Phytoplasma distribution in coconut palms affected by lethal yellowing disease
Abstract
Lethal yellowing (LY), the most devastating disease affecting the coconut palm in America, is caused by phytoplasmas known to be distributed in different parts of infected plants. However, no comprehensive reports exist on the phytoplasma distribution. This study refers to the detection of LY phytoplasma DNA using PCR in different coconut plant parts, throughout the development of the disease. Sample analysis of positive palms taken at different stages of disease development (either symptomatic or symptomless) showed differences in the percentage of LY detection between plant parts. Some parts showed a very high level of LY DNA (stem, young leaves, inflorescences, stem apex and root apex), low levels were found in the intermediate leaves and roots without apex, whereas no LY phytoplasma DNA was detected in mature leaves. The detection percentage of LY phytoplasma DNA was lowest in symptomless-infected palms for all parts, except the stem, where phytoplasma accumulations were consistently detected. This pattern of detection among parts is consistent with the hypothesis that phytoplasmas move from photosynthate source tissues to sink tissues via the phloem mass flow process. The accumulations in the (lower) stem, prior to the appearance of symptoms, suggest that this part of the palm is where phytoplasmas first move from leaves after foliar feeding by vectors and in which they probably multiply and distribute to other palm parts, including roots. Embryos from infected palms were analysed by nested-PCR and 28% of 394 embryos were positive. Phytoplasma DNA was detected in embryos from fruit on any of the fruiting bunches regardless the age, but no pattern of quantitative distribution throughout the bunch developmental stages was observed. Germination of seeds from LY-positive symptomatic palms was 58% and from LY-negative symptomless palms were 71%. No phytoplasma was detected in seedlings tested from both symptomatic and non-symptomatic palms. Seedlings tested after 2 years did not develop LY symptoms or eventually died.
Introduction
Phytoplasmas are unculturable, cell wall-less bacteria of bead-like, filamentous or multi-branched appearance when observed by electron microscopy (EM) (Thomas, 1979; Thomas & Norris, 1980). They preferentially colonise phloem tissues of host plants and are known to cause diseases in numerous economically important plant species worldwide (McCoy et al., 1989; Kirkpatrick, 1992; Seemüller et al., 2002). In palms, they are associated with lethal yellowing (LY) disease in America (Harrison & Oropeza, 2008) and similar devastating lethal yellowing-like diseases (LYD) of coconut in Africa (Eden-Green, 1997).
Phytoplasmas have been reported to be distributed in different parts of host plants. Accumulation of western aster yellows phytoplasmas was found in graft-inoculated periwinkle (Catharanthus roseus) plants to be highest in actively growing shoots compared to the roots as measured by DNA probe hybridisation (Kuske & Kirkpatrick, 1992). Using enzyme-linked immunosorbent assay and immunocytochemistry, flavescence dorée phytoplasmas were detected first in roots of infected broad bean (Vicia faba) plants, then in the collar and axillary shoots, and preferentially multiplied in the apical tip of the growing shoot (Lherminier et al., 1994). In papaya (Carica papaya) plants affected by dieback disease, phytoplasma DNA was located by PCR assay in sink tissues but not in mature leaves (Siddique et al., 1998). The rate of increase of chrysanthemum yellows phytoplasma (CYP, 16Sr-I) in leaves and roots of the host plant Chrysanthemum carinatum was studied over time, following insect inoculation, using a relative quantification method based on real-time PCR (Saracco et al., 2006). The authors found that CYP was much more concentrated in young apical leaves and in roots compared to old basal leaves, where it was not always detectable. In Euphorbia pulcherrima and Cat. roseus plants, phytoplasmas were found by PCR analysis to accumulate unevenly in the leaf veins, to a lesser extent in their petioles and stems, whereas in roots and sink leaves accumulations were small or non-detectable (Christensen et al., 2004). According to these reports, it seems that phytoplasmas most commonly accumulate in growing sink tissues than in mature tissues of invaded plants.
