Role of Soybean GmbZIP132 under Abscisic Acid and Salt Stresses
Supported by the National Natural Science Foundation of China (30490254) and the State Key Basic Research and Development Plan of China (2004CB117200).
Abstract
Plant basic-leucine zipper (bZIP) transcription factors play important roles in many biological processes. In the present study, a bZIP gene, GmbZIP132, was cloned from soybean and its biological function under abiotic stresses was studied. The transcription of GmbZIP132 was induced by drought and high salt treatments. Among all of the organs analyzed, its expression was the highest in cotytledon and stems. GmbZIP132 could weakly bind to the GCN4-like motif (GLM) (5′-GTGAGTCAT-3′) in yeast one-hybrid assay. Compared with wild-type (WT) Arabidopsis plants, transgenic plants overexpressing GmbZIP132 showed reduced abscisic acid sensitivity and increased water loss rate. At the stage of germination, transgenic plants were more tolerant to salt treatment than wild-type plants. The expression of some abiotic stress-related genes, such as rd29B, DREB2A, and P5CS, were upregulated in the transgenic plants. These results indicated that GmbZIP132 was an abiotic stress-related gene, and its overexpression could increase the salt tolerance of transgenic Arabidopsis plants during germination, yet no significant difference of tolerance to abiotic stresses was found between transgenic and wild type plants at the seedling stage.
Proteins with basic region/leucine zipper (bZIP) domains are present in all eukaryotes and belong to one of the most extensively investigated transcriptional factor families. Members of the bZIP family are involved in various biological functions such as pathogen defense, light and stress signaling, seed maturation and flower development (Jakoby et al. 2002). Structurally, bZIP proteins have a basic region that binds to DNA and a leucine zipper dimerization motif. Besides the bZIP domains, there are other conserved domains including proline-rich, glutamine-rich and acidic domains that may function as transcriptional activation domains (Schindler et al. 1992; Vettore et al. 1998). In the Arabidopsis genome, there are at least 75 distinct members of the bZIP family and these identified proteins were clustered into ten groups according to sequence similarities of their basic regions (Jakoby et al. 2002). Members of a given group might have similar expression patterns and share functions.
During their whole lives, plants will encounter various abiotic stresses such as cold, high salt and drought. One of the molecular strategies for plants to adapt to these various adverse environmental conditions is to regulate the expressions of a large amount of abiotic stress-related genes. Recent studies have confirmed that some bZIP proteins are among these regulated genes and could play important roles in abiotic stress tolerance. The expressions of AREB1 (ABA-responsive element binding protein 1)/ABF2 (ABA-responsive element binding factors 2), ABF3/DPBF5 (Dc3 promoter binding factors 5) and AREB2/ABF4 were induced by abscisic acid (ABA), drought and high salt in vegetative tissues, whereas ABF1 expression was induced by cold treatment (Choi et al. 2000). Overexpression of ABF3 or ABF4 in Arabidopsis caused ABA hypersensitivity and enhanced drought tolerance of transgenic plants (Kang et al. 2002). AREB1 was reported to be an essential component of glucose signaling and its overexpression affected multiple types of stress tolerance including drought, salt, and heat (Kim et al. 2004).
Plant hormone ABA plays an important role under abiotic stresses. Most of the genes responding to drought, high salt and cold stresses can also be induced by exogenous ABA (Kang et al. 2002; Cheong et al. 2003). However, some genes induced by water stress were not responsive to exogenous ABA treatment, indicating that both ABA-dependent and ABA-independent regulatory systems are involved in stress-responsive gene expression (Shinozaki and Yamaguchi-Shinozaki 2000). There are two major cis-acting elements: ABRE (ABA-responsive element) and DRE/CRT (dehydration responsive element/C-repeat), which function in ABA-dependent and ABA-independent gene expressions in abiotic stress responses, respectively (Yamaguchi-Shinozaki and Shinozaki 2005). Relevant studies have reported that bZIP proteins related to abiotic stresses could bind ABRE and their expressions were induced by ABA (Kang et al. 2002). However, as we know, there is still no relevant study reporting that bZIP genes related to abiotic stresses are independently or negatively regulated by ABA.
