Zophiuma lobulata (Hemiptera: Lophopidae) causes Finschhafen disorder of coconut and oil palms
Abstract
Finschhafen disorder (FD) affects coconut and oil palms in Papua New Guinea (PNG). It is characterised by yellow-bronzing of fronds which begins at the tips and progresses towards the petiole. Although the planthopper Zophiuma lobulata (Hemiptera: Lophopidae) has been posited as a cause of FD, the basis of the relationship has not been established. Studies conducted previously on FD predate the availability of DNA-based techniques to test for the involvement of plant pathogens such as phytoplasmas that cause yellows-type diseases in many plant taxa and are transmitted by the order of insects to which Z. lobulata belongs. In this study, polymerase chain reaction (PCR) assays found no evidence of phytoplasmas or bacteria-like organisms (BLOs) in tissues of coconut and oil palm symptomatic for FD and from Z. lobulata feeding on these plants. Further studies involved releasing Z. lobulata adults and nymphs onto caged, potted coconut and oil palms and onto palm fronds enclosed in mesh sleeves. In both experiments, chlorotic symptoms on the palms were observed in the presence of Z. lobulata. Insect-free control palms did not exhibit chlorotic symptoms of FD. In the frond sleeve experiment, only the fronds where Z. lobulata fed developed chlorosis indicating that the disorder is not systemic. Unlike most yellows-type diseases associated with Hemiptera, this study indicates that FD is because of a direct feeding effect on palms by Z. lobulata rather than transmission of a pathogen.
Introduction
Oil palm (Elaeis guineensis Jacq.) and coconut (Cocos nucifera L.) are the most valuable crops in the Pacific (Bourke & Harwood, 2009) and are important as oil and food sources in other regions of the world including Africa, Central and South America. Finschhafen disorder (FD) was reported in 1960 on coconut palms near Finschhafen, Morobe Province in Papua New Guinea (PNG) (Ghauri, 1967). For nearly three decades after its detection, FD was confined to coconut palms on mainland PNG. In 1994, however, it was observed on oil palms on the island of New Britain for the first time (Prior et al., 2001). FD has now been widely reported from oil palm (I. Orrell, personal communication) and is a threat to production in PNG (Prior et al., 2001). Oil palm is PNG's major cash crop after its introduction to the country in the 1920s.
Previous studies on FD focused exclusively on coconut palm (Smith, 1980a,b; Prior et al., 2001). The disorder is characterised by yellow-bronzing of fronds with chlorosis extending towards the petiole as it progresses (Fig. 1). Advanced symptoms appear as senescence on leaflets with pronounced and accelerated chlorosis followed by necrosis and then death of the entire frond (Smith, 1980a,b). According to Smith (1980a,b), approximately one-third of coconut palms that are affected by FD may be lost. A native PNG planthopper, Zophiuma lobulata Ghauri (Hemiptera: Lophopidae) has been implicated in FD because of its presence in relatively high numbers where the disorder is prevalent (Smith, 1980a; Prior et al., 2001). Smith (1980b) showed that yellow-bronzing symptoms associated with FD were induced on 8-month-old coconut palms after holding Z. lobulata adults and nymphs in captivity together with potted coconut palms for 7 months. The disorder, however, affects both young and old palms (Prior et al., 2001).

An oil palm frond (leaf) with leaflets showing Finschhafen disorder (FD) symptoms. Chlorosis begins at the tips and progresses towards the petiole (Photo: G.M. Gurr).
Hemiptera comprise a large and diverse group of insects widely implicated in vectoring plant pathogens (Kaloshian & Walling, 2005). Yellows-type diseases, associated with phytoplasmas and transmitted by leafhoppers and planthoppers (Ploetz et al., 1999; Weintraub & Orenstein, 2004; Weintraub, 2007; Gitau et al., 2009), have been studied extensively. The insects deposit phytoplasmas into the phloem during feeding (Howard & Thomas, 1980; Solomon, 1997; Harrison et al., 2008) resulting in yellowing of leaves in the infected tissues. Chlorosis on leaves results from inhibition of sugar transportation in the phloem of affected leaf tissues (Hogenhout et al., 2008). Other studies have shown that the presence of phytoplasmas in plants leads to a decrease in chlorophyll content which interferes with photosynthetic activity resulting in yellowing and rapid senescence of leaf tissue (Lepka et al., 1999; Junqueira et al., 2004). Independent of pathogen transmission, some sap feeders induce plant disorders (Howard et al., 1984a; Backus et al., 2005) through cell destruction when the insect stylet pierces plant tissues (Kabrick & Backus, 1990; Backus et al., 2005, 2007) or because of reaction of plant tissues to hopper salivary secretions (Ecale & Backus, 1995; Walling, 2009).
