Isolation and Molecular Characterization of High Molecular Weight Glutenin Subunit Genes 1Bx13 and 1By16 from Hexaploid Wheat
Supported by the National Natural Science Foundation of China (30671293) and the High-Tech Research and Development (863) Program of China (2006AA100102).
Abstract
The high molecular weight glutenin subunit (HMW-GS) pair 1Bx13 + 1By16 are recognized to positively correlate with bread-making quality; however, their molecular data remain unknown. In order to reveal the mechanism by which 1By16 and 1Bx13 creates high quality, their open reading frames (ORFs) were amplified from common wheat Atlas66 and Jimai 20 using primers that were designed based on published sequences of HMW glutenin genes. The ORF of 1By16 was 2 220 bp, deduced into 738 amino acid residues with seven cysteines including 59 hexapeptides and 22 nanopeptides motifs. The ORF of 1Bx13 was 2 385 bp, deduced into 795 amino acid residues with four cysteines including 68 hexapeptides, 25 nanopeptides and six tripeptides motifs. We found that 1By16 was the largest y-type HMW glutenin gene described to date in common wheat. The 1By16 had 36 amino acid residues inserted in the central repetitive domain compared with 1By15. Expression in bacteria and western-blot tests confirmed that the sequence cloned was the ORF of HMW-GS 1By16, and that 1Bx13 was one of the largest 1Bx genes that have been described so far in common wheat, exhibiting a hexapeptide (PGQGQQ) insertion in the end of central repetitive domain compared with 1Bx7. A phylogenetic tree based on the deduced full-length amino acid sequence alignment of the published HMW-GS genes showed that the 1By16 was clustered with Glu-1B-2, and that the 1Bx13 was clustered with Glu-1B-1 alleles.
High molecular weight glutenin subunits (HMW-GS) play an important role in determining the viscoelastic properties of wheat flour (Shewry et al. 1992, 2003b). HMW-GSs are endosperm storage proteins encoded by genes located at the Glu-1 loci (1A, 1B, 1D) on the long arms of the group 1 chromosomes of hexaploid wheat (Payne 1987; Shewry et al. 1992). Each locus consists of two tightly linked genes, x and y, which encode the high Mr x-type and the low Mr y-type subunit, respectively (Payne et al. 1981). The structures of HMW-GS comprise three distinct domains, a central large repetitive domain flanked by short N- and C-terminal non-repetitive domains (Shewry et al. 1995). The central repetitive domain is composed of tandem and interspersed repeats of hexapeptide, nanopeptide and tripeptide (in x-type subunits only) motifs. These motifs are rich in glutamine, glycine and proline, which account for over 70% of the total amino-acid residues (Shewry et al. 1992, 2002). Differences in subunit size result mainly from variation in the central repetitive domain, in particular, numbers of hexapeptides and tripeptide (Anderson and Greene 1989; Shewry et al. 1992; D'Ovidio et al. 1995).
The number and position of cysteine residues are important features in the structure of HMW-GSs (Shewry et al. 2003b). Though cysteine belongs to the minor amino acids of HMW glutenin subunits, it plays an important role in the structure and functionality of gluten (Wieser 2003; Wieser et al. 2006). In general, most y-type subunits contain seven cysteines (five in the N-terminal domain and one in each of the repetitive and C-terminal domains), the x-type subunits possess four cysteines (three in the N-terminal domain and one in the C-terminal domain) (Shewry et al. 1997). While the subunit 1Dx5 has five cysteines (three in the N-terminal domain, and one in each of the repetitive and N-terminal domains) and 1Bx20 and 1Bx14 both have only two cysteines (one in the N-terminal domain and one in the C-terminal domain) (Shewry et al. 2003a; Li et al. 2004). The cysteines form either intra-chain disulphide bonds within a HMW-GS or inter-chain disulphide bonds between HMW-GS and other proteins. In addition, extra covalent bonds formed during bread-making are tyrosine-tyrosine cross-links between gluten proteins (Tilley et al. 2001). Therefore, tyrosine is also an important amino acid composition for flour processing quality.
The covalent structure of the gluten network was superimposed by non-covalent bonds such as hydrogen bonds, ionic bonds and hydrophobic bonds (Wieser et al. 2006). Studies based on nuclear magnetic resonance imaging indicated that the high glutamine content can stabilize the polymeric structure of glutenin through forming more hydrogen bonds (Belton et al. 1994, 1995; Gilbert et al. 2000). The specific structure of glutamine can form hydrogen bond with other amino acid residues, so the glutamine content is an important index for an HMW-GS quality evaluation.