To investigate the role of soybean bZIP genes under abiotic stresses, several stress-inducible bZIP genes were cloned and their stress tolerance was analyzed. The expression profiles of soybean bZIP genes under salt, drought, cold, and exogenous ABA treatments were investigated. One of them was named GmbZIP132. The expression pattern of GmbZIP132 in soybean seedlings under abiotic stress treatments and in specific tissues, its binding ability, and functional analysis of transgenic Arabidopsis plants overexpressing GmbZIP132 under abiotic stresses were further investigated.
Results
Identifying and cloning of GmbZIP132 from soybean (Glycine max)
Using the Arabidopsis bZIP proteins as queries, 130 bZIP genes were identified by searching the 56 147 soybean unigenes identified in our previous study (Tian et al. 2004). The expression patterns of these genes under drought, cold, salt, and exogenous ABA treatments were examined using reverse transcription-polymerase chain reaction (RT-PCR). Several bZIP genes inducible by various stress treatments were cloned. Of particular interest was one gene named GmbZIP132. Database Basic Local Alignment Search Tool (BLAST) analysis identified several homologous cDNA sequences sharing 20.0% to 73.9% amino acid identity with GmbZIP132 (Figure 1). Among them are three homologous Arabidopsis bZIP sequences (AtbZIP20, AtbZIP45 and AtbZIP47). They belong to the group D bZIP transcription factors, which participate in two different processes: defense against pathogens, and development (Jakoby et al. 2002). GmbZIP132 contains two protein domains: PD388003 and PD004114, as defined by ProDom (Servant et al. 2002). Protein domain PD388003 has been annotated as a tumor-related protein-like domain because it was found in the protein sequence (accession number BAA05470) from tobacco hybrid Nicotiana glauca×Nicotiana langsdorffii, which shows tumorous cell growth. PD004114 is known as DNA-binding nuclear transcription factor bZIP activator coiled coil regulation leucine domain, which is present in the group D bZIP transcription factors.

Comparison of the deduced amino acid sequence of GmbZIP132 (ABI34670) with homologous sequences from Medicago truncatula (ABD32966), from Arabidopsis thaliana AtbZIP20 (P43273), AtbZIP45 (BAD44579), AtbZIP47 (NP_201324) and DOG1 (ABK81213) and from Nicotiana glauca×Nicotiana langsdorffii HBP-1b (BAA05470).Amino acids identical in at least two proteins are highlighted in black. Gaps are introduced to maximize the alignment.
The expression of GmbZIP132 was monitored by RT-PCR. Its expression was strongly induced by salt and drought treatments, and weakly induced by cold. It was slightly induced for the first 3 h of ABA treatment and came back to the pre-treatment level (Figure 2A). Expression of GmbZIP132 was also investigated in different soybean organs including cotyledons, roots, stems, leaves and flowers. The results showed that the GmbZIP132 mRNA accumulated in an organ-specific manner with the strongest expression in cotyledons and stems, comparatively lower expression in roots and flowers, and barely detectable expression in leaves (Figure 2B).

Expressions of GmbZIP132 in soybean.(A) Expression of GmbZIP132 in response to abscisic acid (ABA), cold, drought and salt treatments.(B) Expression of GmbZIP132 in different soybean organs.Water treatment was carried out as a control treatment. Tubulin gene was amplified as a loading control. AS (asparagine synthetase) was carried out as a positive control which was induced by ABA, salt and drought treatments.
DNA-binding specificity of GmbZIP132 protein
One of the characteristics of bZIP proteins is that they can bind ABRE or G-box (Jakoby et al. 2002). Therefore, the binding ability of GmbZIP132 protein was examined by analyzing its binding ability to three known bZIP binding elements in the yeast one-hybrid assay system (BD Clontech, Mountain View, CA, USA). The GCN4-like motif (GLM; 5′-GTGAGTCAT-3′) (Onate et al. 1999), ABA responsive elements (ABRE; 5′-CCACGTGG-3′) (Jakoby et al. 2002) and the prolamin box (PB; 5′-TGAAAA-3′) (Onate et al. 1999), and their corresponding mutant sequences mGLM (5′-GTGAAAAAT-3′), mABRE (5′-CCAAAAGG-3′) and mPB (5′-TGACGT-3′), were synthesized and inserted into the plasmid pHIS2 containing the reporter gene HIS3. A minimal promoter was present downstream of the cis-elements but upstream of the HIS3 gene. Reporter pHIS2 plasmids harboring different elements plus the reporter gene HIS3, together with the effector plasmid pAD-GmbZIP132 were transformed into yeast cells. Growth of the transformants on 3-AT medium indicates the activation of the HIS3 gene and binding of the GmbZIP proteins to the corresponding cis-elements. Figure 3 shows that all transformants grew well on the yeast extract-peptone-dextrose plus adenine (YPAD) medium. But only transformants harboring the effector plasmids pAD-GmbZIP132 and the reporter plasmid containing the GLM, grew well on the 3-AT medium, indicating that this protein could bind to the sequence GLM; a GCN4-like motif.