Detection and identification of non-culturable plant pathogens such as phytoplasmas is widely conducted using molecular biology techniques. DNA-based techniques such as polymerase chain reaction followed by restriction fragment length polymorphism (PCR- RFLP) or DNA sequencing are now routinely used (Harrison et al., 1994; Lee et al., 1998; Heinrich et al., 2001; Crosslin et al., 2006). Disease association is, in addition, widely studied using transmission experiments (Howard et al., 1984b; Arocha et al., 2005; Bressan et al., 2007). Suspected or known insect vectors are allowed to feed on healthy plants. In this approach, evaluation and analysis of disease symptoms not only allows confirmation of a given species as the disease vector, but also leads to an understanding of mechanisms in which disease transmission and association take place. Previous studies on FD predate the availability of DNA-based molecular techniques. In this study, we used PCR followed by sequencing to investigate whether phytoplasmas are involved in FD. In addition, we investigated the relationship of Z. lobulata with FD by conducting experiments that involved releasing known numbers of Z. lobulata into (a) large cages that enclosed entire, potted coconut and oil palms and (b) into sleeved oil palm fronds.
Materials and methods
Samples for molecular assays
Bark, leaf, inflorescence and palm frond bases were collected from symptomatic and asymptomatic palms in West New Britain (WNB) over a period of four weeks (4.vii.2008–2.viii.2008). Three replicates of each of the tissues were collected from Dami (5°17′S, 150°24′E), and Numundo (S5°30′, W151°02′E). These were the areas in WNB where FD and Z. lobulata are prevalent. A total of nine palms were sampled for each of the symptomatic and asymptomatic coconut and oil palms. Bark tissues were collected from plantation-grown coconut and oil palm stems using a hand augur. From each of the nine palms, a piece of the stem core measuring one centimetre in length was cut off, immediately placed in a microcentrifuge tube (Eppendorf, Scientific Specialities Inc., Lodi, CA, USA) and flooded with propylene glycol. A section of palm leaflet (one centimetre in length), frond base (0.5 cm) and one inflorescence were similarly preserved. New scalpel blades were used for each leaflet and bark tissue sample. The augur bit was wiped with cotton wool soaked in bleach between stem core samples, dipped in 70% ethanol and flamed before collecting samples into Eppendorf tubes.
Additional leaf samples were collected from oil palm leaflets on which Z. lobulata had been contained. Four Z. lobulata were individually enclosed in a ‘clip cage’ and allowed to feed on the leaflets until after four days, the minimum time the planthoppers survived in confinement. The portion of frond on which the planthoppers fed was excised using a sterile blade and preserved in propylene glycol. The palm samples and dead Z. lobulata that were used in the clip cages were transported to Industry and Investment laboratories in Wagga Wagga, NSW, Australia, for molecular assays.
Molecular assays
Samples were tested for phytoplasmas and bacterium-like organisms (BLOs) as a literature search showed that these pathogens are transmitted to plants by Hemiptera.
For phytoplasmas, a portion of Z. lobulata adults, nymphs and plant samples that were previously collected from the field and preserved in propylene glycol was used. Head, leg and abdomen of 14 individual Z. lobulata were used separately in order to detect possible false positives resulting from the presence of plant pathogens in the gut of the insect. In addition, the following samples were assayed: sugar solution aliquots on which individual Z. lobulata fed (N = 12), samples of palm leaflets on which Z. lobulata fed in ‘clip cages' and the individuals feeding on the leaflets (N = 8), samples of palm leaflets from symptomatic palms (N = 14), cuts of leaflets from asymptomatic palms (N = 9). Four symptomatic and four asymptomatic samples of bark of palm, inflorescence and frond base were also assayed. DNA extraction was conducted using a Corbett Robotics CAS-1820 robotic DNA platform (Corbett Robotics, Mortlake, NSW, Australia) and the manufacturer's recommended DNA extraction kit (Sigma-Aldrich, Castle Hill, NSW, Australia).
For BLOs, DNA of two Z. lobulata, two asymptomatic palm tissue and four symptomatic palm tissue samples were sent to the Plant Health and Environment Laboratory, MAF Biosecurity New Zealand, where tests were conducted.