The molecular structure of HMW glutenin subunits and their amino acid composition play particularly important roles in functional properties of dough (Hassani et al. 2005). The wheat variety Jimai 20 with strong gluten (1Ax1, 1Bx13 + 1By16, 1Dx5′ + 1Dy12) bred by Shandong Provincial Academy of Agricultural Sciences, had good bread-making and noodle-making qualities in the regional tests of 2003. Its sodium dodecyl sulfate (SDS) -sedimentation was 52.9 mL, water absorption was 61.2%, dough developing time was 11.7 min, and the stable time was 24 min (Luo et al. 2006). Therefore, we focused on the role of the pair 1Bx13 + 1By16 and their molecular characteristics. Although the HMW-GS pair 1Bx13 + 1By16 is recognized as having a positive correlation with bread-making quality (Lukow et al. 1989; Branlard and Dardevet 1985; Branlard et al. 2001), their molecular information has still been unknown until now. We isolated and sequenced the open reading frames (ORFs) of 1Bx13 and 1By16, and further analyzed their molecular structures, amino acid composition and hydrophobicity profiles. They were also compared with the published HMW-GS genes, and anticipated molecularly, revealing the possible mechanisms of good quality for the subunit pair 1Bx13 and 1By16.
Results
SDS-PAGE analysis of HMW-GSs in Atlas66 and PCR amplification of their coding genes
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed that the HMW-GS components were (1Ax2*, 1Bx13 + 1By16, 1Dx2 + 1Dy12), (1Ax1, 1Bx13 + 1By16, 1Dx5′ + 1Dy12) and (1Bx14 + 1By15, 1Dx5′ + 1Dy12) for Atlas66, Jimai20 and Yanzhan 1, respectively (the subunit 1Dx5′ was testified as a new subunit; B Pang, unpubl. data, 2007). It was obvious that the mobility of subunit 1By16 from Atlas66 and Jimai 20 was faster than the subunit 1By15 from Yanzhan 1, slower than the subunit 1By8 from CS, and the mobility of subunit 1Bx13 was faster than that of subunit 1Bx7 from CS and slower than that of subunit 1Bx14 from Yanzhan 1 (Figure 1A). Theoretically the molecular weight of subunit 1By15 should be higher than that of the subunit 1By16, and the molecular weight of subunit 1Bx13 should be higher than that of subunit 1Bx14, and lower than that of subunit 1Bx7. The expression level of 1By16 was apparently lower than that of 1By8. The PCR amplification indicated that the products amplified from Jimai 20 and Atlas66 genomic DNA were consistent (Figure 1B). The size verification showed that the ORF of 1By13 was about 2.4 kb, and the ORF of 1By16 was about 2.2 kb (Figure 1C), which was consistent with the sequencing results.
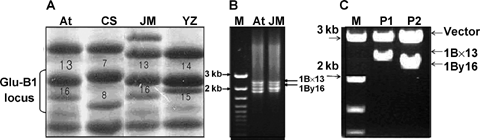
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-analysis of high molecular weight glutenin subunit (HMW-GS) components in wheat varieties (A), and polymerase chain reaction (PCR) amplification and size identification of the HMW-GS enconding genes (B, C).(A) At, Atlas66; CS, Chinese Spring; JM, Jimai 20; YZ, Yanzhan1.(B) The target fragments of 1By16 and 1Bx13 were confirmed by BLAST results. A single end of each fragment was sequenced and located according to basic local alignment search tool (BLAST) results through the NCBI website (http://www.ncbi.nlm.nih.gov).(C) M, DNA marker; P1 and P2, Plasmid pGEM-T-1By16 and pGEM-T-1Bx13 were digested by EcoRI (the vector pGEM-T-easy including two EcoRI sites at the two sides of the insertion site), respectively. The biggest fragments in P1 and P2 were the lined vector (pGEM-T-easy) with 3 kb, the small one in P1 was the open reading frame (ORF) of 1By13; and the smaller one in P2 was the ORF of 1By16.
