Nitric Oxide Reduces Hydrogen Peroxide Accumulation Involved in Water Stress-induced Subcellular Anti-oxidant Defense in Maize Plants
Abstract
Nitric oxide (NO) is a bioactive molecule involved in many biological events, and has been reported as pro-oxidant as well as anti-oxidant in plants. In the present study, the sources of NO production under water stress, the role of NO in water stress-induced hydrogen peroxide (H2O2) accumulation and subcellular activities of anti-oxidant enzymes in leaves of maize (Zea mays L.) plants were investigated. Water stress induced defense increases in the generation of NO in maize mesphyll cells and the activity of nitric oxide synthase (NOS) in the cytosolic and microsomal fractions of maize leaves. Water stress-induced defense increases in the production of NO were blocked by pretreatments with inhibitors of NOS and nitrate reductase (NR), suggesting that NO is produced from NOS and NR in leaves of maize plants exposed to water stress. Water stress also induced increases in the activities of the chloroplastic and cytosolic anti-oxidant enzymes superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR), and the increases in the activities of anti-oxidant enzymes were reduced by pretreatments with inhibitors of NOS and NR. Exogenous NO increases the activities of water stress-induced subcellular anti-oxidant enzymes, which decreases accumulation of H2O2. Our results suggest that NOS and NR are involved in water stress-induced NO production and NOS is the major source of NO. The potential ability of NO to scavenge H2O2 is, at least in part, due to the induction of a subcellular anti-oxidant defense.
Numerous studies have demonstrated that plants under water stress may be exposed to oxidative stress through the production of reactive oxygen species (ROS), such as superoxide radical (O2•−), hydroxyl radical (HO·) and hydrogen peroxide (H2O2) (Iturbe-Ormaetxe et al. 1998; Munné-Bosch and Penũelas 2003). Plant cells are normally protected against the detrimental effects of ROS by a complex anti-oxidant system (Smirnoff 1993). During water stress, increased ROS levels activate expression of genes for anti-oxidant enzymes. The activated anti-oxidant systems are beneficial for plant performance under water stress, because adequate capacity of anti-oxidant enzymes and other anti-oxidant metabolites may help in the removal of excess ROS and inhibit lipid peroxidation (Mittler 2002; Wang et al. 2004). The formation of excessive ROS and decreases in the capacity of anti-oxidant defense systems in plants under severe water stress can result in cellular oxidative damage.
Nitric oxide (NO) is proposed to be one of the important second messengers in plant cells (Beligni et al. 2002). In animal systems, most of the NO is synthesized by nitric oxide synthase (NOS) (Mayer and Hemmens 1997). In plants, however, the enzyme has not yet been isolated, but the activity of NOS for NO synthesis can be detected (Neill et al. 2003). Immunological analysis suggests that NOS-like proteins probably localize in the cytoplasm of maize (Ribeiro et al. 1999). Application of exogenous NO can also mediate various physiological processes to abiotic stresses. Application of the NO donor sodium nitroprusside (SNP) confers resistance to salt (Uchida et al. 2002), drought (Mata and Lamattina 2001), heavy metals (Hsu and Kao 2004) and chilling (Neill et al. 2002) stresses. NO itself is an active nitrogen species and its effects on different types of cells have been proved to be either protective or toxic, depending on the concentration and situation. In fact, NO often functions together with ROS in various ways and plays an important role in environmental stresses, especially in plant–pathogen interactions (Delledonne et al. 2001). This conclusion was indirectly supported by the previous views in which NO, O2•−and H2O2 were regarded as important early upstream signaling components in response to UV-B radiation (Mackerness 2000; Mackerness et al. 2001). Several studies have shown that the protective effect of NO against abiotic stresses is closely related to the NO-mediated reduction of ROS in plants (Kopyra and Gwózdz 2003; Zhang et al. 2003; Hsu and Kao 2004). Under these conditions, NO appears to serve as an anti-oxidant agent able to scavenge H2O2 to protect plant cells from oxidative damage (Laxalt et al. 1997). H2O2 generation and effects are not necessarily delimited within the same cellular compartment. Moreover, distinctive sources seem to be responsible for H2O2 generation under different stress conditions. Chloroplasts, mitochondria and peroxisomes have been believed to be important sources of H2O2 in plant cells under water stress (Mittler 2002; Noctor et al. 2002; Foyer and Noctor 2003; Bartoli et al. 2004; Luna et al. 2004; Mittler et al. 2004). Superoxide dismutase (SOD), ascorbate peroxidase (APX) and glutathione reductase (GR) are major anti-oxidant enzymes in plant cells. These anti-oxidant enzymes have multiple molecular forms of isoenzymes, and are located in different cellular compartments (Mittler 2002). These isoenzymes are expressed by distinct regulatory mechanisms in response to various environmental stresses or cell conditions, and play co-operative roles to protect each organelle and minimize tissue injury (Mittler 2002; Shigeoka et al. 2002).