In the case of LY disease there are reports, using different techniques, that these phytoplasmas are distributed within different organs and tissues of infected plants. Electron microscopy observations of LY-diseased palms of three species (Veitchia merrillii, Pritchardia pacifica and Cocos nucifera) showed that phytoplasmas were present in unexpanded inflorescences, partly expanded spear leaves, but not found in fully expanded inflorescences, leaves or stems (Parthasaraty, 1974). Intracellular accumulation of phytoplasma DNA in phloem sieve elements of coconut palms, as determined by the use of the fluorescent probe 4′-6′-diamidino-2-phenylindole, was revealed in root tips, petiole of young, unemerged leaves surrounding the apical meristem and unopened inflorescences (Deutsch & Nienhaus, 1983). Using DNA probes, LY phytoplasma DNA was reliably detected in young unemerged leaves of the stem apex, root apex and unopened inflorescences (Harrison et al., 1992, 1994; Escamilla et al., 1995). Similar findings have been obtained by PCR (Harrison et al., 1994), as well as locating the phytoplasmas in stem tissues (Harrison et al., 1999). In the case of LYD, Nipah et al. (2007) showed the occurrence of phytoplasmas in stems and various parts of the inflorescences of coconuts affected with Cape St. Paul wilt disease (CSPWD). Other studies have also reported phytoplasma DNA in coconut embryos (Harrison & Oropeza, 1997; Harrison et al., 1999; Nipah et al., 2007), a finding supported by in situ PCR techniques (Cordova et al., 2003). These data collectively show that both LY and LYD phytoplasmas accumulate primarily in sink tissues of palms. However, no single piece of research exists that has attempted to study the phytoplasma distribution, in most plant parts and throughout the development of disease symptoms. Therefore, in order to obtain a better understanding of the in planta distribution of LY phytoplasma within coconut palms during the course of disease development, this study focused on the detection of phytoplasma DNA by PCR within diseased palms, including growing sink and source tissues, throughout the development of the disease symptoms.
Materials and methods
Sample collection and storage
Samples were collected from naturally infected coconut palms (Coc. nucifera L., Atlantic Tall ecotype) growing in a coconut grove at Sabancuy in Campeche State, Mexico (18°59′ N; 91°11′ W). Sampled palms were at different stages of symptom development, as defined by McCoy et al. (1983): (0) symptomless, (1) nut fall, (2) necrotic inflorescences, yellowing (3) in lower leaves, (4) middle leaves, (5) younger leaves and (6) all leaves. For each stage of symptom development including symptomless palms, three batches of palms (each with five palms) were sampled, and one sample of each tissue type was collected from each palm. Also coconut palms were sampled in an LY-free area at Paraiso in Tabasco State (18°25′ N, 93°14′ W) and were used as DNA-negative control. For each case, samples of the following tissues were collected: youngest leaf (spear leaf), mature leaves from the mid and lower canopy, stem apex, inflorescences (+1 to +3, given that 0 is the most mature unopened inflorescence), primary roots, primary root apex and the interior of the lower stem. In the case of leaves, the sample included only the lamina.