Characterization of the ORF of 1Bx13
The ORF of 1Bx13 was 2 385 bp (including the six nucleotide acids coding the tandem stop codons at the end of the ORF), was deduced into 795 amino acid residues. The estimated molecular weight of the subunit 1Bx13 (without the signal peptide) was 83.3 kDa. The repetitive domain of the 1Bx13 contained 68 hexapeptides (consensus PGQGQQ and SGQGQQ), 25 nanopeptides (consensus GYYPTSPQQ) and six tripeptide (consensus GQQ) motifs. The deduced amino acid sequence of 1Bx13 possessed a signal peptide of 21 amino acid residues, an N-terminal domain of 81 amino acid residues followed by a repetitive domain of 651 amino acid residues and the C-terminal domain of 42 amino acid residues.
Comparison of the deduced amino acid sequence of Glu-B1-1 alleles
Compared with the reported coding sequences of Glu-B1-1 alleles including 1Bx13, 1Bx7, 1Bx14, 1Bx20, 1Bx7OE, 1Bx23 and 1Bx17 (Figure 2), the deduced amino acid sequences of 1Bx13, 1Bx20, 1Bx14, 1Bx23 and 1Bx7OE all consisted of 795 amino acid residues except for 1Bx17 of 789 and 1Bx7 of 753 amino acid residues. The 1Bx13 had the same structure as 1Bx23 (only distributed in Triticum turgidum, Yang et al. 2006) and 1Bx7OE, exhibited 19 and 20 amino acid substitutes from 1Bx23 and 1Bx7OE, respectively. The 1Bx7 had a hexapeptide (PGQGQQ) deletion in the end of repetitive domain compared with 1Bx7OE, which was the only difference between them. The 1Bx14 had the same structure as 1Bx20, but only five amino acid substitutes exist between them. The 1Bx17 was the smallest one among the Glu-B1-1 alleles, having a deletion of 36 and six amino acid residues in the repetitive domain. The size variation of Glu-1B-1 alleles was mainly determined by the N-terminal domain and central repetitive domain. Glu-B1-1 alleles, excluding 1Bx14 and 1Bx20, had two cysteines in each of N- and C- terminal domains, and all contained four cysteines; three in the N-terminal domain and one in the C-terminal domain. The respective cysteine position was very consistent.
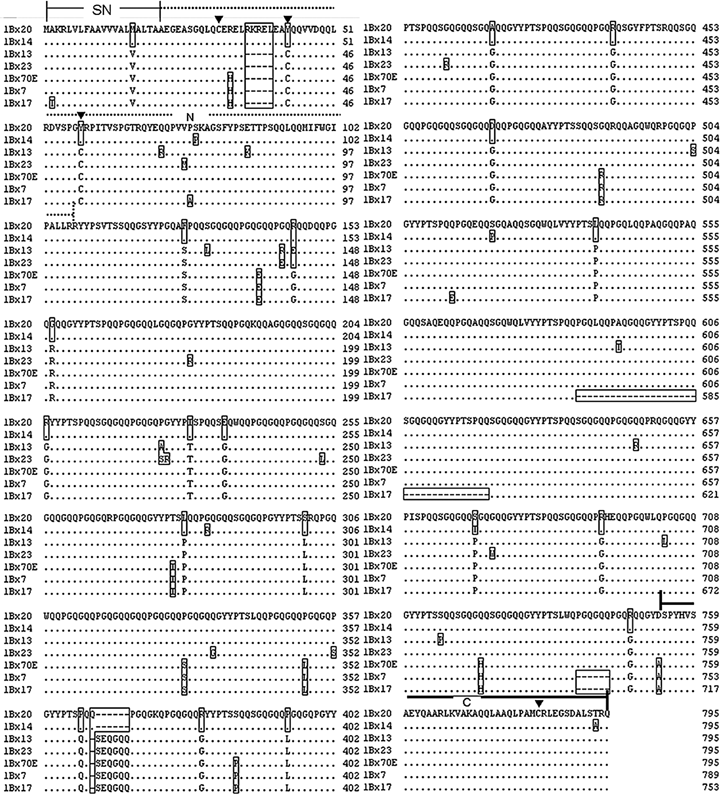
Multiple alignment of the deduced amino acid sequences of 1Bx13 (EF540764), 1Bx7 (X13927), 1Bx14 (AY367771), 1Bx20 (AJ437000), 1Bx7OE (DQ119142), 1Bx23 (AY553933) and 1Bx17 (JC2099).Dots and dashes indicate the same sequences with consensus 1Bx20 and deletion. The sequences that are different from other 1Bx alleles are boxed. ▾ indicates the cysteine position. SN, signal peptide, N and C represent the N-terminal domain and C-terminal domains, respectively. Repetitive domain is between the N-terminal domain and C-terminal domain.