DNA-binding specificity of GmbZIP132 by yeast one-hybrid system.CK- indicates growth of cells harboring the effector plasmids pAD-GmbZIP132 and the reporter plasmid pHIS2 (without DNA elements). Growth of cells containing both the corresponding effector plasmids and the reporter plasmids with different DNA elements are shown.
Functional analysis of transgenic Arabidopsis plants overexpressing GmbZIP132 under ABA, salt, and freezing treatments
The expression of GmbZIP132 under salt, drought, cold and ABA treatments suggested that it might be involved in the tolerance of plants to abiotic stresses. To characterize the function of GmbZIP132 in planta, GmbZIP132 in the sense orientation was inserted into a binary vector pBIN438 under the control of the 2 × CaMV 35S promoters and transformed into Arabidopsis using the vacuum infiltration method. Two homozygous transgenic lines with high target gene expression (Figure 4A) were used for further analysis. While growing on Murashige and Skoog (MS) agar plates or in the soil, neither transgenic lines showed a meiotically stable abnormal phenotype compared with wild-type plants grown under identical conditions (data not shown). Therefore we examined the performance of these two transgenic lines under various abiotic stresses.
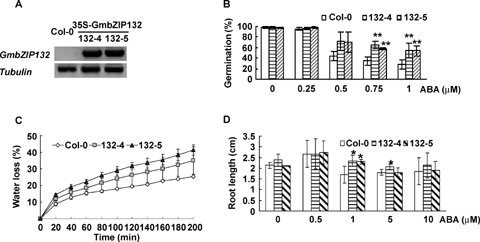
Abscisic acid (ABA) sensitivity of the transgenic plants overexpressing GmbZIP132.(A) Expression of GmbZIP132 in two transgenic lines as revealed by reverse transcription-polymerase chain reaction (RT-PCR). Tubulin gene was amplified as a loading control.(B) Germination of the 35S-GmbZIP132-transgenic seeds under various ABA concentrations. The treatments lasted for 3 d at 22 °C. Each data point represents the mean of triplicate measures and each measurement has more than 100 seeds.(C) Comparison of water loss in different transgenic lines and Col-0 plants. Leaves at similar developmental stages were excised and weighed at various times after the detachment. Each data point represents the mean of triplicate measures and each measurement has nine leaves.(D) Growth of roots in different transgenic lines and Col-0 plants under different concentration of ABA.
Since ABA plays an important role in abiotic stresses and the expression of GmbZIP132 was weakly induced by exogenous ABA treatment, the ABA sensitivity of transgenic plants overexpressing GmbZIP132 was first examined. The seeds of all of the transgenic plants and wild type (WT) plants were germinated in the medium containing various concentrations of ABA. Figure 4B shows that the germination rates in transgenic plants were significantly higher than that in WT plants under the concentrations above 0.5 μM ABA. The water loss rates of both transgenic lines were also higher than those of the WT plants (Figure 4C). Three-day-old seedlings after germination were transferred to MS medium containing different concentrations of ABA. The root lengths were measured after 7 d, and the root lengths of transgenic plants were longer than those of WT under the concentrations of 1 and 5 μM ABA (Figure 4D). These results implied that GmbZIP132 might reduce ABA sensitivity of transgenic plants.
Next, the salt, drought and freezing tolerances of the transgenic plants were examined. The seeds of all of the transgenic and WT plants were germinated in the medium containing various concentrations of NaCl. Figure 5A shows that the germination rates were significantly higher in transgenic plants than in WT plants under the concentrations over 100 mM NaCl. Two-week-old seedlings from the MS medium were transferred to soil, and treated with increasing concentrations of NaCl or high salt by sitting pots in 600 mM NaCl. No differences in average plant height (Figure 5B) and survival rate (Figure 5C) were observed between transgenic and WT plants. No significant difference between transgenic and WT plants in tolerance of transgenic plants to drought treatment was observed either (data not shown). These results implied that GmbZIP132 might improve salt tolerance of transgenic plants at the germination stage, but not at the seedling stage.