Polymerase chain reaction assays for phytoplasma detection
Polymerase chain reactions were performed with an Eppendorf thermocycler (model: eps) in a total volume of 15 µL. The reaction mixture contained 1 µL of genomic DNA, PCR buffer (20 mM Tris–HCl, pH 8.4; 50 mM KCl), 3 mM MgCl2, 0.2 mM dNTPs (0.3 µL of 10 mM stock), 0.375 units of Platinum Taq® DNA polymerase (all reagents supplied by Invitrogen, Mount Waverly, Australia) and 1.5 pmol (0.3 µL of 5 µM stock) of each primer. All PCR assays used sterile water as a negative control. DNA extracted from phytoplasma-infected periwinkle Catharanthus roseus (Apocynaceae) was used as a positive control in PCRs using universal phytoplasma primers. Thermal cycling programmes were as described in the papers cited for each primer (Table 1), except in a few cases where our PCR optimisations suggested that higher annealing temperatures should be used to reduce false positives.
| Type of PCR | Primer Pair | Specificity of Phytoplasma Primers | Number of Test PCR Samples (n) | Positive Bands | Expected Size (bp) | References | |
|---|---|---|---|---|---|---|---|
| NPCR | P1/P7 | fU5/m23sr | Universal | 61 | 0 | 1469 | Padovan et al., 2000 |
| NPCR | P1/P7 | fU5/rU3 | Universal | 61 | 4 | 880 | Batlle et al., 2008 |
| NPCR | P1/P7 | 16r758f/M23Sr | Universal | 31 | 2 | 1050 | Padovan et al., 2000 |
| NPCR | P1/P7 | P1/Tint | Universal | 61 | 4 | 1600 | Smart et al., 1996 |
| NPCR | P1/P7 | Pc399/P1694 | Universal | 30 | 3 | 1100 | Skrzeczkowski et al., 2001 |
| NPCR | P1/P7 | R16F2n/R16R2 | Universal | 30 | 0 | 1239 | Gundersen et al., 1994; Lee et al., 1998 |
| DPCR | fU5/rU3 | Universal | 30 | 0 | 880 | Gibb et al., 2003 | |
| DPCR | R16F2n/R16R2 | Universal | 31 | 0 | 1239 | Gundersen et al., 1994 | |
| DPCR | PC399/P1694 | Universal | 31 | 0 | 1200 | Skrzeczkowski et al., 2001 | |
| DPCR | PA2F/R | Universal | 31 | 0 | 1187 | Heinrich et al., 2001 | |
| NPCR | PA2F/R | NPA2F/NPA2R | Universal | 31 | 18 | 485 | Heinrich et al., 2001 |
| NPCR | P1/P7 | P1m/LY16-23Sr | Lethal yellowing Phytoplasma-specific | 31 | 0 | 1000 | Harrison et al., 2008 |
| NPCR | P1m/LY16-23Sr | LY16Sf2/LY16-23Sr2 | Lethal yellowing Phytoplasma-specific | 31 | 0 | 1600 | Harrison et al., 2008 |
| NPCR | P1/P7 | LY16Sf/LY16-23Sr | Lethal yellowing Phytoplasma-specific | 31 | 0 | 1700 | Harrison et al., 2008 |
| NPCR | P1/P7 | LY16Sf/LY16Sr | Lethal yellowing Phytoplasma-specific | 31 | 0 | 1500 | Brown et al., 2006 |
A range of universal phytoplasma rDNA primers was used in the PCRs, as follows: the products of an initial P1/P7-primed PCR (Deng & Hiruki, 1991, Smart et al., 1996) were diluted to 1:10 and/or 1:40 with sterile water and re-amplified using nested primer pairs fU5/rU3, Pc399/P1694, P1/Tint, 16r758f/M23Sr and fU5/m23sr (Smart et al., 1996; Batlle et al., 2008). In addition, the universal primers fU5/rU3, PA2F/R and PC399/P1694 were used in direct PCRs (Heinrich et al., 2001; Skrzeczkowski et al., 2001). As some of these PCRs produced false positives (see results) we also tested primers specific for palm lethal yellowing (LY) phytoplasmas. These PCRs used primers LY16Sf2/ P1m LY in the initial step, followed by semi-nested PCR using either LY16-23Sr2 or LY16-23Sr as the reverse primer (Harrison et al., 2008). In addition, P1/P7 PCR products were used in nested PCRs with P1m/ LY16-23Sr for detection of palm LY phytoplasmas (Harrison et al., 2008). PCR products were analysed on 1.2% agarose gels stained with ethidium bromide and photographed under ultraviolet (UV) illumination.