Molecular characterization of the ORF of 1By16
The ORF of 1By16 was 2 220 bp (including six nucleotides coding the tandem stop codons at the end of the ORF), deduced into 738 amino acids residues. The estimated molecular weight of the subunit 1By16 (without the signal peptide) was 77.3 kDa. It contained 59 hexapeptides (consensus PGQGQQ), and 22 nanopeptides (consensus GYYPTSPQQ and GQYPASQQQ) motifs in the repetitive domain. The deduced amino acid sequence possessed a signal peptide of 21 amino acid residues, an N-terminal domain of 104 amino acid residues, followed by a repetitive domain of 571 amino acid residues and the C-terminal domain of 42 amino acid residues. The deduced amino acid sequence of 1By16 contained seven cysteines; five in the N-terminal domain, one in the repetitive domain close to the C-terminal domain, and one in the C-terminal domain.
Comparison of Glu-B1-2 alleles and certification of the gene 1By16
Compared with the reported ORF of Glu-B1-2 alleles including 1By16, 1By15, 1By8 and 1By9 (Figure 3), the 1By16 consisted of 738 amino acid residues, 1By15 consisted of 723, the 1By8 consisted of 720 and 1By9 consisted of 705. Apparently the 1By16 was the largest one among the Glu-B1-2 alleles. The cysteine position for the Glu-B1-2 alleles was completely consistent. The multiple alignment results of the deduced amino acid sequence for Glu-B1-2 alleles indicated that the 1By16 had 36 amino acid residues inserted, and 1By8 and 1By9 had only 18 amino acid residues inserted in the repetitive domain, compared with 1By15. Compared with other 1By alleles, the 1By15 had 15 and 6 amino acid residues inserted in the repetitive domain, and deletion of 15 amino acid residues existed in 1By9 in the repetitive domain. In 1By16, 12, 9 and 39 amino acid substitutes existed from 1By8, 1By9 and 1By15, respectively. All four 1By alleles possessed the same number of amino acids in the N-terminal and C-terminal domain. The size difference of 1By alleles was only determined by the central repetitive domain.

Multiple alignment of the deduced amino acid sequences of 1By16 (EF540765), 1By15 (DQ086215), 1By9 (X61026) and 1By8 (AY245797).Dots and dashes indicate the same sequences with consensus 1By16 and deletion. The sequence different from other 1By alleles is boxed. ▾ indicates the cysteine position. SN, signal pepetide, N and C represent the N-terminal domain and C-terminal domain, respectively, and the repetitive domain is between the N-terminal domain and C-terminal domain.
A large fragment inserted in the repetitive domain of the putative 1By16 gene compared with other Glu-1B-2 alleles required further identification. When the mature putative 1By16 (without signal peptide) was expressed in bacterial cells, it yielded a polypeptide showing an electrophoretic mobility identical to the subunit 1By16 extracted from the seeds (Figure 4A), and the Western-blot test using a monoclonal antibody specific for HMW-GS further testified that the putative 1By16 encoded HMW-GS (Figure 4B). Therefore, the putative 1By16 sequence was exactly the same as the ORF of the 1By16.
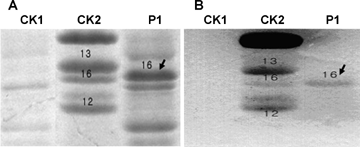
Verification of high molecular weight glutenin subunit (HMW-GS) 1By16 by expression in Escherichia coli.(A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. CK1, a negative control with the pET-30a plasmid in the isopropylthio-β-D-galactoside (IPTG) induced bacterial culture; CK2, Atlas66 (1Ax2*, 1Bx13 + 1By16, 1Dx2 + 1Dy12); P1, overexpression of the mature 1By16 protein in E. coli. Electrophoretic mobility of 1By16 overexpressed in E. coli (arrow in P1) was identical to that extracted from the atlas66 seeds (CK2).(B) Western-blot by the mono-clonal antibody specified to HMW-GS further testified the gene expressed was the open reading frame (ORF) of HMW-GS 1By16 (P1).
