluded that the buds from medial sections developed more rapidly than those of either the upper or basal positions, whereas Cline (1996) believed that only the highest lateral buds grew out following decapitation. De Vries and Dubois (1992) suggested that the effect of bud position accounted for approximately 30% of the differences observed in flower yield. Therefore, it was very interesting to investigate the different re-growth ability of fragments at different lengths and of nodes from different positions after apex removal.
In order to investigate the effect of apex removal, fragment length and bud position effect on fragment re-growth (including reproduction and colonization) in submerged plants, Potamogeton crispus was chosen as a study model. This species is often used as a pioneer species in lake re-vegetation projects for its wide range of climatic habitats and effective nutrient reduction ability (Ren et al. 1997; Zhou et al. 2000; Lauridsen et al. 2003). Just like other aquatic macrophytes, it plays a great role in many aquatic ecosystems (Vermaat et al. 2000; Kufel and Kufel 2002). In the present study, aseptic environments and shoot fragments were used for excluding other biotic influences (Xie et al. 2005), and the following hypotheses were to be tested: (i) fragment reproduction and colonization ability would be greatly enhanced after apex removal; (ii) longer fragments had stronger reproduction and colonization ability; and (iii) nodes from different positions would have different re-growth capabilities.
Results
Growth variations between shoot fragments with and without apices
There was great difference between the growth of shoot fragments with apices and those without apices (Table 1). Dry weight of shoot fragments without apices was significantly greater than the ones with apices (P < 0.05). However, the treatment of decapitation did not show a great effect on biomass allocation to below-ground and above-ground organs, as the root ratios were not significantly different between fragments with and without apices (P > 0.05). Fragments without apices had a significantly greater shoot number compared with the ones with apices (P < 0.05), implying that decapitation greatly enhanced the reproduction ability of fragments. Root number and total root length were significantly greater for the fragments without apices (P < 0.05), which implied the enhanced colonization ability after decapitation.
| Treatments | Dry weight (mg) | Root ratio (%) | Shoot number (ind) | Root number (ind) | Total root length (cm) |
|---|---|---|---|---|---|
| Without apex | 194.100 ± 15.200a | 3.380 ± 0.760 | 14.000 ± 1.060 | 42.000 ± 3.600 | 65.970 ± 4.750 |
| With apex | 71.700 ± 5.300 | 2.720 ± 0.370 | 8.330 ± 0.760 | 25.970 ± 1.380 | 37.270 ± 3.770 |
| P values | 0.000 | 0.485 | 0.003 | 0.003 | 0.001 |
- aMean ± SE; n = 5 for both treatments; root ratio calculated as root dry weight divided by dry weight of the whole individual. Independent-Samples T test was used at the 0.05 significance level.
Growth variation of decapitated shoot fragments at different length levels
Dry weight significantly increased with the increase of the fragment length among all treatments at the fragment level (Table 2). However, length treatment had little effect on biomass allocation (P > 0.05), including root ratio, stem ratio and leaf ratio. Shoot number showed significance at the fragment level among three length treatments (P < 0.05), and it was greatest for fragments of 6 cm in length compared with the other two treatments. Root number and total root length showed slight variations among all length treatments (P > 0.05).
| Dependent variables | Fragment lengths | Tests | ||||
|---|---|---|---|---|---|---|
| 2 cm | 4 cm | 6 cm | df1 | df2 | P | |
| Dry weight (g) | 0.190 ± 0.020b | 0.250 ± 0.030b | 0.320 ± 0.020a | 2 | 12 | 0.006 |
| Root ratio (%) | 3.380 ± 0.760 | 3.400 ± 0.070 | 3.610 ± 0.340 | 2 | 6 | 0.935 |
| Stem ratio (%) | 40.310 ± 1.330 | 43.390 ± 0.400 | 44.050 ± 2.050 | 2 | 6 | 0.223 |
| Leaf ratio (%) | 56.310 ± 0.580 | 53.210 ± 0.430 | 52.340 ± 1.700 | 2 | 6 | 0.085 |
| Shoot number (ind) | 14.000 ± 1.060b | 17.330 ± 2.510b | 22.750 ± 0.750a | 2 | 12 | 0.036† |
| Root number (ind) | 42.000 ± 3.600 | 51.200 ± 4.840 | 56.400 ± 5.390 | 2 | 12 | 0.129 |
| Total root length (cm) | 65.970 ± 4.750 | 76.100 ± 9.130 | 88.700 ± 7.460 | 2 | 12 | 0.132 |
- Values are mean ± SE; df1, df2 represent degrees of freedom between groups and within groups, respectively. Root/stem/leaf ratio was calculated as root dry weight divided by dry weight of the whole individual. The different upper case letters indicate significant differences among treatments by Duncan's test at the 0.05 significance level. † Shoot number was tested by Kruskal-Wallis H test at the 0.05 significant level as the homogeneity of variances was not assumed by Levene's test.