Polymerase chain reaction assays for BLO detection
Infection of symptomatic palms by BLOs was investigated by PCR employing primer pair fD2/rP1 which amplifies the 16S rRNA gene of prokaryotes as well as plant organelles. (Weisburg et al., 1991). The 16S rRNA gene of prokaryotes typically contains an EcoRI restriction enzyme site, whereas those of plant organelles do not. Therefore, the production of two smaller fragments when the fD2/rP1 amplicon is digested with EcoRI, suggests that prokaryotic DNA has been amplified from the DNA extracts. Thus, the amplicon produced by the primer pair fD2/rP1 from DNA of symptomatic palms was digested with EcoRI. To determine the origin of the EcoRI restriction fragments, the fD2/rP1 amplicon was cloned into the pCR 4-TOPO vector (Invitrogen, Carlsbad, CA, USA) followed by transformation into One Shot TOP10 chemically competent Escherichia coli according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). Resulting colonies were PCR amplified with the M13F/M13R primers and the resulting amplicon digested with EcoRI. Plasmids from the colonies that produced two EcoRI restriction fragments were purified using the QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA, USA) and sequenced using the M13F/M13R primers.
Sequence analysis
Approximately half the visible DNA bands from the universal-primer PCRs, including at least one sample from each primer combination which yielded a positive result, were sequenced in both directions using the ABI PRISM® BigDye™ Terminator v3.1 Ready Reaction Cycle Sequencing Kit and an ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). These suspected phytoplasma rDNA sequences were edited for base-calling accuracy then used in BLAST searches of the Genbank nucleotide database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) in order to establish their similarity.
Collection and handling of insects used in potted palms and sleeved frond experiments
Z. lobulata egg masses, nymphs and adults were collected from coconut, oil palm and betel nut (Areca catechu L.) and occasionally from bananas (Musa sp.) and taro (Colocasia sp.). The collection sites were Hoskins (5°27′S 150°24′E); Dami (5°32′S 150°20′E); Kimbe (5°33′S 150°09′E); Numundo (5°31′S 150°05′E) and Kavui (5°35′S 150°18′E). The collections were performed at least once every week from 10.vi.2008 to 28.iv.2009 and the planthoppers were transported to Dami Research Station, West New Britain, PNG. Randomly selected specimens from these collections were preserved for later molecular assays. Live adults and nymphs were held in a mesh cage (1.8 m × 1.8 m × 2.5 m) that contained one healthy 6-month-old coconut and oil palm each. Planthoppers remained in the ‘holding cage’ for at least 24 h before they were released into experimental cages to allow insects to acquire from the palms any possible pathogen that is present in the field. The field-collected egg masses were scraped from leaf surfaces into a clean glass vial using a camel hair brush and held in the laboratory at ambient temperatures (25–30°C) until emergence of Z. lobulata nymphs or parasitoids. The newly enclosed Z. lobulata nymphs were used in potted palm experiments.
Potted palm experiment
Thirty-two cages, similar to the ‘holding cage’ above were constructed using wooden frames covered with a polyester netting material with mesh size 600 × 280 µm (MegaView Science©, Taichung, Taiwan). Preliminary tests showed the fabric successfully contained Z. lobulata nymphs. The cages were sited beneath a 25% transmittance green shade netting canopy. Personnel entry to cages was via a full length zip. Each cage contained a potted six-month-old coconut palm and oil palm. Soil was fertilised every two weeks with 24 g pot−1 ammonium sulphate and every month with 10 g pot−1 of N:P:K:Mg (12:12:17:2). Cages were arranged in four rows, each with eight cages. Two replicates of four treatments were randomised to the cages in each row. Treatments one and two received field-collected Z. lobulata that would carry any pathogen residing in the field population. Z. lobulata were however hand-removed from treatment two cages in order to assess symptom remission two months before full assessment of symptoms. Treatment three received nymphs that hatched in the laboratory from field-collected egg masses and were therefore assumed free of any plant pathogen that is acquired by feeding upon infected plants. Transovarial transmission of phytoplasmas has been recorded for some Hemiptera (Hanboonsong et al., 2002) and not others (Weintraub & Beanland, 2006). Treatment four was without insects (control). Measurements were made on each frond of each palm within each cage. Thus the experiment utilised a split-plot design with rows as blocks, cages as whole plots, palms as sub-plots and fronds as sub-sub-plots. The first release of Z. lobulata into the experimental cages was on 10.vi.2008. Further releases occurred until 28.iv.2009 after which insects reproduced without more addition. Full assessment of symptoms was taken on 10.v.2009 for all palms in the four treatments.