Recent studies have shown that ABA induces increases in the production of NO and H2O2, which are involved in anti-oxidant defense (Jiang and Zhang 2002a, 2002b, 2003; Hu et al. 2005; Zhang et al. 2006, 2007). As a signaling molecule, NO is believed to be one of the first anti-oxidants during early life evolution (Dat et al. 1998), which can upregulate anti-oxidants (Levine et al. 1994; Beligni and Lamattina 1999; Zhou et al. 2005) and indirectly mediate H2O2 levels in the cascade of events leading to alterations of anti-oxidant gene expression (Leshem and Haramaty 1996). However, the role of NO and the relationship between NO and H2O2 in water stress-induced activities of subcellular anti-oxidant enzymes are not clear. Given the ability of NO to capture ROS, we tried to determine whether NO could protect maize plants from cellular injury caused by water stress.
Results
Water stress induces NO generation in maize leaves
In order to investigate the production of NO in leaves of maize plants exposed to water stress, the leaf segments were loaded with the NO-specific fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2DA) and confocal laser scanning microscopy (CLSM) was used to monitor changes in NO-induced fluorescence in mesophyll cells of maize plants. Treatment with 10% polyethylene glycol (PEG) led to a rapid increase in DAF-2DA fluorescence (data not shown). The DAF-2DA fluorescence was visible as early as 1 h, maximized at 4 h and then decreased after 6 h of water stress treatment (Figure 1A). Replacing DAF-2DA with 4-aminofluorescein (4AF-DA, the negative control for DAF-2DA) did not give rise to fluorescence in mesophyll cells of maize leaves (data not shown). Pretreatments with 2-4-carboxypheny l-4, 4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide (c-PTIO), a specific scavenger of NO, NG-nitro-L-Arg methyl ester (L-NAME) and S,S’-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea (PBITU), two well-known inhibitors of NOS, and potassium cyanide (KCN) and sodium azide (NaN3), two widely used inhibitors of nitrate reductase (NR), markedly reduced the fluorescence intensity induced by water stress (Figure 1B), indicating that water stress does induce an increased generation of NO in maize leaves.
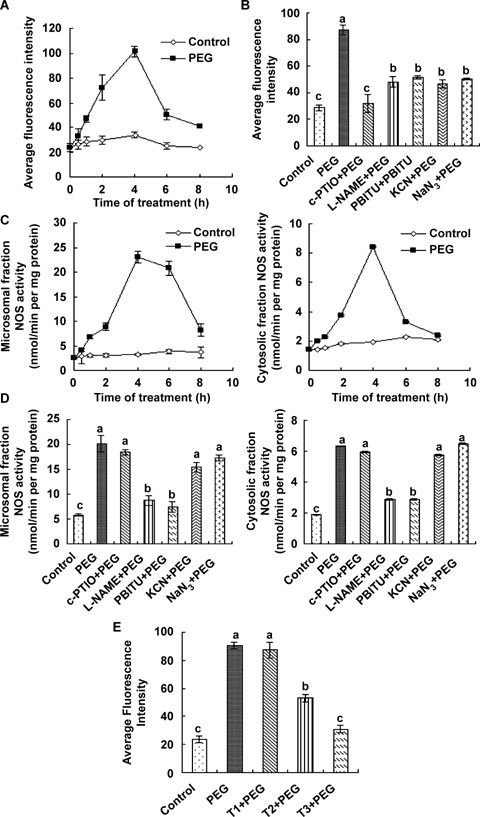
Water stress-induced nitric oxide (NO) production and nitric oxide synthase (NOS) activity in maize leaves.(A) Time course of water stress-induced NO production in maize leaves. The detached plants were treated with distilled water (control) and 10% polyethylene glycol (PEG) for various lengths of time.(B) Effects of pretreatments with the NO scavenger, the NOS inhibitors and the nitrate reductase (NR) inhibitors on water stress-induced NO production. The detached plants were pretreated with distilled water, 200 μM 2-4-carboxypheny l-4, 4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide (c-PTIO), 200 μM NG-nitro-L-Arg methyl ester (L-NAME), 5 mM S,S’-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea (PBITU), 2 mM potassium cyanide (KCN) or 100 μM sodium azide (NaN3) for 4 h, respectively, and then exposed to 10% PEG for 4 h.(C) Water stress-induced changes in the activity of NOS in cytosolic and microsomal fractions of maize leaves. The detached plants were treated with distilled water (control) and 10% PEG for various lengths of time.(D) Effects of pretreatments with the NO scavenger, the NOS inhibitors and the NR inhibitors on water stress-induced NOS activity in cytosolic fraction and microsomal fraction of maize leaves. The detached plants were pretreated with distilled water, 200 μM c-PTIO, 200 μM L-NAME, 5 mM PBITU, 2 mM KCN or 100 μM NaN3 for 4 h, respectively, and then exposed to 10% PEG for 4 h.(E) Effects of pretreatments with different concentrations of tungstate (T) (500 μM, 1 mM and 2 mM) on water stress-induced NO production. The detached plants were pretreated with 500 μM tungstate (T1), 1 mM tungstate (T2) or 2 mM tungstate (T3) for 4 h, respectively, and then exposed to 10% PEG for 4 h. Detached plants treated with distilled water under the same conditions throughout the period of the experiment served as controls.In (A) (B) and (E) experiments were repeated at least three times with similar results. In (C) and (D) the values are the means ± SE (n = 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
To further elucidate the relationship between NOS and NO production, the activity of NOS in cytosolic and microsomal fractions of maize leaves was determined. Figure 1C showed the activity of NOS maintained at a continuously ascending trend during 4-h of water stress treatment. After 4 h of water stress treatment, the activity of NOS in cytosolic and microsomal fractions reached maximum values of 4.3-fold and 7.2-fold, respectively; higher than those in the controls. These results suggest that the activity of NOS in cytosolic and microsomal fractions is remarkably induced by water stress, and the activity of NOS in microsomal fraction was higher and more susceptible to water stress than that in cytosolic fractions. Pretreatments with L-NAME and PBITU completely inhibited the increases in the activity of NOS in cytosolic and microsomal fractions induced by water stress treatment, but pretreatments with c-PTIO, KCN and NaN3 hardly affected the activity of NOS in cytosolic and microsomal fractions induced by water stress treatment (Figure 1D).