Phylogenetic tree
The phylogenetic tree was constructed based on the multiple alignment of the full-length amino acid sequence (including the signal peptide) of wheat HMW glutenin genes at Glu-1 loci (Figure 5). The European Molecular Biology Laboratory (EMBL) Nucleotide Sequence Database (http://www.ebi.ac.uk/embl) accession numbers for 1Ax1, 1Ax2*, 1Bx7OE, 1Bx23, 1Bx20, 1Bx14, 1Bx7, 1Bx17, 1By8, 1By9, 1By15, 1Dx2.2, 1Dx2.2*, 1Dx5, 1Dx2, 1Dy10, 1Dy12 are: X61009, M22208, DQ119142, AY553933, AJ437000, AY367771, X13927, JC2099, AY245797, X61026, DQ086215, AY159367, AJ893508, X12928, X03346, X12929 and X03041, respectively. The phylogenetic tree was obviously separated into three clades; one only composed of the Glu-1Bx alleles, the second of Glu-1Dx and Glu-1Ax alleles, and the third of Glu-1By and Glu-1Dy alleles. The 1By16 was clustered with Glu-1By alleles, and the 1Bx13 was clustered with Glu-1Bx alleles. This added further testimony that the classification for 1By16 and 1Bx13 was correct.
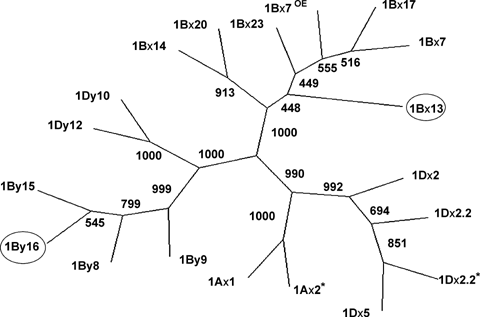
Phylogenetic tree generated from the amino acid sequence alignment of published high molecular weight glutenin genes.
Amino acid composition comparison for HMW glutenin genes
The amino acid composition of HMW-GS genes are summarized in Table 1. The mature 1By16, 1By15, 1By8 and 1By9 contained 717, 702, 699 and 684 amino acid residues, respectively, and the molecular weight of the mature 1By16 was apparent in the largest among the y-type subunits. The mature 1Bx13, 1Bx14, 1Bx20, 1Bx23, 1Bx7OE all consisted of 774 amino acid residues; the mature 1Bx7 of 768, and the mature 1Bx17 of 732.
| Subunit | Hydrophobic AA | Tyr | Cys | Asp | Glu | Gly | His | Lys | MET | Asn | Pro | Gln | Arg | Ser | Thr | Total AA | IP | MW (Daltons) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Dy12 | 79 | 34 | 7 | 4 | 17 | 116 | 13 | 9 | 5 | 0 | 69 | 211 | 13 | 42 | 25 | 639 | 7.19 | 68 712.65 |
| 1Dy10 | 75 | 34 | 7 | 4 | 17 | 113 | 13 | 8 | 4 | 0 | 71 | 208 | 14 | 42 | 26 | 634 | 7.19 | 67 474.18 |
| 1By9 | 82 | 38 | 7 | 4 | 15 | 129 | 11 | 7 | 4 | 2 | 68 | 233 | 17 | 48 | 25 | 684 | 8.13 | 73 516.71 |
| 1By8 | 85 | 39 | 7 | 4 | 15 | 131 | 11 | 7 | 4 | 2 | 69 | 239 | 18 | 49 | 25 | 699 | 8.30 | 75 158.48 |
| 1By16 | 83 | 39 | 7 | 4 | 17 | 134 | 11 | 8 | 4 | 2 | 70 | 249 | 19 | 48 | 28 | 717 | 8.30 | 77 282.65 |
| 1By15 | 83 | 40 | 7 | 4 | 17 | 125 | 11 | 7 | 4 | 2 | 72 | 241 | 19 | 50 | 25 | 702 | 8.13 | 75 734.91 |