Insects were placed into the cages allocated to treatments one and two, from the previously described holding cage using the following procedure. Prior to release, saliva samples were collected from each insect by placing them singly in tubes measuring 10 cm × 3 cm within which they were able to feed on 5% sucrose solution (1.25 mM EDTA and 5 g sugar dissolved in 100 mL sterile DNAase-free water) through a Parafilm™ layer sealing the mouth of a smaller vial (5 cm × 1 cm). The sucrose solution was stored for later PCR assays for detection of possible plant pathogens. The following day, live insects were allocated randomly to the 16 cages in treatments one and two. Numbers of planthoppers released into each cage were recorded during each of the introductions. Z. lobulata that died prior to being introduced into cages were preserved in propylene glycol. This procedure was repeated every week in an additive process. Seventy-one (minimum) and 165 (maximum) Z. lobulata were released into the cages. For treatment three, between 691 and 1537 newly enclosed nymphs were released into the eight cages. The variation in numbers of planthoppers released was in response to weekly monitoring of numbers of Z. lobulata in each cage and aimed at giving palms in each cage consistent feeding pressure. In cages that had a high turnover, Z. lobulata numbers were adjusted in subsequent releases for homogeneity.
Total number of fronds produced by both coconut and oil palm were counted to assess if feeding by Z. lobulata reduced plant vigour. During the experiments, leaflets and frond bases were frequently inspected for egg masses to assess if Z. lobulata exhibits oviposition preference on either palm. The following counts were made during symptom assessment: (a) leaflets per frond (b) chlorotic leaflets (c) necrotic leaflets and (d) leaflets with chlorotic spots.
Sleeved fronds experiment
This experiment was similar to the potted palm experiment except that insect pressure was greater and confined to single fronds to test if the disorder was systemic. Sixteen six-month-old nursery-grown oil palm seedlings were arranged under shade trees in four rows each with four palms. The fourth youngest frond of each palm was enclosed in a sleeve measuring 1.2 m × 0.8 m made from a fine netting material (600 × 280 µm) impervious to Z. lobulata nymphs. Four treatments were randomised to the palms in each row. In treatment one, 100 field-collected Z. lobulata adults and nymphs were released into each sleeve once every week for 20 weeks. In treatment two 100 field-collected Z. lobulata were released in each cage each week until chlorosis was observed on tips of leaflets on the sleeved fronds. Insects and sleeves were then removed to allow monitoring for possible remission. In treatment three, sleeves were in place but no Z. lobulata were added until chlorosis was observed on leaflets of palms in treatment one and two. Thereafter, 110 field-collected Z. lobulata were released weekly for nine weeks. The sleeve was firmly tied to the frond base in all the three treatments to stop insects from escaping. Treatment four was the control; no insects were released into the four sleeves. The experiment was terminated when chlorosis appeared on tips of leaflets on the sleeved fronds in treatment three. Chlorosis was assessed every month for the entire five months period using a similar procedure as that used in the potted palm experiments.
Data analysis
GenStat (2009), 12th Edition, VSN International, Hemel Hempstead, UK, was used for all statistical analyses. In the potted palm experiments, the number of (a) chlorotic leaflets (b) necrotic leaflets (c) leaflets with chlorotic spots compared to the total leaflets on each frond was modelled assuming a Binomial distribution, fitting a generalised linear mixed model (GLMM) with Treatment × Palm Species as fixed effects and Row/Cage/Palm Species/Frond as random effects. ‘×’ indicates the main effects and interaction of the two fixed effects' factors, and ‘/’ indicates nesting of the random effects factors. The four treatments were included in models as three orthogonal contrasts: (a) means of treatments with insects versus no insects (b) means of treatments with adults versus treatment with newly hatched nymphs (c) means of treatments with early removal of insects versus treatments to full term.