To analyze the extent to which NR contributes to the NO production in response to water stress, the addition of tungstate experiments was conducted. It is well known that tungstate can be substituted for Mo and inhibit the NR activity by preventing formation of an active molybdenum cofactor, indispensable for the catalytic activity of nitrate reductase (Notton and Hewitt 1971). Figure 1E shows that water stress-induced fluorescence intensity decreased with the increase of tungstate concentration. Pretreatment with 2 mM tungstate almost completely inhibited water stress-induced DAF-2DA fluorescence by 70% compared with water stress treatment alone. These results suggest that NR is also responsible for NO production under water stress in maize leaves.
Histochemical and cytochemical detection of the effects of NO on H2O2 accumulation under water stress
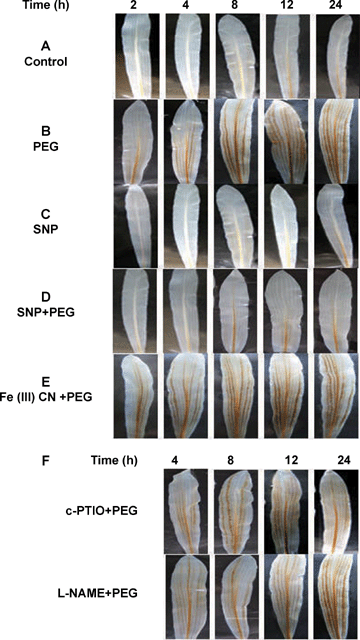
A pevious study showed that water stress induced the apoplastic H2O2 accumulation in maize leaves (Hu et al. 2005). To test a possible effect of NO on H2O2 accumulation in the leaves of maize plants exposed to water stress, a histochemical method for H2O2 detection was used. Figure 2C shows that no visible H2O2 accumulation was observed within 24 h in the leaves of maize plants exposed to SNP, a NO-releasing chemical, which is widely used in biological studies. Water stress treatment led to an obvious accumulation of H2O2. H2O2 is detectable as early as 2 h after the beginning of water stress treatment (Figure 2B). Pretreatment with SNP significantly decreased the level in accumulation of H2O2 during 24-h water stress treatment (Figure 2D). In order to confirm that exogenous NO was effective in reducing water stress-induced H2O2 accumulation, sodium ferricyanide [Fe(III)CN] was used as a negative control in experiments. Pretreatment with Fe(III)CN, which shares many structural features with SNP but lacks a nitroso group and thus the ability to generate NO (Bethke et al. 2006), did not reduce H2O2 accumulation induced by water stress (Figure 2E). The role of NO in the accumulation of H2O2 induced by water stress was further examined by a cytochemical technique with CeCl3, which reacts with H2O2 to produce electron-dense deposits of cerium perhydroxides (Bestwick et al. 1997). Figure 3B shows that pretreatment with SNP abolished most of the water stress-induced H2O2 accumulation detected with the CeCl3 staining, while pretreatment with Fe(III)CN did not decrease the formation of CeCl3 precipitates (Figure 3G), indicating that Fe(III)CN had a very little effect on the accumulation of H2O2 induced by water stress. Pretreatments with c-PTIO and L-NAME did not affect the water stress-induced H2O2 accumulation (Figures 2F, 3H,I). Moreover, treatments with SNP, [Fe(III)CN], c-PTIO or L-NAME alone did not affect H2O2 production in control leaves (Figure 3C–E,J). These results clearly suggest that the effect of NO donor SNP is attributable to NO released, and NO has the ability to reduce the accumulation of H2O2 induced by water stress.