For the evaluation of the presence of LY phytoplasma DNA in embryos, fruit were collected in coconut groves affected by LY at Sabancuy (same site as above) and two additional groves at Chicxulub (21°17′ N; 89°35′ W) and San Miguel (21°18′ N; 89°33′ W) on the northern coast of Yucatan State, Mexico. All groves sampled were not commercially maintained at the time of the study. For Sabancuy and Chicxulub, the fruit were collected from the ground at the base of infected and non-infected palms (as determined by direct-PCR). Sourced-infected palms were already showing early symptoms of LY (nut fall) and the other LY symptoms developed during the study. In the case of San Miguel, fruit were collected to evaluate if fruit from different bunches (maturity stages +15 to +9) could be infected. Twelve palms were sourced in two batches. The first batch included nine palms showing first LY symptom (nut fall) and positive direct-PCR detection. Fruit collected were that still attached to the bunches as not all of them had fallen at the moment of collection. Fruit from the ground were not collected because it was going to be impossible to associate each of these fruit with a particular bunch. For this reason, in the second batch of palms the approach chosen had the purpose of collecting most of the nuts from each bunch. This second batch included three asymptomatic palms with positive direct-PCR detection and fruit were marked to associate them when fallen, with each particular individual bunch (developmental stages +15 to +9, given that 0 is the most mature unopened inflorescence). After the nut fall symptom occurred, fruit were collected from the ground as well as those still attached to the palm, the latest accounted for 87% of the total collected. For the purpose of analysis the results obtained from the two batches of fruit were pooled together and dealt with as a single batch of 12 palms.
While still in the field, fruit collected were cut transversely to excise the endosperm cylinder enclosing the embryo using a cork borer (1.6 cm diameter). Immediately after excision, endosperm cylinders were placed for 20 min in a 0.6% (v/v) NaOCl solution (diluted commercial bleach), subsequently surface-sterilised for 3 min with 70% ethanol, rinsed three times with sterile distilled water, washed with agitation for 20 min with a 3% NaOCl solution and finally rinsed three times with sterile distilled water.
For the determination of viability, coconut fruit were harvested from infected and uninfected palms in the disease-affected area of Chicxulub, Yucatan. A total of 15 symptomless palms that tested positive for phytoplasma infection by nested-PCR served as a source of mature seeds shortly after the initial LY symptom of nut fall commenced. A total of 12 symptomless palms that tested negative by nested-PCR served as a source of uninfected fruit.
All samples once obtained were stored on ice for transportation from the field to the laboratory where they were stored at −80°C prior to DNA extraction.
DNA extraction
DNA was extracted from 1 g of each tissue sample according to the cetyltrimethyammonium bromide (CTAB) method described by Doyle & Doyle (1990) as modified by Harrison et al. (1994). Final nucleic acid extracts were precipitated with ethanol, pelleted by centrifugation, resuspended in 100 µl of Tris-ethylenediaminetetraacetic acid (EDTA) buffer (1 mM Tris, 0.1 mM EDTA, pH 8) and incubated with RNAse for 1 h at 37°C. Aliquots of resulting DNA preparations were used as template for PCR. The quantity of DNA in each extract was determined by spectrophotometry (OD260) and diluted as required for a final concentration of 50 ng of DNA per reaction.
Phytoplasma detection by PCR and analysis of the PCR products
For LY phytoplasma-specific direct-PCR, amplifications were performed in 50 µL reaction volumes each containing 2 µL of DNA template, 50 ng of each primer, 125 µM of each dNTP, 1U Taq DNA polymerase and standard PCR buffer containing 1.5 mM MgCl2. Assays were was performed in a programmable thermocycler (GeneAmp PCR system 2000, Applied Biosystems, Foster City, CA, USA) for 35 cycles using primer pair LYF1/LYR1 as previously described (Harrison et al., 1994). For nested-PCR assays, phytoplasma universal 16S rRNA gene primer pairs P1 (Deng & Hiruki, 1991) and P7 (Smart et al., 1996) were used for initial amplifications. Resulting products were then diluted 1:40 with ultrapure water before 2 µL of each dilution was re-amplified for 35 cycles using LY-specific 16S rRNA gene primer pair 503f/LY16Sr (Harrison et al., 1999). Previously characterised DNA samples from LY-diseased and healthy coconut palms were included in each assay as positive and negative controls, respectively. Aliquots (10 µL) of each 50 µL final reaction mixture were electrophoresed through 1% agarose gels using Tris-acetate-EDTA (40 mM Tris-acetate, 1 mM EDTA, pH 8) buffer, stained with ethidium bromide, visualised by ultraviolet transilluminator and photographed.