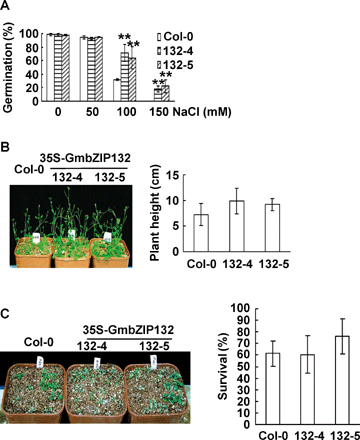
Salt tolerance of the transgenic plants overexpressing GmbZIP132.(A) Germination of the transgenic seeds under salt stress. Seeds were plated on filter saturated with various NaCl concentrations, and then treated for 3 d at 4 °C. Germination was scored after 3 d. Experiments were performed in triplicate and each measurement has more than 100 seeds.(B) Effect of salt stress on plant inflorescence height in soil. Two-week-old seedlings were transferred to soil for 1 week, and then treated with increasing concentrations of NaCl. Inflorescence height was measured after 20 d. Each data point represents the mean of triplicate measures and each measurement has 11 plants.(C) Survival of the transgenic plants under high salt treatment. Two-week-old seedlings were transferred to soil for 1 week, and then treated with 600 mM NaCl for 14 d by sitting the pots in a tray containing 600 mM NaCl. The survival rate of the transgenic plants under high salt treatment was examined. Each data point represents the mean of triplicate tests and each test has 18 plants.
Many studies have shown that accumulation of proline in plants can improve salt and freezing tolerance of plants (Khedr et al. 2003; Vannini et al. 2004). We thus measured the proline contents in 16-day-old WT and transgenic plants under normal conditions. Figure 6 showed that proline accumulation of GmbZIP132 transgenic plants was higher than that of WT plants.
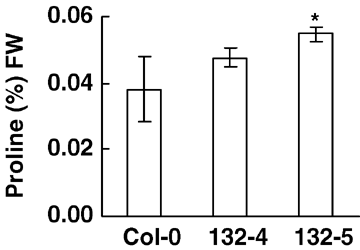
Proline content in different transgenic lines.Two-week-old seedlings were used for experiments. Each data point represents mean of three replicates.
Finally, some genes related to abiotic stresses were examined to see whether they were regulated by GmbZIP132 in transgenic plants. As shown in Figure 7, though expressions of rab18, COR15a and RD28 were not changed, expressions of rd29B, ICK1, DREB2A, RD17, P5CS, and ERD10 were upregulated in the transgenic plants. This result indicated that GmbZIP132 could regulate or interact with these abiotic-related genes.
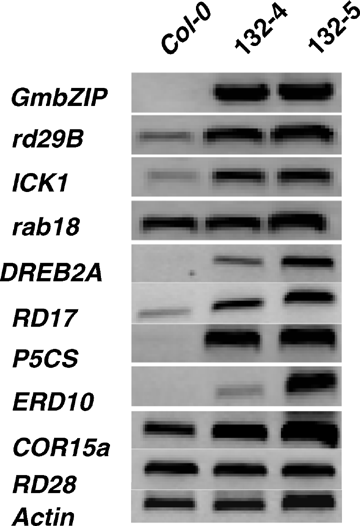
Expression of stress-responsive genes in GmbZIP132 transgenic plants.mRNA transcript levels of the stress-responsive genes were examined by reverse transcription-polymerase chain reaction (RT-PCR). cDNA were synthesized using total RNAs from 2-week-old plants grown on Murashige and Skoog (MS) plates. Actin gene was amplified as a loading control.
Discussion
Being sessile, plants have the capability to respond and adapt to adverse environmental stresses such as drought, cold, and high salt, through not only physiological and biochemical processes, but also molecular and cellular processes (Kang et al. 2002; Yamaguchi-Shinozaki and Shinozaki 2005). The expression of a large number of plant genes is regulated by abiotic environmental stresses such as drought, high salt and cold. GmbZIP132 was one of them because the expression of GmbZIP132 was strongly induced by salt and drought treatments, and weakly induced by cold and ABA (Figure 2A).