Histochemical detection of H2O2 with 3,3-diaminobenzidine tetrachloride (DAB) staining in maize leaves.(A,B) The detached plants were treated with distilled water (control) and 100 μM sodium nitroprusside (SNP) for various lengths of time.(C–E) The detached plants were pretreated with distilled water (control), 100 μM SNP or 100 μM Fe(III)CN for 12 h, and then exposed to 10% PEG for 2, 4, 8, 12 and 24 h, respectively.(F) Effects of pretreatments with the nitric oxide (NO) scavenger 2-4-carboxypheny l-4, 4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide (c-PTIO) and the nitric oxide synthase (NOS) inhibitor NG-nitro-L-Arg methyl ester (L-NAME) on water stress-induced H2O2 accumulation. The detached plants were pretreated with 200 μM c-PTIO or 200 μM L-NAME for 12 h, and then exposed to 10% PEG for 4, 8, 12 and 24 h, respectively.All experiments were repeated at least five times with similar results.
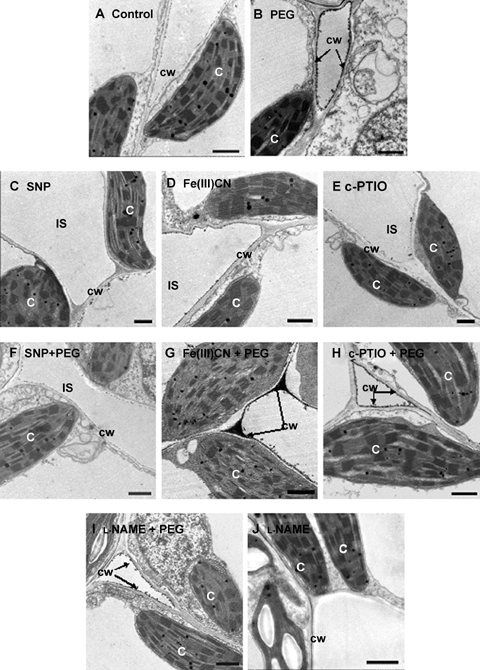
Effects of pretreatments with sodium nitroprusside (SNP), Fe (III)CN, the nitric oxide (NO) scavenger and the nitric oxide synthase (NOS) inhibitor on water stress-induced H2O2 accumulation in maize leaves with CeCl3-staining and transmission electron microscopy.The detached plants were pretreated with distilled water, 100 μM SNP, 100 μM Fe(III)CN, 200 μM 2-4-carboxypheny l-4, 4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide (c-PTIO) or 200 μM NG-nitro-L-Arg methyl ester (L-NAME) for 12 h, respectively, and then exposed to 10% polyethylene glycol (PEG) treatment for 12 h. Detached plants treated with distilled water under the same conditions throughout the period of the experiment served as controls. All experiments were repeated at least three times with similar results. C, chloroplast; cw, cell wall; IS, intercellular space. Bar, 1 μm.
Effects of NO on the activities of water stress-induced anti-oxidant enzymes in chloroplasts and cytosol
To test the effect of NO on the activities of subcellular anti-oxidant enzymes in maize leaves, the detached plants were pretreated with Fe(III)CN or SNP, and then exposed to water stress treatment. Those leaves were assayed after 4, 8, 12 and 24 h of treatment. As shown in Figure 4, water stress treatment led to significant changes in the activities of anti-oxidant enzymes GR, SOD and APX in chloroplasts and cytosol. During the 24-h treatment, pretreatment with SNP resulted in remarkable increases in the activities of chloroplastic and cytosolic anti-oxidant enzymes GR, SOD and APX induced by water stress, but pretreatment with Fe(III)CN reduced the activities of anti-oxidant enzymes induced by water stress. In chloroplasts, a significant increase in the activities of GR (Figure 4B) and APX (Figure 4D) occurred within the first 12 h of SNP + PEG treatment, but the increase in SOD (Figure 4F) occurred at 4 h of SNP + PEG treatment. In cytosol, a marked increase in the activities of GR (Figure 4A), SOD (Figure 4E) and APX (Figure 4C) occurred within the 4 h of SNP + PEG treatment. At the 24 h of treatment, the activities of all these anti-oxidant enzymes in leaves of maize plants exposed to SNP + PEG remained still higher than those exposed to Fe(III)CN + PEG or PEG. Treatment with SNP + PEG for 12 h enhanced the activities of chloroplastic GR, SOD and APX by 27%, 4% and 46%, respectively, and the activities of these cytosolic anti-oxidant enzymes by 12%, 3% and 21%, respectively, compared with the PEG treatment values. These results indicate that the changes in the activities of anti-oxidant enzymes GR, SOD and APX in chloroplasts were more sensitive than those in cytosol in response to SNP treatment under water stress. Treatment with Fe(III)CN +PEG for 12 h reduced chloroplastic GR (Figure 4B), SOD (Figure 4F) and APX (Figure 4D) by 20%, 20% and 3%, respectively, and cytosolic GR (Figure 4A) SOD (Figure 4E), APX (Figure 4C) and by 6%, 2% and 15%, respectively, compared with the PEG treatment values. The results confirmed that NO, and no other derived compound, was responsible for the increases in the activities of three key anti-oxidant enzymes.