Determination of the viability of fruit from infected and uninfected coconut palms
In disease-free conditions at Centro de Investigación Científica de Yucatán (CICY) in Merida, Yucatan, 75 nuts from each group of palms (LY infected and uninfected) were germinated in three seedbeds (25 seeds per bed) using standard techniques (Santos et al., 1996). The nursery beds were regularly sprayed with insecticides at 14-day intervals to prevent contact with insects. The rates of germination were recorded every week over a 15-week period. Seedling plants obtained after this period were sampled for nested-PCR analysis. The upper parts of apical leaves were sectioned as close as possible to the growing point without killing the seedling and crushed in CTAB buffer for DNA extraction. Plants were kept for a further period of 2 years in the beds and monitored monthly for LY symptom development.
Statistical analysis
To determine the statistical difference in the percentages of seed germination between the two treatments, a Student's t-test was carried out using the Sigma Stat package (Jandel Scientific Software, Chicago, IL, USA). χ2 analysis was carried out to determine if there was a significant effect of bunch maturity stage on percentage of detection.
Results
Detection of lethal yellowing phytoplasma DNA
Lethal yellowing phytoplasma DNA was detected by direct-PCR (Harrison et al., 1994) in extracts obtained from tissue samples from all symptomatic palms collected from Sabancuy, Campeche, a region affected by LY disease. No LY phytoplasma DNA was detected in any samples from coconut palms collected from Paraiso, Tabasco, a region that was free from LY disease. It can be concluded that LY was not present in Paraiso and that the PCR techniques were not producing any false positive readings.
Distribution of lethal yellowing phytoplasmas within infected coconut palms
Tissue type affected the percentage of LY-positive samples determined by a LY-specific PCR with the primer pair LYF1/LYR1 (Fig. 1). While mature palm leaves were consistently devoid of detectable quantities of LY phytoplasma DNA, stem tissues were uniformly positive in all stages of disease development, including at stage 0 when visible symptoms on palms were absent. The percentage of phytoplasma positives varied among all other palm tissues and according to the stage of symptom development. Excluding mature leaves and stems, phytoplasma detections increased in all other tissues over time and peaked at stages 1–3 for immature leaves, inflorescences and stem apexes; stages 2–5 for primary root apex and intermediate leaves; and stages 4–5 for primary root distal to the root apex. Detections then declined by stage 6 in all tissues, except stems. Positive results were also obtained at low frequency in immature leaves, inflorescences and the stem apex of symptomless palms (stage 0, presumably while phytoplasma incubation).
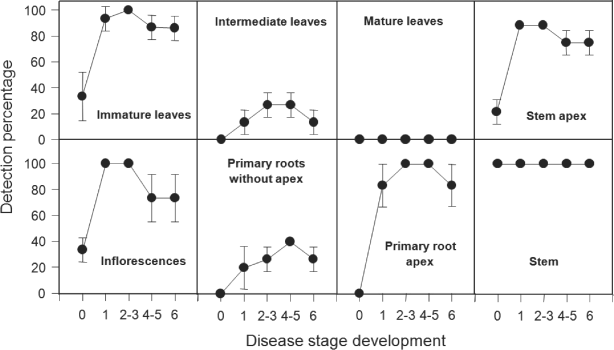
Detection of phytoplasma DNA in different parts of coconut palms affected with lethal yellowing (LY) and determined at different stages of the disease development: 0, no symptoms; 1, nut drop; 2–3, appearance of necrosis in inflorescences; 4–5, yellowing of leaves in base and middle of the crown; 6, yellowing of leaves in top of the crown. Direct-PCR amplification was carried out using the LYF1/LYR1 primer pair. Data shown are means of the detection percentages of three batches of palms (each of five palms) with standard deviations.