The Arabidopsis plants overexpressing GmbZIP132 showed a little higher salt tolerance than WT plants (Figure 5A), but it did not significantly improve the tolerance of transgenic plants to salt. This is consistent with the phenomenon of AtMYB2. The expression of AtMYB2 was induced by dehydration, high salt stress and exogenous ABA (Urao et al. 1993), but no plant stress tolerance was reported in overexpression of AtMYB2. However coexpression of AtMYB2 with AtMYC2 conferred moderate stress tolerance (Abe et al. 2003). A recent study showed that overexpressing SCOF-1 (soybean cold-inducible factor-1), which is a cold-inducible zinic finger protein gene from soybean, induced cold-regulated gene expression and enhanced cold-tolerance of transgenic plants. But SCOF-1 did not bind directly to either CRT/DRE or ABRE. SCOF-1 enhanced ABRE-dependent gene expression and mediated cold-regulated gene expression via protein-protein interaction with SGBF-1 (Soybean G-box binding factor 1), a soybean G-box binding bZIP transcription factor (Kim et al. 2001). The expression of the intact AREB1 gene on its own is insufficient to lead to expression of downstream genes under normal growth conditions and have no growth phenotypes between the wild-type and AREB1 overexpressing plants, but plants overexpressing AREB1ΔQT, an activated form of AREB1, showed ABA hypersensitivity and enhanced drought-tolerance (Fujita et al. 2005). The overexpression of GmbZIP132 was sufficient to lead to the expressions of downstream genes (Figure 7), but GmbZIP132 did not bind AREB in yeast one-hybrid assay. These results indicated that coexpression of or interaction with other genes might be required for GmbZIP132 to improve the tolerance of transgenic plants.
Studies showed that overexpression of some ABA-related genes such as ABF3 or ABF4 can accelerate stomatal closure and water loss (Kang et al. 2002). A typical phenotype of ABA deficient (aba) and ABA-insensitive (abi) mutants has less stomatal closure (Xiong et al. 2001; Cominelli et al. 2005), which would lead to acceleration of water loss by transpiration. Moreover, physiological and genetic studies had shown that ABA play a critical role in seed dormancy (Finch-Savage and Leubner-Metzger 2006; Liu et al. 2007). Mutants with defects in ABA biosynthesis show reduced seed dormancy (Léon-Kloosterziel et al. 1996). Conversely, the ABA supersensitive mutant era1 confers enhanced seed dormancy (Cutler et al. 1996). Overexpression of DOG1 (Delay Of Germination 1) can contribute to inducing seed dormancy and sensitivity to ABA at the seed germination stage, but the seed germination of its mutant dog1 has no obvious difference with that of Ler (Bentsink et al. 2006). The GmbZIP132 transgenic plants showed reduced ABA sensitivity. Under ABA treatment, the seeds of transgenic plants overexpressing GmbZIP132 showed higher germination rates compared with those of WT plants (Figure 4B). Under normal conditions, transgenic plants overexpressing GmbZIP132 showed increased water loss compared with WT plants (Figure 4C). These results, together with the fact that the expression of GmbZIP132 in cotyledons was the highest among the organs investigated (Figure 2B), indicate that GmbZIP132 might be related to seed dormancy.
ICK1 is a cyclin-dependent protein kinase inhibitor interacting with both Cdc2a and CycD3, and its expression is induced by ABA (Wang et al. 1998). ERD10 was isolated from a cDNA library from 1-h-dehydrated plants of Arabidopsis thaliana. The expression of ERD10 was induced by dehydration, cold and ABA (Kiyosue et al. 1994). DREB2A was isolated by using the yeast one-hybrid screening technique. It functions as a trans-acting factor in the signal transduction pathway under dehydration conditions. Its expression could be induced by dehydration (Liu et al. 1998). RD17 is a low-temperature-induced dhn/lea/rab-like gene. Its transcript has been shown to accumulate in response to low temperature, ABA and dehydration (Welin et al. 1995). rd29B is a well-studied dehydration responsive gene. It contains at least one cis-acting element that is involved in ABA-response, slow induction. Under conditions of dehydration, high salt, or low temperature, its expression was markedly increased (Yamaguchi-Shinozaki and Shinozaki 1994). The P5CS (delta 1-pyrroline-5-carboxylate synthase) gene, which catalyzes the rate-limiting step in the proline biosynthesis pathway, is induced by salt, drought, and ABA stresses (Liu and Zhu 1997). Proline accumulation or treatment by exogenous proline can improve the salt tolerance of plants (Khedr et al. 2003). In GmbZIP132 transgenic plants, the expression of these stress response marker genes were upregulated (Figure 7). Overexpression of GmbZIP132 could increase the salt-tolerance of transgenic Arabidopsis plants during seed germination but not at the seedling stage. A close look at the expression of GmbZIP132 and these related stress marker genes in different developmental stages would reveal more details on the relationship between the function of GmbZIP132 and the performance of the transgenic plants.