| 1Bx14 | 75 | 56 | 2 | 6 | 14 | 137 | 2 | 7 | 4 | 0 | 96 | 262 | 25 | 65 | 28 | 774 | 9.22 | 84 014.27 |
| 1Bx20 | 74 | 56 | 2 | 6 | 14 | 138 | 2 | 7 | 4 | 0 | 94 | 262 | 24 | 68 | 28 | 774 | 9.15 | 83 911.05 |
| 1Bx23 | 71 | 54 | 4 | 5 | 14 | 142 | 2 | 6 | 4 | 0 | 93 | 264 | 21 | 69 | 29 | 774 | 8.75 | 83 541.57 |
| 1Bx13 | 72 | 54 | 4 | 5 | 13 | 142 | 2 | 7 | 3 | 0 | 93 | 263 | 20 | 67 | 30 | 774 | 8.86 | 83 249.29 |
| 1Bx7OE | 73 | 54 | 4 | 4 | 14 | 145 | 4 | 6 | 3 | 0 | 94 | 264 | 17 | 68 | 28 | 774 | 8.28 | 83 122.06 |
| 1Bx7 | 73 | 54 | 4 | 4 | 14 | 143 | 4 | 6 | 3 | 0 | 93 | 261 | 17 | 68 | 28 | 768 | 8.28 | 82 526.44 |
| 1Bx17 | 72 | 50 | 4 | 4 | 15 | 137 | 4 | 6 | 3 | 0 | 86 | 247 | 17 | 65 | 27 | 732 | 8.05 | 78 610.29 |
| 1A2* | 83 | 49 | 4 | 6 | 24 | 140 | 4 | 7 | 2 | 0 | 95 | 275 | 21 | 61 | 28 | 794 | 5.8 | 86 334.96 |
| 1Ax1 | 85 | 49 | 4 | 6 | 24 | 146 | 4 | 6 | 2 | 0 | 98 | 282 | 20 | 60 | 28 | 809 | 5.38 | 87 678.35 |
| 1Dx2 | 88 | 46 | 4 | 4 | 17 | 163 | 4 | 7 | 3 | 0 | 107 | 291 | 11 | 49 | 25 | 815 | 5.45 | 86 807.54 |
| 1Dx5 | 92 | 46 | 5 | 4 | 15 | 162 | 5 | 8 | 3 | 0 | 108 | 292 | 10 | 46 | 26 | 818 | 6.20 | 87 189.08 |
| 1Dx2.2 | 95 | 54 | 4 | 4 | 17 | 194 | 4 | 7 | 4 | 0 | 128 | 345 | 10 | 58 | 30 | 950 | 5.23 | 100 885.34 |
| 1Dx2.2* | 104 | 53 | 4 | 5 | 16 | 207 | 5 | 7 | 3 | 0 | 137 | 372 | 10 | 55 | 29 | 1003 | 5.40 | 106 597.39 |
- Arg, arginine; Asn, asparagines; Asp, aspartic acid; Cys, cysteine; Gln, glutamine; Glu, glutamic acid; Gly, glycine; His, histidine; Hydrophobic AA, valine, tryptophan, leucine, alanine, isoleucine, phenylalanin; IP, isoelectric point; Lys, lysine; Met, methionine; MW, molecular weight of mature subunit; Pro, proline; Ser, serine; Thr, threonine; Total AA, total amino acids of mature HMW-GS gene; Tyr, tyrosine.
Two asparagines were obvious specific characters of 1By alleles. The total content of glycine, proline and glutamine in individual mature HMW-GS genes (without signal peptides) ranged from 61.8% (1Dy10) to 71.4% (1Dx2.2*), and among them, the content of glutamine ranged from 32.8% (1Dy10) to 37.1% (1Dx2.2*), the glycine ranged from 17.6% (1Ax2*) to 20.6% (1Dx2.2*), and the proline ranged from 9.8% (1By16) to 13.7% (1Dx2.2*). The glutamine content of the mature 1By16 accounted for 34.7%, which was the highest among the y-type alleles. The glutamine content of the mature 1Bx13 was 34.0%, and little significant difference was observed between the other 1Bx alleles.