To assess whether feeding by Z. lobulata influenced plant vigour a GLMM was fitted to the number of fronds assuming a Poisson distribution. To assess whether Z. lobulata exhibits an oviposition preference a linear mixed model was fitted to log (egg masses per frond+1), the transformation ensuring that the residuals have a normal distribution. In both these models, Treatment × Palm Species were fixed effects and Row/Cage/Palm were random effects. Only the three treatments with insects were included in the oviposition analysis, as the control treatment did not have insects.
In the sleeved frond experiments, two analyses (repeated measures) were performed to examine the proportion of chlorotic leaflets. As the control treatment (excluding insects) resulted in few non-zero counts of chlorotic leaflets whereas treatments that received insects all resulted in a much larger number of non-zero counts, a high proportion of zero counts invalidates standard analysis techniques. Thus, we conducted two analyses: the first analysis examined the effect of insects on presence/absence of chlorotic leaflets using the contrast between the three treatments with insects and the control. The second analysis compared the proportion of chlorotic leaflets in the three treatments that contained insects. Both analyses used GLMMs, assuming a binomial distribution, with Treatment × Month as fixed effects and Plant/Month as random effects.
Results
Molecular assays for phytoplasmas
No rDNA products were obtained from direct PCRs. When Z. lobulata and palm samples were assessed for phytoplasma using nested PCRs with universal primer pairs P1/P7 and PA2F/PA2R followed by second primer pairs in various combinations, no rDNA product was amplified from diseased palms, Z. lobulata tissues, sugar solution or palm tissues on which Z. lobulata fed. Of the 491 optimised PCR assays conducted on Z. lobulata (legs, head and abdomen), sugar solution and palm tissues on which Z. lobulata fed and palm tissues from symptomatic and asymptomatic palms, 31 samples gave positive PCR results (Table 1). From these, 14 strong bands were sequenced. Sequences obtained were deposited in GenBank database under accession numbers HQ596195-HQ596201. BLAST searches identified the closest matches in GenBank with 96–100% sequence identity to bacterial endosymbionts of insects or to bacteria inhabiting gastrointestinal tracts of humans and other mammals. These non target positives were amplified from diseased and asymptomatic coconut and oil palms despite adjustments to thermal cycling conditions (data not shown). LY phytoplasmas, ubiquitous in palms were not detected using LY specific primers on DNA extracted from coconut, oil palm and insect samples (Table 1).
Molecular assays for BLOs
DNA extracted from Z. lobulata and palm tissue was amplified with the fD2/rP1 primer pair and the resulting amplicon digested with EcoRI. The Z. lobulata and one of the asymptomatic palm tissues PCR amplicons produced two smaller DNA fragments of approximately 650 kb and 850 kb as well as the original 1500 bp fragment presumably from the host (data not shown). EcoRI fragments were not visible in the symptomatic palm tissue PCR amplicons. The fD2/rP1 amplicons from the asymptomatic as well as from symptomatic palm tissue were cloned and 18 colonies from each sample were screened with EcoRI. Twelve out of the 18 clones of the fD2/rP1 amplicon from asymptomatic tissue contained an EcoRI site and seven clones were selected for sequence analysis. None of the 18 clones from the symptomatic tissue contained an EcoRI site indicating that no prokaryote was present in this sample. Sequence analysis of the seven clones from the asymptomatic plant revealed that they shared 96–99% sequence identity to the 16S rRNA gene of uncharacterised bacteria previously isolated from environmental samples (for example, GenBank accession numbers AY957948 and EU421878).
Potted palm experiments
Differences between treatments in the proportion of chlorotic leaflets were on the threshold of conventionally accepted statistical significance (F = 2.94; df = 3,26; P = 0.051). Palms in cages that did not receive Z. lobulata (control) had a lower proportion of chlorotic leaflets compared to the three treatments that had insects (F = 5.77; df = 1,34; P = 0.02). The estimated proportions of chlorotic leaflets in the treatments which received insects were not statistically significantly different from each other (Fig. 2). Chlorosis on leaflets did not differ between palm species (F = 1.23; df = 1,25; P = 0.278).