Materials and Methods
Growth of soybean seedlings and various treatments
Soybean (Glycine max L. Merr.) plants were grown on vermiculite under a photoperiod of 16 h/8 h (light/dark) at 25 °C for 2 weeks and subjected to stress treatments. For drought treatment, plants were dehydrated on Whatman No. 3MM paper at 25 °C and with a RH (relative humidity) of 70% for various times. For ABA and high salt treatment, plants were immersed with their roots in the solution of 100 μM ABA and 150 mM NaCl for various periods of time. Cold treatment was carried out under dim light by continuously exposing plants to 4 °C for different times. After treatments, plant leaves were harvested, frozen in liquid nitrogen, and stored at −80 °C for RNA isolation. Various organs from mature soybean plants were also collected for RNA isolation.
RNA isolation and RT-PCR analysis
RNA isolation from soybean plants and transgenic Arabidopsis seedlings was carried out as described (Zhang et al. 1995). Total RNA (5 μg) from each sample was digested with DNase I (Promega, Madison, WI, USA) to get rid of any DNA contamination and then used to synthesize the first-strand cDNA using the cDNA synthesis kit according to the manufacturer's instructions (Promega). One μL of the cDNA mix was used as a template in a 25 μL PCR reaction volume to examine expressions of genes. The PCR condition was 94 °C, 3 min and 30 cycles of 94 °C, 30 s; 56 °C, 1 min; 72 °C, 1 min, with a final extension of 10 min at 72 °C. PCR products were separated on 1% agarose gel containing ethidium bromide and were photographed. Tubulin gene was amplified as a sample loading control. The asparagine synthetase gene from soybean, whose transcript was dramatically induced by salinity, osmotic stress and exogenous ABA, was amplified as a positive control (Hughes et al. 1997; Wang et al. 2005).
Soybean bZIP132 gene cloning
Soybean GmbZIP132 was cloned by RT-PCR from soybean cDNAs using gene specific primers: 5′-CGCGGATCCAGCAACAATGAACAACCCTA-3′; and 5′-CGGGGTACCGCAGCCTAATAGCGAAGGAC-3′. PCR products were cloned into pMD18-T vector (TaKaRa, Otsu, Shiga, Japan) and confirmed by sequencing.
DNA binding ability of the GmbZIP132 by yeast one-hybrid system
Three copies of each cis-element (GLM, mGLM, ABRE, mABRE, PB or mPB) were synthesized (Sengon, Shanghai, China) and annealed. The annealed double-strand including SacI and MluI sites at the two ends were double-digested with restriction enzymes SacI and MluI, and cloned into the pHIS2 reporter plasmid containing the nutritional reporter gene HIS3. The sequence of the insert was confirmed by sequencing. A minimal promoter was present downstream of the cis-elements but upstream of the HIS3 gene.
The coding region of GmbZIP132 was cloned into the DNA-activation domain vector pAD to generate the pAD-GmbZIP132 construct. The pAD-GmbZIP132 was used as effector plasmids. Transformation and screening analyses were carried out according to the manufacturer's instruction (BD Clontech). Both the effector plasmids and the reporter plasmids were transformed into yeast cells (Y187) and the transformants were selected on SD/-Trp-Leu. The transformed yeast cells were dropped onto SD/-Trp-Leu-His plus 10 or 30 mM 3-AT to examine the cell growth. For negative control, pAD-GmbZIP132 and pHIS2 without cis-elements were co-transformed into yeast cells (Y187) and the growth of the transformants was examined on SD/-Trp-Leu-His plus 10 or 30 mM 3-AT medium. The sequence of each original and mutant element was: GLM (5′-GTGAGTCAT-3′) (Onate et al. 1999), ABRE (5′-CCACGTGG-3′) (Jakoby et al. 2002), PB (5′-TGAAAA-3′) (Onate et al. 1999), mGLM (5′-GTGAAAAAT-3′), mABRE (5′-CCAAAAAGG-3′) and mPB (5′-TGACGT-3′).