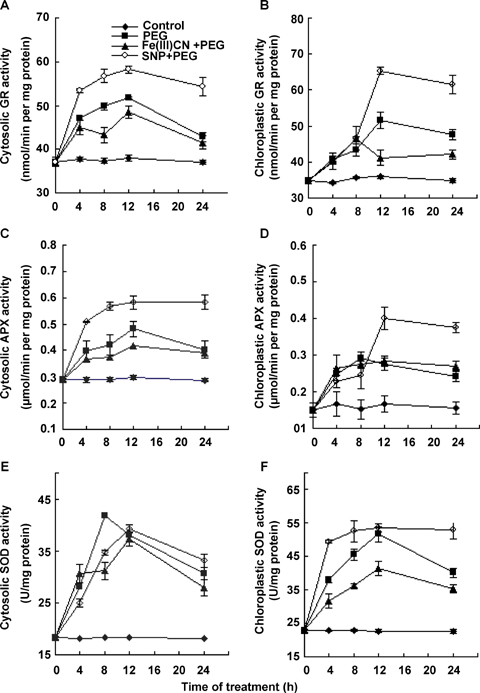
Effects of pretreatments with sodium nitroprusside (SNP), and Fe(III)CN on water stress-induced the activities of cytosolic and chloroplastic anti-oxidant enzymes glutathione reductase (GR) (A, B), ascorbate peroxidase (APX) (C, D), and superoxide dismutase (SOD) (E, F).The detached plants were pretreated with distilled water, 100 μM SNP or 100 μM Fe(III)CN for 12 h, respectively, and then exposed to 10% polyethylene glycol (PEG) treatment for 4, 8, 12, 24 h. Detached plants treated with distilled water under the same conditions throughout the period of the experiment served as control. The values are the means ± SE (n = 6) of three different experiments.
NO is involved in water stress-induced subcellular anti-oxidant defense
In order to determine whether water stress-induced increases in the activities of anti-oxidant enzymes in chloroplasts and cytosol are related to the increased generation of NO induced by water stress, the NO scavenger c-PTIO, the NOS inhibitors L-NAME and PBITU and the NR inhibitors KCN and NaN3 were used. Pretreatments with these inhibitors almost completely blocked increases in the activities of chloroplastic and cytosolic GR, SOD and APX induced by water stress treatment (Figure 5), suggesting that NO is involved in the water stress-induced subcellular anti-oxidant defense in maize leaves.
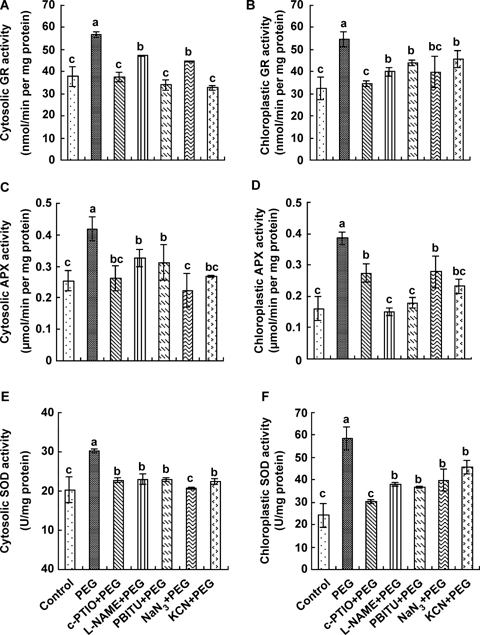
Effects of pretreatments with the nitric oxide (NO) scavenger, the nitric oxide synthase (NOS) inhibitors and the nitrate reductase (NR) inhibitors on the activities of cytosolic and chloroplastic anti-oxidant enzymes glutathione reductase (GR) (A, B), ascorbate peroxidse (APX) (C, D), and superoxide dismutase (SOD) (E, F) in leaves of detached maize plants exposed to water stress.The detached plants were pretreated with distilled water, 200 μM 2-4-carboxypheny l-4, 4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide (c-PTIO), 200 μM NG-nitro-L-Arg methyl ester (L-NAME), 5 mM S’-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea (PBITU), 100 μM sodium azide (NaN3) or 2 mM potassium cyanide (KCN) for 4 h, and then exposed to 10% polyethylene glycol (PEG) 12 h. Detached plants treated with distilled water under the same conditions throughout the period of the experiment served as control. The values are the means ± SE (n = 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
Discussion
Previous studies showed that water stress induces the accumulation of ABA, and ABA can induce the production of H2O2 and NO in maize leaves (Jiang and Zhang 2002c; Hu et al. 2005; Zhang et al. 2007). However, the source of water stress-induced NO production and the role of NO in water stress-induced H2O2 accumulation and subcellular anti-oxidant defense are not clear. In the present study, our data showed that water stress induced NO production in mesophyll cells of maize leaves, and the increase in NO production was substantially reduced by the pretreatments with the NO scavenger c-PTIO, the NOS inhibitors L-NAME, PBITU and the NR inhibitors KCN and NaN3 (Figure 1A,B), suggesting that NOS and NR may be involved in water stress-induced NO production.