Average growth per node of decapitated shoot fragments
Average fresh weight, root number and shoot number per node for fragments at different lengths at different growth times were calculated and statistically analyzed (Figure 1). At the 6th and 12th d of growth, all of these traits showed no significance among all length treatments (P > 0.05). However, at the 9th d of growth, the root number and shoot number varied significantly (P < 0.05). At the 15th or 18th d of growth, there was still no significance for fresh weight per node among treatments (P > 0.05), whereas it was significant for root number and shoot number (P < 0.05). With the increase of fragment length, there was a great decrease in root or shoot number during this growth period.
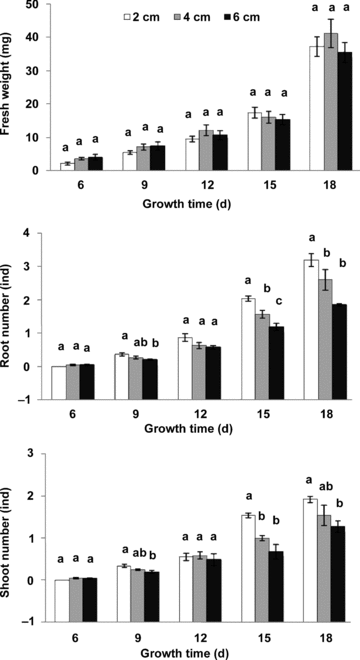
Average fresh weight/root number/shoot number per node at each measuring time.Data at 6, 9, 12, 15 and 18 d of growth were compared. Different letters a, b and c, above the columns at each measuring time indicate the significant variations among the treatments. Kruskal-Wallis H test was used as multiple comparisons for means of average shoot number per node among all treatments at first measuring time, while the others were carried out by Duncan's test. The level of significance was 0.05.
The significant differences for average shoot number and root number per node among length treatments at the final harvest were supplements to the results above during the growing period (P < 0.01) (Figure 2). The average shoot number and root number per node both greatly decreased with increases in the original fragment length. Average dry weight per node also showed significance and was greatest at 2 cm in length (P < 0.01). Length treatment showed a great effect on average total root length per node (P < 0.01).
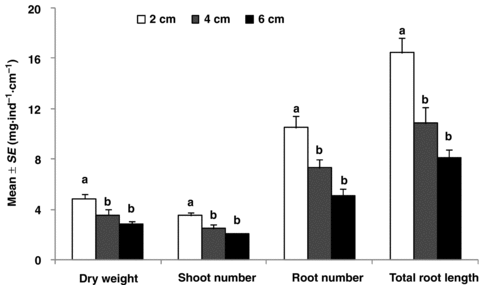
Variations of the average growth per node at final harvest.All of the parameters were at node level, which were calculated as data of fragments divided by node number contained. Dry weight was divided by 10 for the readability of the figure. Different letters, a and b, above the columns of each parameter indicate the significant difference among the treatments. Kruskal-Wallis H test was used as multiple comparisons for means of average shoot number per node among all treatments, while the others were carried out by Duncan's test. The level of significance was 0.05.
Discussion
Apical dominance regulated fragment reproduction and colonization ability
According to our results, fragment reproduction ability (shoot number) was greatly enhanced after the apex removal, just like the early reports in other terrestrial and aquatic plants (Terrados et al. 1997; Salemaa and Sievänen 2002). Therefore, we have convincing evidence that apical dominance does exist in P. crispus. Sadras and Fitt (1997) concluded that a large variability in the degree of apical dominance among cotton genotypes existed that could influence resistance to herbivory. Apical dominance may also be one of the strategies used for adapting to unfavorable environments for this species, like fish herbivory or mechanical damage. The following enhanced outgrowth of lateral buds after decapitation increases the possibility of surviving at the original habitat. Moreover, the enhanced development of root systems favors the re-establishment of the plants. However, the results showed that the total biomass was also greatly increased after escaping from the apical dominance, which was quite different from the early results (Terrados et al. 1997).
Fragments without apices had longer dispersal distances and it would help the species to explore new suitable habitats (Wu et al. 2007). Stronger capability of fragment re-growth after apex removal would greatly promote the possibilities of colonization, and then it greatly affected the distribution of plants in natural aquatic ecosystems. Furthermore, from the early reports, it is generally agreed that polar auxin transport plays a central role in this phenomenon (Cline 1991, 1994), and other phyto-hormones may take part (Li et al. 1995; Wang et al. 2006). Therefore, a further study is needed to investigate the active hormones for apical dominance in P. crispus.