In all samples from those symptomless palms reported above, a re-assessment of the presence of LY DNA was carried out using nested-PCR. The percentage of positive detections increased in all tissues (Table 1), with the exception of primary roots without apexes and mature leaves that again were negative, and stem that had already a percentage of detection of 100% with direct-PCR. All the primary root apex and intermediate leaf samples were negative with direct-PCR, whereas with nested-PCR 20% and 33.3% of them were positive, respectively. The percentages of LY phytoplasma detection in immature leaf, inflorescence and stem apex samples, detection percentages increased from 33.3% to between 80% and 86% respectively. Samples from the mature leaves of symptomatic palms were also re-assessed by nested-PCR, and once again, proved to be all negative for LY phytoplasma DNA (Table 1).
| Plant part | Detection (%)a | |
|---|---|---|
| Standard PCRb | Nested-PCRc | |
| Symptomless palms | ||
| Immature leaf | 33.3 | 80 |
| Intermediate leaf | 0 | 33.3 |
| Mature leaf | 0 | 0 |
| Inflorescence | 33.3 | 80 |
| Stem apex | 33.3 | 86 |
| Primary root apex | 0 | 20 |
| Primary root without apex | 0 | 0 |
| Stem | 100 | 100 |
| Symptomatic palms | ||
| Mature leaf | 0 | 0 |
- aFifteen samples per plant part were assayed.
- bPCR with primer pair LYF1/LYR1.
- cNested-PCR with primer pairs P1/P7 followed by 503f/LY16Sr.
Lethal yellowing phytoplasmas in embryos
Analysis of mature embryos from palms in different sites
Embryos excised from mature fruit collected from the ground adjacent to LY-positive coconut palms at Sabancuy (Campeche state) and Chicxulub (Yucatan state) were analysed by LY-specific direct-PCR. Positive detections were obtained for 7% (5/65) of the embryos of the batch from Sabancuy and for 1% (1/84) of the embryo of the batch from Chicxulub. When analysed with nested-PCR, detection frequency increased to 20% for Sabancuy embryos and to 14% for Chicxulub embryos. These two batches comprise 149 embryos and the average percentage of LY phytoplasma detection by nested-PCR was 17%. If we also consider the batch of embryos (from mature and immature fruit) described in the following section, the total amount of embryos analysed was 394 and the average percentage of LY phytoplasma detection by nested-PCR was 29%, ranging from batch to batch from 14% to 45%.
Analysis of embryos from bunches of different developmental stages
In the analyses reported in the previous section, associating bunch maturity with the fruit collected was impossible. Therefore, this part of the study was conducted to determine if the LY phytoplasma could be present in coconut embryos from bunches of different maturity stages (+15 to +9). Fruit were obtained from 12 palms showing nut fall symptom and that were positive to LY detection by direct-PCR. A number of 245 embryos were obtained from the bunches of 12 palms; 38% of them were positive to LY detection by nested-PCR, and for individual palms percentages varied from 15% to 66%. Positive embryos were found from fruit bunches of each of the different maturity stages (Fig. 2), but for individual palms, LY phytoplasma was not always detected for each bunch (not shown). These results show no apparent correlation between percentage of LY phytoplasma detection and bunch maturity. A χ2 analysis was carried out confirming that there is no significant effect of bunch maturity stage on percentage of detection.
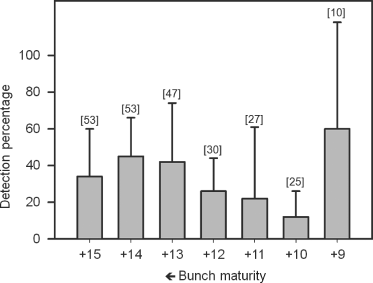
Detection of lethal yellowing (LY) phytoplasma DNA in embryos from bunches of coconut palms of different maturity stages (+15 to +9). Overall detection percentage was 45.5 ± 18.5%. Detection was carried out by nested PCR using the primer pair P1/P7 for first amplification and 503 f/LY16Sr for second amplification. Bars denote standard deviation. Data shown are means of the detection percentages (±SD). Figures in brackets above the bars denote the total number of nuts obtained from all source palms of each bunch maturity.