In plants, the sources of NO production have been the subject of much debate. A growing body of evidence indicates that NO is formed by mammalian-like NOS activity, NR, or nonenzymatic sources (Cooney et al. 1994; Wildt et al. 1997; Ribeiro et al. 1999; Leshem 2001). In plants, pharmacological, biochemical and physicochemical, and immunological evidence indicates the presence of NOS-like activity similar (to a certain extent) to mammalian NOS (Neill et al. 2003; Corpas et al. 2004, 2006; del Río et al. 2004; Lamotte et al. 2005; Crawford 2006; Liu et al. 2007), although no plant NOS similar to the mammalian one has been characterized (Crawford 2006; Zemotjel et al. 2006; Neill 2007). It has been shown that NOS is localized in cytoplasm, chloroplasts, mitochondria, peroxisomes, and nucleus in plant cells (Ribeiro et al. 1999; Corpas et al. 2004; del Río et al. 2004; Guo and Crawford 2005; Liu et al. 2007). In this study, the activity of NOS in the cytosolic and microsomal fractions of maize leaves was determined. Our results showed that water stress induced increases in the activity of NOS in the cytosolic and microsomal fractions, and the activity of NOS in microsomal fraction was higher and more susceptible to water stress treatment than that in the cytosolic fraction of maize leaves (Figure 1C). Pretreatments with the NOS inhibitors L-NAME and PBITU completely blocked increases in the activity of NOS in the cytosolic and microsomal fractions induced by water stress treatment (Figure 1D). Our results clearly suggest that water stress-induced increases in the production of NO are mainly from NOS.
In addition to NOS, plant NR catalyzes the nicotinamide hypoanthine dinucleotide phosphate reduced tetrasodium salt (NAD(P)H)-dependent reduction of nitrite to NO (Yamasaki 2000). Maize NR (NR, EC 1.6.6.1) is a KCN-sensitive enzyme (Solomonson and Barber 1989). Tungstate serves as a molybdenum analog, and the reduction in NR activity in plants is caused by the synthesis of an inactive tungstoprotein (Notton and Hewitt 1971). Water stress-induced NO production was sensitive to pretreatment with tungstate (Figure 1E). Similar results were obtained using KCN and NaN3, two potent inhibitors of NR (Yamasaki 2000; Sakihama et al. 2002). Pretreatments with KCN and NaN3 blocked NO production induced by water stress (Figure 1B). However, tungstate, KCN and NaN3 are non-specific inhibitors in plants. KCN and NaN3 are peroxidase inhibitors, and tungstate is also the inhibitor of ABA synthesis. These results suggest that NR might be the source of water stress-induced NO generation. However, Zhang et al. (2003) reported that NO was produced from NOS and was not produced as a by-product of NR. The discrepancy between the study by Zhang et al. (2003) and our data may be related to the different species, the tissues and the conditions, which had different potential sources of NO.
It is known that H2O2, as a main kind of active oxygen species, accumulates under water stress (Dat et al. 1998; Jiang and Zhang 2002b). The ability of NO to scavenge ROS and therefore avoid cellular damage caused by H2O2 and O2− has been widely reported in animals. In plants, NO often functions together with H2O2 in various ways and plays an important role in environmental stress (Beligni and Lamattina 1999). In this work, we provide evidence for the ability of NO to scavenge H2O2 and slow down the accumulation of H2O2 induced by water stress in maize plants. SNP significantly reduced the accumulation of H2O2 detected by CeCl3 staining in random pieces of maize leaves (Figure 3F) and this result was corroborated in whole leaves by histochemistry with DAB staining (Figure 2D) under water stress. To specify the role of NO, we used Fe(III)CN in the experiments. Results indicated that Fe(III)CN had no effect on water stress-induced H2O2 accumulation in maize plants (2, 3). Although the application of exogenous NO appears to be a useful tool to study its physiological effect, such strategy does not take into account the finding that NO is also produced endogenously by plant cells in response to water stress. To clarify the physiological role of endogenous NO-mediated H2O2 under water stress, the NO scavenger c-PTIO and L-NAME were used. Our work showed that pretreatments with c-PTIO and L-NAME had no obvious reduction in accumulation of H2O2 in leaves of maize plants exposed to water stress (Figures 2F, 3H,I). These results clearly suggest that NO indeed has the ability to reduce H2O2 level in leaves of maize plants exposed to water stress, and endogenous NO production might play a physiological role in accumulation of H2O2 induced by water stress.