Generation of the transgenic Arabidopsis plants
The coding region of the GmbZIP132 was cloned into the BamHI and KpnI sites of the plant expression vector pBin438 and confirmed by sequencing. The gene was controlled by the 35S promoter plus a Ω translation enhancer. The resulting plasmids were transformed into Agrobacterium tumefaciens strain GV3101 and further transformed into Arabidopsis plants by the vacuum infiltration method. Homozygous transgenic lines expressing higher GmbZIP132 gene were used for further analysis. Primers used for plasmid construction were: 5′-CGCGGATCCAGCAACAATGAACAACCCTA-3′; and 5′-CGGGGTACCGCAGCCTAATAGCGAAGGAC-3′.
Stress treatments of the transgenic Arabidopsis plants
Seeds (>100) of the transgenic Arabidopsis plants were planted in triplicate on filter papers saturated with different concentrations of ABA or NaCl, and incubated at 4 °C for 4 d before being placed at 22 °C for germination under a photoperiod of 16 h/8 h (light/dark). Germination (emergence of radicals) was scored 3 d later. The experiment was repeated three times independently. Results from one set of experiments are shown.
For NaCl treatment in soil, 14-day-old seedlings of WT and transgenic seedlings from MS agar medium were moved into potted soil for additional growth of 7 d. Then the plants were watered with 100 mM, 150 mM, and 200 mM NaCl, and lasted for 4 d. The inflorescence height was measured after the treatments. The 14-day-old seedlings were also grown in pots for 7 d and then treated with high salt by placing the pots in a tray containing 600 mM NaCl. After 14 d, the survival of the plants was examined. The experiments were repeated independently three times. Results from one set of experiments are shown.
For freezing tolerance assay, 14-day-old WT and transgenic seedlings from the MS agar medium were grown in potted soil for 7 d, and the plants were subjected to −6 °C for 2 d. After that, the plants were placed under normal conditions. The survival of the seedlings was scored after 7 d. The experiments were repeated independently three times. Results from one set of experiments are shown.
To measure the root length under ABA treatment, 3-day-old seedlings after germination were transferred into the plates containing MS medium with various concentrations of ABA, and then the plates were vertically placed under normal conditions. After 7 d, the root length was measured. Each experiment was repeated three times.
Water loss measurements
For water loss measurements, leaves were detached from plants at the rosette stage and weighed immediately on a weighed paper. The leaves, together with the paper, were then placed at 22 °C, under continuous light and with an RH of 50%. The weight was measured at designated time intervals. There were three replicates for each transgenic line. The percentage loss of fresh weight was calculated based on the initial weight of the plants. The experiment was carried out three times independently. Results from one experiment are shown.
Determination of proline contents
Free proline content in plants was determined following the methods of Bates et al. (1973). Briefly, 2-week-old seedlings (100 mg) were homogenized in 1 mL sulfosalicylic acid (3%) using a mortar and the mixture was mixed and centrifuged at 17 950g for 15 min at 4 °C. The extract (200 μL) was transferred to a new eppendorf tube, and 200 μL of acid ninhydrin and 200 μL of acetic acid were added. The reaction mixture was boiled in a water bath for 1 h, and placed at 4 °C for 30 min. Then 800 μL of toluene was added to the mixture. The tube was vortexed for 15 s and then 700 μL of the toluene phase was taken for the measurement of absorbance at 520 nm on spectrophotometer. Each data point represents the average of three plant samples. Two independent experiments were carried out. Results from one experiment are shown.
Statistic analysis
The data were subjected to statistic analysis, and analysis of variance was carried out using the SPSS 12.0 program. The bars indicate SD. * indicates that the differences between the transgenic line and the Col-0 plants are significant (P < 0.05). ** indicates that the differences between the transgenic line and the Col-0 plants are highly significant (P < 0.01).
(Handling editor: Qi Xie)
Acknowledgements
The authors are very grateful to Professor Rongxiang Fang at the Institute of Microbiology, the Chinese Academy of Sciences for kindly providing the plasmid pBin438.