Embryo germination and seedling analysis
A total of 75 seeds were collected from 15 infected (nested-PCR positive) palms at the start of symptom development and 75 seeds from 12 uninfected (nested-PCR negative and symptomless) palms, both from same site at Chicxulub, Yucatan. After seed collection, all infected source palms were then monitored weekly and subsequent development of the typical LY syndrome occurred in all of them. Germination was monitored over a 15-week period although most nuts germinated within the first 10 weeks (Fig. 3). Overall, germination success was consistently higher for mature seeds from uninfected than from diseased palms, although differences between the two germination curves were significant during 7–15 weeks after the experiment began. By week 15, 59% of seeds from infected palms and 71% of seeds from uninfected palms had germinated. Leaf samples of all seedlings were analysed for phytoplasma infection by nested-PCR after week 15 with consistently negative results. Seedlings were then monitored visually for 2 years and none of the palms developed LY symptoms.
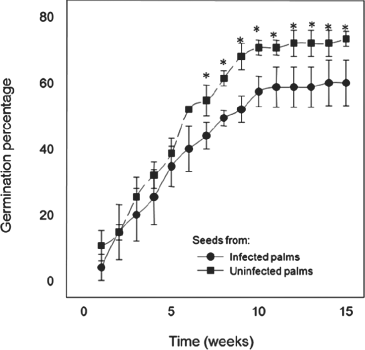
Germination of seeds obtained from coconut-infected (nested-PCR positive) palms at start of symptom development and from uninfected (nested-PCR negative and symptomless) palms. Data shown are means of the germination percentage (±SD) of germination of three batches with 25 nuts each. The asterisk denotes significant differences (P < 0.05).
Discussion
Determining the build-up and distribution of phytoplasmas throughout LY-affected coconut palms during the course of symptom development is important for understanding how colonisation might affect plant function and development (León et al., 1996; Lepka et al., 1999; Maust et al., 2003). It is also important to determine the most effective tissue(s) to sample for diagnostic purposes and to know how LY phytoplasma moves within the embryos/seeds to assess its further impact on germplasm exchange. Analysis by direct-PCR of tissues from palms in Sabancuy, a site in a LY-affected region in the Yucatan Peninsula in Mexico, using LYF1/LYR1 primer pair (Harrison et al., 1994) determined the occurrence of subgroup 16SrIV-A LY (Harrison et al., 1999) in both diseased and adjacent symptomless palms. Conversely, no phytoplasmas were detected upon similar analysis of tissues from symptomless palms in Paraiso, a site in a LY-free region in Tabasco State.
Analysis of samples of separate parts of the positive palms, taken at different stages of disease development (including symptomless, stage 0), showed differences in the percentage of LY phytoplasma detection among parts. Some parts showed a very high level (stem, young leaves, inflorescences, stem apex and root apex), others (intermediate leaves and roots without apex) showed a lower level, whereas no detections were registered for mature leaves. Also, a pattern of detection level was found to be associated with disease development: the detection percentage was lowest in symptomless, but infected, palms in all parts except the stem where phytoplasma accumulations were detected prior to the onset of visible symptoms; then percentages increase, peaking at stages 1–3 before decreasing during the final stages of disease development. In the case of stem, the phytoplasma detection was 100% at any time sampled.
When samples of symptomless but positive palms were re-assessed using a more sensitive nested-PCR assay, the percentage of detection increased, except for samples of mature leaves where it remained 0%. Although the PCR methodology used here is not quantitative, the differences observed between results obtained by direct LY-specific PCR versus nested-PCR indicate that phytoplasma concentration vary within tissues of the plant parts as follows: there is no presence (or a very low non-detectable titre) of phytoplasmas in mature leaves; lower levels in intermediary leaves and primary root without apex; and higher levels in immature leaves, stem apex, inflorescences, primary root apex and the highest level in stem. This might be related to monocot vasculature, that is, large numbers of vascular bundles especially at the periphery of stems (Zimmermann & Tomlinson, 1972; Santos et al., 1996) that would allow the sampling of many zones with abundance of phytoplasmas.