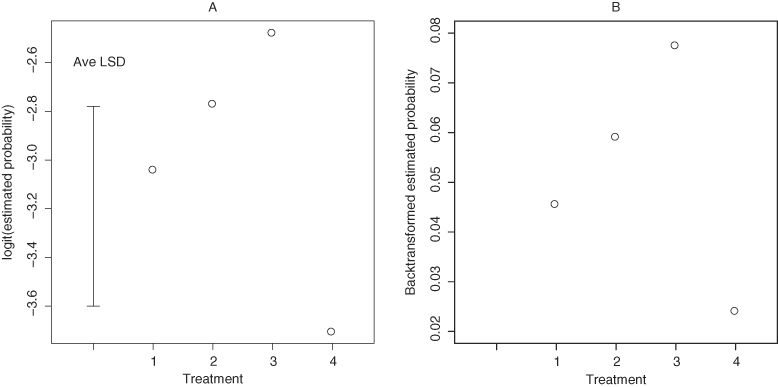
Plots of chlorotic leaflets (A) logistic scale with average Least Significant Difference (LSD) and (B) back-transformed scale in potted palms at each treatment level. Treatment 1 = Z. lobulata collected from the field, Treatment 2 = Z. lobulata collected from the field and removed from cages after nine months, Treatment 3 = newly enclosed Z. lobulata nymphs, Treatment 4 = control (no Z. lobulata).
Proportion of chlorotic leaflets on palms was neither significantly affected by earlier removal of Z. lobulata (F = 0.01; df = 1,22; P = 0.973) nor feeding by newly hatched nymphs (F = 2.62; df = 1,24; P = 0.12).
Coconut palms had a higher proportion of leaflets with necrosis (F = 66.80; df = 1,725; P < 0.001) and chlorotic spots (F = 11.60; df = 1,715; P < 0.001) than oil palm. However, there was no evidence that this was related to presence and feeding by Z. lobulata (Table 2).
| Fixed effects | Chlorosis | Necrosis | Chlorotic Spots | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | P | F | df | P | F | df | P | |
| Treatment | 2.94 | 3, 26 | 0.051 | 1.96 | 3, 25 | 0.145 | 0.81 | 3, 24 | 0.500 |
| Palm Species | 1.23 | 1, 25 | 0.278 | 66.80 | 1, 725 | <.001 | 11.60 | 1, 715 | <.001 |
| Treatment × Palm Species | 1.84 | 3, 34 | 0.159 | 0.85 | 3, 725 | 0.467 | 0.19 | 3, 715 | 0.901 |
The presence and feeding on palms by Z. lobulata did not affect palm vigour as measured by analysis of the number of fronds in which neither the interaction between treatment and palm species (F = 1.28; df = 3,27; P = 0.299) nor the treatment effect (F = 0.27; df = 3,24; P = 0.848) were statistically significant. There were highly significant differences between the two palm species in the number of fronds (F = 1268.24; df = 1,27; P < 0.001) with oil palms having a mean of 17.99 fronds per plant and coconut having 5.26 fronds per plant. Because of this difference between species, the oviposition preference analysis was based on the number of egg masses per frond.
The analysis of log (egg masses per frond +1) found that Z. lobulata prefers coconut palm (F = 9.56; df = 1,21; P = 0.006). The interaction between treatment and palm species was not significant (F = 0.48; df = 2,21; P = 0.469), nor was the treatment effect (F = 0.80; df = 2,18; P = 0.464). The mean number of egg masses per frond was 0.75 for coconut palm and 0.45 for oil palm.
Sleeved frond experiments
Chlorotic symptoms were confined to treatments that had Z. lobulata (F = 6.77; df = 1,68; P = 0.011). Non-sleeved fronds of the same palm did not show symptoms of chlorosis indicating that FD is not systemic and evidence against involvement of a pathogen.
From observed means, the proportion of chlorotic leaflets in treatment one, peaked then decreased after the fourth month, despite having insects added over the entire experimental time period, as chlorotic leaflets died. The percentage of chlorotic leaflets tended to decline from 30% to below 5% in two months when sleeved frond of treatment two stopped receiving insects.
An analysis on proportion of chlorotic leaflets including only the three treatments with insects showed no interaction between treatment and month (F = 1.53, df = 8,36, P = 0.180) and no treatment main effect (F = 1.03, df = 2,9; P = 0.396).