Fragment re-growth of different lengths after apex removal
Different lengths of fragments had little variations on biomass allocation in our experiment. It may be because the same resources were available for all of these fragments, as biomass allocation is very much related to the environmental resources (Xie et al. 2005). However, like other reports, longer fragments had stronger reproduction abilities (Wu et al. 2007). Early reports have found that fragment length had an effect on its dispersal pattern and longer fragments had longer dispersal distances (Santamaría 2002; Wu et al. 2007). Therefore, the stronger ability for reproduction of longer fragments would favor the wider distribution and better survival of plants at new habitats. As we all know, P. crispus generates shoots from the nodes on stems. Therefore, more nodes (resting buds) contained in longer fragments may ensure a stronger reproduction ability. Moreover, such ability may be influenced by the node position effect, which has been discussed above (De Vries and Dubois 1992). In our experiment, no significant difference in root parameters was found (root number and total root length) among different lengths of fragments, which implied that the colonization ability was not greatly varied by length treatments. We may suppose that this was caused by different re-growth ability of nodes from different positions on fragments.
Bud position effect affected the nodal propagation and colonization capability
Average nodal re-growth ability of fragments at different lengths, which represents the growth of nodes from different positions, was quite different after apex removal (Figures 1,2). This difference may prove the existence of the bud position effect in this species. Some researchers have made a conclusion that the buds from the upper side developed more rapidly than the basal side (Zieslin et al. 1976; Marcelis-van Acker 1994), and this was quite in accordance with our experiment. The average nodal re-growth ability of fragments at 2 cm, which represented the node from the upper section, had the greatest shoot number, root number and total root length at the final harvest compared with the ones at 4 cm and 6 cm (Figure 2). However, such a significant difference was not shown until the 15th d of growth (Figure 1), as it needed time for activation as reported elsewhere (Sadras and Fitt 1997). The difference shown at 9 d growth might be caused by random outgrowth as there was no significant difference at the next measuring time. Early reports have found that the shoots yield increased basipetally (De Vries and Dubois 1992). However, this was not the same as the results in our experiment because average dry weight per node became greater with decreases in fragment length (Figure 2).
The existence of a bud position effect may imply an effective strategy for aquatic plants adapting to natural disturbances. As the upper section of the submerged plants would be easier to be detached and washed away by running water, the stronger re-growth ability of nodes in upper sections may benefit the survival and colonization of the species at new habitats. On the other hand, stronger reproduction ability of nodes in the upper sections may favor the formation of canopies in this species, and then benefit the light acquisition and population growth (Nichols and Shaw 1986).
Perspectives of application for in vitro techniques and ecosystem restoration
In most vegetation restoration work, transplantation of propagules or seedlings collected from donor ecosystems is commonly used. This would evidently affect the donor ecosystems and risk bringing nuisance species into target ecosystems (Confer and Niering 1992; Brown and Bedford 1997). Hence, a new plant propagation method has been developed to get large amounts of healthy plant materials for in vitro culture (Zhou et al. 2006). The method was mainly based on the fragment reproduction of these species (Barrat-Segretain et al. 1998; Zhou et al. 2006). According to the findings of apical dominance and bud position effect in the present study, an improved method of in vitro culture can be suggested. In this method, the upper section of the stem is selected and the apex of the shoot fragment is removed. Therefore, we can take full advantage of the great re-growth ability of lateral buds and greatly promote the effectiveness of a vast scale of plant propagation. Moreover, based on the finding of apical dominance, we can manually clip the apex of plants to speed up the process of establishment after colonization in re-vegetation projects. All of these findings would be beneficial to the success of eutrophication restoration programs.
Materials and Methods
Plant materials
The original plant materials of Potamogeton crispus L. were collected from Taihu Lake (30°5′–32°8′N, 119°8′–121°6′E) in March, 2003. Turions were selected, then washed and incubated in tap water for several hours to remove dust and some bacteria on the surface. All turions were then disinfected with the common practice of tissue culture process (Bouman et al. 1995; Martin et al. 2003; Rout et al. 2006). Here 0.1% of mercuric chloride was used for several minutes in the disinfection process, followed by the rinsing procedure in sterile distilled water five times. Then all of the disinfected materials were transferred to autoclaved solid media based on Murashige and Skoog-based media (Murashige and Skoog 1962) plus 3% sucrose (MS). Sterile distilled water was used to cover the materials for protecting them from drought over the solid media. After 15 d of incubation, aseptic seedlings were obtained. Then healthy ones were selected and transferred into the autoclaved liquid media which was also based on MS for clonal propagation. Enough aseptic seedlings were collected after culture for more than six generations in a year. Shoots similar in size to the same generation without roots were used for experiments from 28 June to 25 July 2004. All of the procedures were undertaken in a tissue culture room and kept aseptic. A 12-h light period with 30 μEm−2s−1 fluorescent lights were used in the tissue culture room and the temperature was kept at 25 ± 1 °C.