This pattern of detection among parts is consistent with the hypothesis proposed previously by Parthasarathy (1974) and Zimmerman (1979) that phytoplasmas move from photosynthate source tissues to sink tissues via the phloem, as a result of a mass flow process. Therefore, as in the present case, phytoplasmas would not be detectable or would be less abundant in source tissues like mature and intermediate leaves and would be more abundant and more easily detectable in sink tissues of expanding parts such as immature leaves, stem apex, inflorescences, stem and fruit. Similar patterns of distribution have been reported for phytoplasmas associated with other plant species such as Flavescence dorée phytoplasmas in V. faba (Lherminier et al., 1994), dieback disease phytoplasmas in Car. papaya (Siddique et al., 1998) and CYP in Chr. carinatum (Saracco et al., 2006). In contrast, phytoplasmas in E. pulcherrima and Cat. roseus plants were found to accumulate in source leaves, petioles and stems, but rarely in roots or sink leaves (Christensen et al., 2004). In these cases, phytoplasma infection is not lethal and there are probably two different situations. One situation consisting of lethal infections with phytoplasmas accumulating in sink tissues and not detectable or in low titres in source tissues as reported for V. faba, Car. papaya (Lherminier et al., 1994; Siddique et al., 1998) and the present study; and another situation consisting of non-lethal infections with phytoplasmas accumulating in source tissues rather than in sink tissues as reported for E. pulcherrima and Cat. roseus (Christensen et al., 2004).
With regard to the chronology of events during disease development, flavescence dorée phytoplasmas appear to move from inoculation sites (shoot and leaf petioles) to the roots of V. faba within 2 weeks, where they actively multiply and reach detectable levels during 20–24 days post-inoculation, before invading actively growing shoots and leaves (Lherminier et al., 1994). In this study, phytoplasmas were first detected in stem tissues where they remained throughout all the subsequent stages of the disease, suggesting that this part of the palm is where phytoplasmas first move from leaves after foliar feeding by inoculative vectors and in which they probably multiply and become distributed to other palm parts. This sustained high titre of phytoplasmas in stem might affect photosynthate transport even at the presymptomatic stage, as a previous report from our laboratory showed that carbohydrate production in leaves in coconut palms is not reduced during the presymptomatic stage and actually increases (Maust et al., 2003). Siddique et al. (1998) speculated that in phytoplasma diseases involving a high titre of the pathogen, the physiology of the host plant may be altered because of physical blockage of the sieve elements by the phytoplasma cells leading to resource diversion. Then if LY phytoplasmas reduce phloem transport through the stem, the performance of other parts such as the roots could be affected. We previously reported a reduction in respiration rates (Islas-Flores et al., 1999; Maust et al., 2003), protein (Islas-Flores et al., 1999) and sugar (Maust et al., 2003) content in the roots of LY-diseased coconut palms followed by deterioration of these tissues (Islas-Flores et al., 1999). This in turn could lead to shoot damage and LY symptom development as proposed by León et al. (1996) and Maust et al. (2003). Dyer & Sinclair (1991) had already suggested root damage as a common feature of diseases caused by phytoplasmas.