Discussion
This study confirms the association of Z. lobulata with FD. Results revealed that FD occurs as a consequence of concentrated and direct feeding on palms by the planthopper without molecular evidence of involvement of neither a phytoplasma nor BLO. Chlorotic symptoms can be induced within 3–12 months on both coconut and oil palm. The disorder is non-systemic as evidenced by the presence of chlorotic symptoms on sleeved fronds and absence of the symptoms on non-sleeved fronds of the same palm. Smith (1980a,b) reported symptoms after seven months on coconut palms. Similar chlorotic symptoms have been reported for other yellows-type disorders of palms. LY is by far the most prevalent and destructive disease on palms in the Caribbean and Americas while Cape St. Paul Wilt and Awka diseases are common in Africa (Eden-Green & Tully, 1979; Schuiling & Mpunami, 1992; Harrison et al., 1994; Tymon et al., 1997). Despite symptomological similarities with LY, the current study indicates that FD is not associated with a pathogen. Although LY is characterised by a rapid spread in the Caribbean (McCoy, 1976), the pattern of spread for FD has been slow, patchy and sporadic. FD was first reported on coconut in 1960. Three decades later, it was observed on oil palm at Dami Research Station and surrounding areas of WNB province. Currently, FD has progressively spread on oil palm in Northern (Oro) Province and Milne Bay Province of mainland PNG (I. Orrell, personal communication).
Bacterial DNA present in our samples was amplified using PCR assays that employed a range of universal primers. Occurrence of bacteria while screening for phytoplasma in DNA of plant and insects was previously reported by Heinrich et al., (2001) and Skrzeczkowski et al. (2001). We used universal primers located in the 16S rDNA and the 23S rDNA region as well as primers specific for the LY-phytoplasma (Heinrich et al., 2001; Harrison et al., 2008). The present study neither found phytoplasmas nor BLOs in Z. lobulata tissues, the sugar solution and palm tissues on which the planthoppers fed. Such pathogens were also absent in samples that were collected from symptomatic palms where FD was prevalent. Moreover, newly enclosed nymphs that hatched without contact with palm material or contaminants induced FD symptoms on the palms, verifying the non-involvement of a pathogen in FD. This result however contradicts studies by Hanboonsong et al. (2002) who reported presence of the white leaf phytoplasma in egg and nymph stages of the leafhopper Matsumuratettix hiroglyphicus (Matsumura).
A high density of Z. lobulata is required for expression of symptoms on oil palm. Palms in cages with newly hatched nymphs had 5% more chlorotic leaflets when compared to those where field-collected Z. lobulata were released. Furthermore, oil palm leaflets in treatment one which had field caught Z. lobulata had a similar proportion of chlorotic leaflets as the control. The numbers of Z. lobulata surviving on palms in treatment one cages may not have been high enough to damage the palms. The numbers of Z. lobulata in the newly hatched nymph cages remained relatively high because of slower deaths of the neonates and this could have maintained a higher feeding pressure resulting in higher chlorotic symptoms on the palms in this treatment compared to treatments with field collected, late instar nymphs and adults. This result is corroborated by the sleeved frond experiments where chlorosis was sustained for the first four months of the experiment on the treatment that had insects throughout the experiment, until the chlorotic leaflets started dying off. When present in high densities, sap feeders are known to cause various disorders on leaves (Howard et al., 1984a; Backus et al., 2005). FD may have been induced by wound responses triggered by movement of the insect stylets in the plant tissues, as is the case with the grapevine leafhoppers Empoasca spp. (Cicadellidae: Typhlocybinae) (Kabrick & Backus, 1990; Backus et al., 2007). We hypothesise that the chlorotic symptoms on coconut and oil palms were caused by destruction of tissues during the feeding process or perhaps, a possible toxin in the planthoppers' saliva (Walling, 2009). These hypotheses, however, remain to be tested.
Results from this study indicate that Z. lobulata does not have a feeding preference for coconut, its original host or oil palm. This implies that the planthopper is polyphagous and can exploit several alternative hosts. Biotic and abiotic factors that favour an increase in Z. lobulata populations would mean a parallel increase in FD incidence. Because of the intermittent, slow spread of FD and long period before FD symptoms are expressed on palms, findings from this study suggest that a control strategy for Z. lobulata is the key to curbing FD spread.
Acknowledgements
Funding for this work was provided by the Australian Centre for International Agricultural Research (ACIAR) grant CP/2006/063. We thank Holger Löcker (I&I NSW, Orange Agricultural Institute), Dr David Gopurenko and Anandan Anandan (I&I NSW, Wagga Wagga Agricultural Institute) for help with molecular biology protocols. The authors appreciate support from Ian Orrell, Managing Director PNGOPRA, Dami, West New Britain, PNG. The entomology team at PNGOPRA, Deane Woruba, Serah Waisale, Simon Makai, Seset Komda and Paul Mana are thanked for their technical support.