Hydrophobicity profiles and secondary structure comparison within Glu-B1 alleles
The signal peptide region for the first 21 amino acids of the Glu-B1 alleles had an obvious high score in the hydrophobic region (data not shown). The four 1By alleles had similar hydrophobicity profiles except the 1By16 possessed an evident strong hydrophobic region in the N-terminal domain. Its amino acid sequence had one mutant from the non-hydrophobic T to the hydrophobic I at the 91st amino acid residue from the first methionine, and this mutant led to the creation of the stronger hydrophobic region. The alpha helical region prediction showed that the 1By16 alleles had seven alpha helical regions, and the 1By8, 1By9 and 1By15 alleles had only six alpha helical regions. The seven 1Bx alleles were very similar in hydrophobicity profiles, the 1Bx20, 1Bx13 and 1Bx14 had six helical regions, but 1Bx23, 1Bx7, 1Bx17 and 1Bx7OE had only five helical regions.
Discussion
In this paper, we analyzed the molecular characterization of 1By16 and 1Bx13. The estimated molecular weight of the mature 1By16 (without the signal peptide) based on the deduced amino acid sequence was 77.3 kDa, which is consistent with Li (2006) based on matrix-assisted laser desorption ionization time of flight mass spectrometry. Zhao et al. (2005) reported a novel HMW-GS 1By gene that was cloned by reverse transcription-polymerase chain reaction (RT-PCR) in the hybrid line II-1-3 of an asymmetric somatic hybrid between Triticum aestivum L. and Agropron elongatam (Host) Nevisk, which had a similar mobility to the subunit 1By16 in SDS-PAGE gel, the novel 1By deduced 712 amino acid residues, whose size is between the 1By15 and 1By16. The mature 1Bx13 and 1Bx7OE both contained 774 amino acid residues, had similar structure to a hexapeptide (PGQGQQ) insertion in the end of their central repetitive domain compared with 1Bx7, and the 1Bx13 exhibited 20 amino acid substitutes from the 1Bx7OE.
Although the mobility of the subunit 1By16 was faster than that of the subunit 1By15, 1Bx14 was faster than 1Bx13, and 1Bx13 was faster than 1Bx7 in the SDS-PAGE. In fact, the molecular weight of mature 1By16 (77.3 kDa) based on the deduced amino acid sequence was higher than that of mature 1By15 (75.7 kDa), 1Bx14 higher than 1Bx13, and 1Bx13 higher than 1Bx7. The anomalous migration phenomenon also occurred in other subunits, such as 1Dx2 and 1Dx5, 1Dy12 and 1Dy10 (Shewry et al. 1992). The mobility of subunit 1By16 from seed extracts was identical to that extracted from the bacterial cell, obtaining the same results in different transcription and translation systems suggests that the discrepancy between the mobility and molecular weight may be not related to post-translational modification of protein, but related to different spatial structure or carrying a different charge of the individual allele. The fact is that 1By16 contains an additional strong hydrophobic region in the N-terminal domain and an additional helix in the repetitive region compared with subunit 1By15, and the charge they carried was slightly different, so these two specific structures may be responsible for the faster mobility of 1By16.
William (1995) testified that the anomalous migration of the HMW-GS in gel is due to a decreased binding of SDS to these proteins. More SDS could completely wrap proteins and exclude the influence of charge and break the spatial structure through destroying hydrogen bonds. The isoelectric points of 1Bx14, 1Bx13 and 1Bx7 calculated based on the deduced amino acid sequence were 9.22, 8.86 and 8.28, respectively, which means that they will carry different charges in the same pH separation gel. At the same time, the 1Bx14 and 1Bx13 both possessed an additional helix in their central repetitive domain compared with other 1Bx alleles. So the different charge and different spatial structure maybe lead to the anomalous migration of the subunits 1Bx14, 1Bx13 and 1Bx7.
There is evidence from mixing studies that larger HMW subunit proteins have a greater positive effect on dough strength than smaller subunits (Békés et al. 1995), and the size difference of HMW-GS is determined by the size of its repetitive region (Shewry et al. 1992) that is rich in glutamine. Therefore, a larger HMW-GS means that it has higher glutamine content. The high glutamine content through forming more hydrogen bonds can stabilize the polymeric structure of glutenin (Gilbert et al. 2000). 1By16 is the largest y-type HMW glutenin gene with higher glutamine content, and 1Bx13 was the largest gene of the Glu-B1-1 alleles containing four cysteines in common wheat. Therefore, the largest 1By16 gene combining the larger 1Bx13 theoretically is the best combination at the Glu-B1-1 locus. However, the expression levels of 1By16 and 1Bx13 are not high enough, so increasing their expression level via transformation would be a worthy exercise for improving the processing quality of common wheat.