Water stress is related to the oxidative stress in plants (Bartoli et al. 1999; Boo and Jung 1999). Water stress can disturb the balance between cellular pro-oxidants and anti-oxidants. If the former prevails over the latter, cellular oxidative damage may occur. H2O2 is a well-know oxidant and can be an index of oxidative damage. In this work, we demonstrated that NO slowed down the accumulation of H2O2 induced by water stress. This could also account for the result that the low NO level is able to partially protect from oxidative damage under water stress. However, it is not clear how NO mediates the accumulation of H2O2 in leaves of maize plants exposed to water stress. In contrast to biotic stress, in which necrotic zones can be visualized at the point of pathogen infection, no toxicity symptoms were observed in water stressed-plants, which makes the study of plant response at subcellular level more difficult. GR, SOD and APX are three key enzymes for the removal of H2O2. These anti-oxidant enzymes have many isoenzymes, and are located in different subcellular compartments. Furthermore, NOS and NR, the sources of NO production, are located in different subcellular compartments. NR is located in the cytosol in mesophyll cells and plastid of maize leaves (Becker et al. 1993). NR activity is also dependent on chloroplasts and the loss of chloroplasts causes the loss of NR activity (Mohr et al. 1992). In addition, NOS-like proteins have been localized in the cytosol and nucleus of maize root tips (Ribeiro et al. 1999), and in peroxisomes and chloroplasts of pea leaves (Barroso et al. 1999). So, we selected the whole leaf chloroplasts and cytosol as a model to investigate the effect of NO on water stress-induced activities of anti-oxidant enzymes in these cellular compartments (Figure 4). The activities of chloroplastic and cytosolic anti-oxidant enzymes SOD, APX and GR in the presence of SNP were much higher than those in the presence of Fe(III)CN or the absence of SNP under water stress, suggesting that NO elevates the activities of water stress-induced subcellular anti-oxidant enzymes, which is able to further enhance the capacity of water stress. On the other hand, our results indicated clearly that NO is involved in water stress-induced subcellular anti-oxidant defense (Figure 5). In fact, NO might have well been one of the first biological signaling molecules, which is involved not only in cell-cell adhesion mechanisms, but also in intercellular communication (Dat et al. 1998). NO production increased by 162% at 4 h in the leaves of maize plants exposed to water stress (Figure 1A). The NO generated rapidly might cross biological membranes and trigger biological responses in a short period of time. Thus, the capacity of NO to scavenge H2O2 may be due to its ability to easily cross membranes and enter different subcellular compartments, and then upregulate subcellular anti-oxidant enzymes, resulting in the prevention of H2O2 overproduction-induced oxidative damage in plants exposed to water stress.
In conclusion, the present study provides evidence for the involvement of NOS and NR in water stress-induced NO production of which NOS is the major source. The potential ability of NO to scavenge ROS is, at least in part, due to the induction of subcellular anti-oxidant defense, which is probably through multiple factors that directly or indirectly protect plants from oxidative damages.
Materials and Methods
Plant material and treatments
Seeds of maize (Zea mays L. cv. Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a light chamber at a temperature of 22–28 °C, with a photosynthetic active radiation (PAR) of 200 μmol/m2 per s and a photoperiod of 14/10 h (day/night), and watered daily. When the second leaf was fully expanded, the plants were collected and used for all investigations. The plants were excised at the base of the stem, and placed in distilled water for 1 h to eliminate wound stress. After treatment, the cut ends of stems were placed in beakers wrapped with aluminum foil containing 10% PEG solution for 24 h at 22 °C with a continuous light intensity of 200 μmol/m2 per s. In order to study the effects of inhibitors and scavengers, the detached plants were pretreated with 200 μM c-PTIO (Sigma, St. Louis, MO, USA), 200 μM L-NAME (Sigma, St. Louis, MO, USA), 5 mM PBITU (Merck, Darmstadt, Germany),100 μM NaN3 and 2 mM KCN for 4 h and then exposed to PEG treatment under the same conditions as described above. After treatments of detached maize plants, the second leaves were sampled and immediately frozen under liquid N2.
Isolation of chloroplasts
Chloroplasts were isolated from leaves using the method as described by Munné-Bosch and Alegre (2003). Briefly, after grinding 5 g leaves in isolation buffer [0.33 Msorbitol, 2 mM ethylenediaminetetraacetic acid (EDTA), 50 mM Hepes-KOH (pH 7.5), 0.1% (w/v) bovine serum albumin (BSA), 1 mM MgCl2, 1 mM MnCl2, 1 mM dithiothreitol (DTT), the homogenate was filtered through four layers of cheesecloth and centrifuged at 2 °C and 200g for 1 min. The pellets were resupended in isolation buffer and then centrifuged at 2 °C and 2 500g for 5 min. Chloroplasts were purified by resuspending the pellets in isolation buffer, laying on 12.5 mL 25% (v/v) of Percoll (in isolation buffer), and centrifuging at 2 °C and 15 800g for 20 min. The intact chloroplasts in the lower layer were resuspended in isolation buffer without BSA and MnCl2, centrifuged at 2 °C and 2 500g for 5 min. The chloroplast pellets were lysed in lyses buffer (50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 1 mM MgCl2), and used immediately for analyses. On the basis of the specific activities of G-6-P dehydrogenase cytosol contaminations were about 4%. To detect APX activity, an independent organelle-isolation procedure was used (20 mM sodium ascorbate added to the extraction medium), and all other solutions also contained 2 mM ascorbic acid to prevent the possible inactivation of APX.