Experimental designs
Two experiments were undertaken to examine the apical dominance (recorded as experiment 1), fragment growth and bud position effect (recorded as experiment 2) simultaneously (Table 3). In experiment 1, shoot fragments of 2 cm in length with apices were tested for the apical dominance effect contrasting to the growth of 2 cm fragments without apices. All shoot fragments were cultured in 150-mL flasks with 80 mL autoclaved MS liquid medium in the tissue culture room mentioned above. Five flasks (five replicates) were used for both treatments. Each flask contained three fragments. Therefore, 15 fragments altogether were used for both treatments. All of the media were renewed every 3 d. In experiment 2, the growth of shoot fragments beheaded at three length levels and the average growth per node was compared. The fragments were at 2 cm, 4 cm and 6 cm in length, which contained 4, 7 and 11 nodes, respectively. Three at 2 cm or 4 cm in length were taken as one group cultured in one flask, and two for 6 cm. Five replicates were also used for each group; therefore, 15 fragments in total for both 2 cm and 4 cm treatments, and 10 fragments for 6 cm treatments were tested. All of these shoots were also cultured in 150-mL flasks with 80 mL autoclaved MS liquid medium, and the media were renewed every 3 d until harvest. Shoots of 2 cm in length and without apices were shared in both experiments.
| Apex treatment | Fragment length (cm) | Node contained (ind) | Replicates | Node positiona |
|---|---|---|---|---|
| Apical dominance experiment (experiment 1) | ||||
| With apex | 2 | 4 | 5 | |
| Without apex | 2 | 4 | 5 | |
| Fragment growth and bud position effect experiment (experiment 2) | ||||
| Without apex | 2 | 4 | 5 | Upper |
| Without apex | 4 | 7 | 5 | Middle |
| Without apex | 6 | 11 | 5 | Lower |
- aAverage growth per node in fragment of 2 cm represents the growth of nodes from relative upper sections in the shoot compared with the fragment of 4 cm or 6 cm, for it has more percentage of upper-side nodes. Similarly, the average growth per node in fragments of 4 cm and 6 cm represent the growth of the node from the relative middle and lower sections, respectively. Two fragments of 6 cm were taken as one group because of the limited culture spaces.
Plant harvest and data collection
For experiment 1, all individuals were harvested after 27 d of growth, then carefully washed in running tap water and measured. Shoot number and root number were counted. The length of each root was measured, and thus the total root length of each individual was calculated. Finally, three individuals of both treatments were randomly chosen from different flasks, and each was divided into roots, leaves and stems. Each part was weighed freshly, and then oven dried at 60 °C with the remaining individuals. Dry weights were collected.
In experiment 2, shoot numbers and root numbers of each individual were counted every 3 d when the media was renewed except for the first time. Meanwhile, the fresh weight of each group was obtained by weight differences of flasks with media before and after transplantation after removing all of the surface solution of plants. After 18 d of growth, the media were still renewed without collecting any data until final harvest after 27 d of growth. Like experiment 1, all individuals were harvested and measured in the same way with the same parameters.
Dry (or fresh) weight, root ratio, stem ratio and leaf ratio were used as the traits of growth in biomass accumulation and allocation. Shoot number was used as the trait of propagation. Root number and total root length were used as the traits of colonization ability.
Data analysis
Means of individual growth parameters, including dry weight, shoot number, root number and total root length in each replicate for all treatments were calculated for later statistical analyses in SPSS 13.0, together with root ratio, stem ratio and leaf ratio. Normal distribution was confirmed by Kolmogorov-Smirnov Test. Levene's test was carried out to examine the homogeneity of variances. In experiment 1, all of the growth parameters were compared by independent-samples T test between the two treatments. In experiment 2, one-way anova was used to compare the variations among treatments. When the requirement of homogeneity of variances was met, multiple comparisons of the means of fresh weight/shoot number/root number per node at each measuring time during growth were carried out using Duncan's test. However, when it was not, for example, average shoot number per node at first measuring time, Kruskal-Wallis H test (non-parameter test) was used as multiple comparisons. Data of the final harvest at fragmental and nodal levels were analyzed in the same way. All of the tests were at 0.05 significant levels.
(Handling editor: Jianxin Sun)
Acknowledgements
The authors thank Wenwen Wang for assistance with some laboratory work, and also express great thanks to Dr Li Zhang for valuable suggestions for this paper.