Materials and Methods
Plant materials
Triticum aestivum L. cv. Chinese Spring (CS), Atlas66, Jimai 20 and Yanzhan 1 were used in this experiment. Single seeds with full and equal weight for each variety were used to quantify the expression level of individual alleles by SDS-PAGE of HMW-GS components, and CS (1Bx7 + 1By8, Dx2 + 1Dy12) was used as control.
SDS-PAGE and Western-blot analysis
High molecular weight glutenin subunit proteins were extracted from seeds for SDS-PAGE and Western-blot analysis as previously described (Wang et al. 2005; Wang and Zhang 2006). A monoclonal antibody (Patent No. ZL01144781.8) specific binding to HMW-GS was used to identify the mature ORF of 1By16 expressed in Escherichia coli. The image of the SDS-PAGE and Western-blot of HMW-GS components were captured by a HP 3 570 Scanjet (Beijing, China).
Cloning and sequencing of HMW-GS 1By16 and 1Bx13
Genomic DNA of Atlas66 and Jimai 20 was isolated from seedlings as described by D'Ovidio et al. (1992). The primers designed based on the conserved N- and C-terminal nucleotide sequences of the published HMW-GS gene sequences for cloning the complete ORF of HMW-GS 1By16 and 1Bx13 are F1 (5′-ATGA/GCTAAGCGC/GCTGGTCCTCTTTG-3′), and R1 (5′-CTATCACTGCCTGGT/CCGACAATGCG-3′). Genomic DNA (120 ng) was added to a 20 μL reaction mixture, containing 1 × PCR buffer (TaKaRa, Otsu, Shiga, Japan), 1.6 mM MgCl2, 0.5 μM of each primer, 250 μM dNTP and 1.0 U Taq polymerase. Thermal cycling was predenatured at 94 °C for 3 min, 29 cycles of denaturation at 94 °C for 30 s, annealing and extension at 68 °C for 3.5 min, followed by a final cycle of extension at 72 °C for 10 min. The amplified products were separated in 1.0% agarose gels. The target DNA fragments were recovered from the agarose gels. After being purified with a DNA purification kit (BioTeKe, Beijing, China), they were ligated into the pGEM-T easy vector (Promega, Madison, WI, USA). The clone with the 1By16 ORF was named pGEM-T-1By16. To sequence the complete ORF of 1By16, serial subclones from pGEM-T-1By16 were prepared with a nested deletion kit method (Fermentas, St. Leon-Rot, Germany) and sequenced. The full-length nucleotide sequence was obtained by assembling the nine subclones in sequence using SeqMan software (DNAstar, Madison, WI, USA).
Bacterial expression of the ORF of 1By16
The primers exF1By16 (5′-ACCCATATGGAAGGTGAGGCCTCTA-3′) and exP1By16 (5′-CTA GAATTCCTATCACTGCCTGGTC-3′) were used for amplifying the mature 1By16 DNA sequence removing the signal peptide and introducing the Nde I and EcoR I sites and an additional methionine for commencing the translation of mature 1By16 (Liu et al. 2003). The PCR conditions were identical to those described above except that the template was the plasmid DNA pGEM-T-1By16. The PCR products and pET-30a vector (Novagen, Madison, WI, USA) were both completely digested by Nde I and EcoR I, and then they were ligated together. The plasmid pET-1By16 was selected for expression of the mature protein of the subunit 1By16 in the bacterial cells.
Phylogenetic analysis and secondary structure
Multiple sequence alignments were conducted using Clustal X (Illkirch Cedex, France) version 1.83 (Saitou and Nei 1987; Thompson et al. 1997). Maximum parsimony trees were built using the Phylip package (Seattle, WA, USA) (Felsenstein 1993). The number of bootstrap replicates was 1 000. The trees were presented using TreeView (Glasgow, UK) (Page 1996). Sequences were analyzed by the EditSeq program (DNAstar, Madison, WI, USA), the secondary structure prediction used the Protean program (DNAstar, Madison, WI, USA), the hydrophobicity profile was analyzed by DNAman (Berkeley, CA, USA).
Accession numbers
The complete nucleotide sequences and deduced amino acid sequences of 1Bx13 and 1By16 reported in this paper have been deposited in GenBank with the accession numbers EF540764 and EF540765, respectively.
(Handling editor: Yong-Biao Xue)