Preparation of cytosolic fraction
Cytosolic fraction was isolated according to the method as described by Yang and Komatsu (2000). Leaf segments (0.5 g) were homogenized in a mortar and pestle in 5 mL homogenization buffer containing 50 mM phosphate buffer (pH 7.5), 0.25 M sucrose, 10 mM ethyleneglycol-bis(2-amino ethyl ether)-N,N,N′,N′-teraacetic acid (EGTA), 1 mM DTT and 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenates were centrifuged at 10 000g for 10 min. The supernatants were centrifuged at 100 000g for 1 h, and the cytosolic fraction was obtained by collecting the supernatant. On the basis of the content of chlorophyll, the contamination of chloroplasts was about 3%.
Enzyme assays
The activities of anti-oxidant enzymes were determined as previously described (Jiang and Zhang 2001). Total SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium. One unit of SOD activity was defined as the amount of enzyme that was required to cause 50% inhibition of the reduction of nitro blue tetrazolium as monitored at 560 nm. APX activity was measured by monitoring the decrease in absorbance at 290 nm as ascorbate was oxidized. GR activity was measured by following the change in absorbance at 340 nm as oxidized glutathione (GSSG)-dependent oxidation of NADPH.
Histochemical detection of H2O2
Hydrogen peroxide was visually detected in the leaves of plants by using 3,3-diamino-benzidine (DAB) as substrate (Orozco-Cárdenas and Ryan 1999). Briefly, plants were excised at the base of stems with a razor blade and supplied through the cut stems with a 1 mg/mL solution of DAB (pH 3.8) for 8 h under light at 25 °C, and then exposed to distilled water, Fe(III)CN, SNP, L-NAME or c-PTIO plus PEG treatments. After these treatments, the second leaves were decolorized by immersion of leaves in boiling ethanol (96%) for 10 min. This treatment decolorized the leaves except for the deep brown polymerization product produced by the reaction of DAB with H2O2. After cooling, the leaves were extracted at room temperature with fresh ethanol and photographed.
Cytochemical detection of H2O2
Hydrogen peroxide was visualized at the subcellular level using CeCl3 for localization (Bestwick et al. 1997). Electron-dense CeCl3 deposits were formed in the presence of H2O2 and were visible by transmission electron microscopy. Tissue pieces (∼1 to 2 mm2) were excised from the treated and untreated leaves and incubated in freshly prepared 5 mM CeCl3 in 50 mM 3-(N-morpholino) propanesulfonic acid (Mops, Amresco, Solon, OH, USA) at pH 7.2 for 1 h. The leaf sections were then fixed in 1.25% (v/v) glutaraldehyde and 1.25% (v/v) paraformaldehyde in 50 mM sodium cacodylate buffer, pH 7.2, for 1 h. After fixation, tissues were washed twice for 10 min in the same buffer and postfixed for 45 min in 1% (v/v) osmium tetroxide, and then dehydrated in a graded ethanol series (30–100%; v/v) and embedded in Eponaraldite (Agar Aids, Bishop's Stortford, UK). After 12 h in pure resin, followed by a change of fresh resin for 4 h, the samples were polymerized at 60 °C for 48 h. Blocks were sectioned (70–90 nm) on a Reichert-Ultracut E microtome, and mounted on uncoated copper grids (300 mesh). Sections were examined using a transmission electron microscope at an accelerating voltage of 75 kV.
NO detection by CLSM
Nitric oxide measurement was carried out with the specific NO dye DAF-2DA (Sigma), using the method as described by Corpas et al. (2004) with slight modifications. Leaf segments of approximately 0.5 cm2 were incubated in loading buffer (0.1 mM CaCl2, 10 mM KCl, 10 mM MES-Tris, pH 5.6) and DAF-2DA at a final concentration of 10 μM for 30 min in the dark at 25 °C, followed by washing twice in the same loading buffer for 15 min each. All images were visualized using a confocal laser scanning microscopy (excitation 495 nm, emission 515 nm). Images acquired were analyzed using LEICA IMAGE software. Data are presented as average fluorescence intensity.
Preparation of microsomal fraction and NOS activity assay
Crude enzyme preparation was carried out according to Ninnemann and Maier (1996) with some modification. The frozen leaf tissues were homogenized in 5 mL of homogenization buffer (50 mM triethanolamine hydrochloride, pH 7.5, containing 0.5 mM ethylene-diamine tetraacetic acid, 1 mM leupeptin, 1 mM pepstatin, 7 mM glutathione, 0.2 mM phenylmethyl sulfonyl fluoride). After centrifuging at 9 000g for 30 min (4 °C), the supernatant was collected and recentrifuged at 100 000g for 1 h (4 °C). The supernatant was used as cytosolic fraction. The pellet was resuspended in 1 mL homogenization buffer and used as a microsomal fraction. NOS activity was analyzed by hemoglobin assay as previously described (Hevel and Marletta 1994; Murphy and Noack 1994). Protein content was determined according to method of Bradford (1976) with BSA as standard.
Statistical analysis
The results presented were the mean of six replicates in the enzyme assays. Means were compared by one-way analysis of variance and Duncan's multiple range test at 5% level of significance.
(Handling editor: Xiangdong Fu)