We previously reported the occurrence of LY phytoplasma DNA in zygotic embryos from infected coconut palms (Harrison & Oropeza, 1997; Harrison et al., 1999; Cordova et al., 2003). Also Nipah et al. (2007) reported the presence of phytoplasma DNA in embryos of coconuts with CSPWD. In this study, we initially obtained embryos from fruits collected from the ground in two different sites, Sabancuy and Chicxulub in the Yucatan Peninsula, and LY phytoplasma DNA was detected in samples from both sites. The percentages of LY phytoplasma detection were higher when assayed by nested-PCR (17%) than by direct-PCR (4%), showing as reported for other detection cases (Grote et al., 2002; Khan et al., 2004) that the nested-PCR assay was more sensitive than the direct-PCR assay for the detection of LY phytoplasma DNA. Hence nested-PCR was used to evaluate the occurrence of phytoplasmas in the embryos of fruit from bunches of different maturity stage of diseased palms in another site, San Miguel also in the Yucatan Peninsula. The results showed that phytoplasma DNA could be present in embryos of fruit from any of the bunches of any of the nine maturity stages studied. Although an apparent pattern of larger percentage detection associated with more mature bunch stages was observed, there was no statistically significant difference in percentage of detection at different maturity stages. Regarding the overall LY detection in embryos by nested-PCR, taking all four fruit batches studied from the three sites sampled, the percentage was 29%. Therefore, the presence of LY phytoplasmas in coconut embryo tissues seems to be very frequent.
Then in order to determine if this LY phytoplasma presence in embryos could lead to a risk for the dispersion of LY, we evaluated if seed germination could be affected and if the resulting seedlings were harbouring the LY phytoplasma. Seeds from LY-positive symptomatic palms had reduced germination (58%) compared with seeds from LY-negative symptomless palms (71%). In a similar experiment but with embryos from CSPWD-affected palms, Nipah et al. (2007) found the opposite, a higher percentage of germination for seeds from infected palms than from uninfected palms. They concluded that mature embryos infected with phytoplasma were still able to germinate. However, it is important to consider that as reported by these authors, the infected palms were at a site 100 km away from where uninfected palms were, and we cannot disregard differences between sites in the local environmental conditions or in the palms or both could account for the difference in germination percentages observed. In the present case, seeds of infected and uninfected palms were collected at the same site, and as the germination percentage of seeds from infected palms was lower, we cannot conclude that infected embryos retain their ability to germinate. Whether this resulted from a direct effect of the phytoplasmas on the embryo germination capacity or not needs further study.
All seedlings obtained from the germination of both types of seeds after 15 weeks were analysed by nested-PCR and all yielded negative PCR results. Symptom development was monitored on palms for over a 2-year period. Palms either did not develop LY symptoms or died. Likewise, Nipah et al. (2007) were unable to detect phytoplasmas in germinating seedlings from CSPWD-affected palms. Nipah et al. (2007) referred to previous findings (Romney, 1983), where 600 seedlings grown from seeds collected from LY-diseased Jamaican tall palms and planted in disease-free areas remained disease free. They concluded that there was as yet no evidence that the CSPWD phytoplasmas can be transmitted through the seedling to cause disease in the resultant palm. On the basis of our results, we have arrived at the same conclusion for the LY phytoplasmas.
The present results are relevant because they extend the understanding of how LY phytoplasmas interact with coconut host plants, in time and space, distributing to different plant parts, apparently from photosynthate source tissues to sink tissues via the phloem. This information combined with that from previous reports on the biochemistry and physiology of LY infected palms (León et al., 1996; Isla-Flores et al., 1999; Maust et al., 2003) help us to generate a clearer view of how phytoplasmas could affect the functions of diseased palms. These results are also important from a practical point of view because they show that sampling for detection of LY can be carried out reliably from different plant parts, such as inflorescences, young leaves and stem apex, particularly during early leaf yellowing. However, the best part was stem with best yields for LY phytoplasma detection, even before symptom appearance. Stem sampling has the additional advantage that it is non-destructive and simple to carry out without a ladder or any other mechanical aids, needed with older plants to reach other plant parts. Therefore stem sampling combined with nested-PCR will be an excellent tool, both for early detection and to facilitate further studies on the pathogenicity and epidemiology of LY.
Acknowledgements
The authors wish to thank Lucely Alpizar and Javier Mijangos for technical collaboration and statistical analysis, respectively. Partial funding was provided by CONACYT-SISIERRA (No. 980107).




