Characterisation and phylogeny of a phytoplasma inducing sandal spike disease in sandal (Santalum album)
Abstract
Sandal (Santalum album) is an industrially important forest species in India, where it is devastated by sandal spike (SAS) disease. Diseased S. album trees show characteristic witches’ broom symptoms suspected to be caused by phytoplasma. Since the first report of occurrence of this disease at the end of 19th century, studies mainly have been carried out to detect SAS phytoplasma through various approaches. The causative agent, however, has remained poorly characterised at a molecular level. The present investigation was aimed to characterise the pathogen at this level. In nested PCR, a 1.4-kb 16S rDNA fragment was amplified and analysed by restriction fragment length polymorphism using 17 restriction enzymes. The patterns were identical to those of strains AY1 and APh of the aster yellows subgroup 16SrI-B, except for BfaI, which gave a different pattern. After cloning and sequencing, a phylogenetic analysis revealed the closest relationship to aster yellows subgroup 16SrI-B members. Nucleotide sequence identity ranged from 99.2% to 99.5% with this subgroup. On the basis of these results, the SAS phytoplasma was classified as a member of subgroup 16SrI-B.
Introduction
Sandal (Santalum album), a hemi-root parasitic tree, is one of the most important forest species in India. With its limited distribution, the species is confined to India and Indonesia. Sandal has a great utility in industries because of its high-valued heartwood and scented oil. India has been the main exporting country of sandalwood and its oil (Anon, 1972).
Sandal spike (SAS), a destructive bacterial disease of sandal, has attracted worldwide attention. The characteristic witches’ broom symptoms consist of reduction in the size of the leaves, which become narrow, pale-green or yellow and finally reddish just before the death of the tree. The affected leaves stand out stiffly from the branch that displays spike-like appearance. Because of the shortening of internodes, the leaves get crowded on the branches. As the disease advances, the newly emerged leaves become smaller and smaller and their lateral decrease is much more than the longitudinal ones. The symptoms start from certain parts, which gradually spread over the entire tree. Furthermore, there is an abnormal erect growth and sprouting of normally dormant buds. Phyllody of flowers on an otherwise healthy looking branch is an early symptom. The sandal trees of all ages and sizes are affected by SAS disease. The life of a diseased tree is only a few years after the onset of symptoms.
Owing to its economic importance, three international groups had independently established the aetiological nature of SAS disease. It was shown that mycoplasma-like organisms (now termed as phytoplasma) occurred in the sieve tubes of infected sandal leaves and twigs (Dijkstra & Ie, 1969; Hull et al., 1969; Varma et al., 1969). Remission of the disease symptoms following tetracycline treatment further confirmed the association of a phytoplasma with SAS (Raychaudhri et al., 1972). There are reports on insect vectors and alternate hosts to SAS phytoplasma (Raychaudhri & Varma, 1988), but they need confirmation.
Detection of SAS phytoplasma has mainly relied upon electron microscopy (Dijkstra & Ie, 1969; Hull et al., 1969; Varma et al., 1969). Various other approaches, such as light microscopy (Hiruki & Dijkstra, 1973), DNA-binding fluorochrome, 4,6,diamidino-2-phenylindole (Thomas & Balasundaran, 1998), enzyme-linked immunoassay (Thomas & Balasundaran, 2001) and nested PCR (Khan et al., 2004) have been applied for its detection.
Although much work has been carried out on its detection, SAS phytoplasma has remained poorly characterised at the molecular level. 16S rDNA of phytoplasma has significantly contributed for identification, characterisation and detection of a number of phytoplasmas. Schneider et al. (1993) amplified the full-length 16S rDNA, performed restriction fragment length polymorphism (RFLP) and were able to place the SAS phytoplasma in the aster yellows group phytoplasma. Furthermore, it was shown that it could hybridise to chromosomal probes derived from the aster yellows group phytoplasmas but not to that of clover phyllody or vaccinium witches’ broom phytoplasma (Schneider & Seemüller, 1994). Following restriction digestion of partially amplified 16S rDNA, it was shown that the SAS phytoplasma belonged to group I (Thomas & Balasundaran, 1999).
RFLP analysis of 16S rDNA with a range of restriction enzymes is an effective, reliable and well-accepted method to differentiate and classify unexplored phytoplasmas (Lee et al., 1998). The universal primers, capable of amplifying all known phytoplasma 16S rDNA, have greatly facilitated this approach (Deng & Hiruki, 1991; Ahrens & Seemüller, 1992; Gundersen & Lee, 1996). Moreover, nucleotide sequence data of the 16S rDNA fragments of phytoplasma isolates have allowed study of their phylogenetic analysis and the classification of unidentified phytoplasma into 20 major phylogenetic group or subclades (Gundersen et al., 1994; Seemüller et al., 1998; Lee et al., 2000).
The aim of the present study was to characterise and classify the phytoplasma associated with SAS disease. A preliminary report tentatively placing it in Candidatus Phytoplasma asteris group has been published (Khan et al., 2006). Samples derived from two SAS diseased trees (designated SAS1 and SAS5) were selected as representatives for characterisation and identification of the associated phytoplasma in this study.
Materials and methods
Phytoplasma strains
Symptomatic leaves from two S. album diseased trees (henceforth referred to as SAS1 and SAS5) showing characteristic symptoms of SAS disease and those of healthy trees were collected in the state of Karnataka (India). Reference phytoplasma strains used in direct comparison of RFLP pattern were as follows: AY1 (Maryland aster yellows, accession number AF322644) and BB (Tomato big bud, accession number AY180955) and APh (Aster phyllody). The phytoplasma strains AY1 and BB were kindly provided by I.-M. Lee (USA) and strain APh by Jana Fránová (Czech Republic) in DNA forms.
Polymerase chain reaction
Total DNA was isolated from 1 g of naturally infected leaves each from diseased trees, namely SAS1, SAS5 as well as healthy looking trees of S. album following the procedure described by Ahrens & Seemüller (1992). Two sets of universal primers were used to amplify phytoplasma DNA in PCR that comprised of two steps, that is direct PCR followed by nested PCR. A primer pair P1/P7, located in the 16S rDNA and 23S rDNA region of the phytoplasma (Deng & Hiruki, 1991; Schneider et al., 1995), was employed in direct PCR. The second set of primers R16mF2/R16mR1 was nested within the P1/P7 PCR product (Gundersen & Lee, 1996).
In direct PCR, template consisted of total DNA from leaves of SAS-infected (SAS1 and SAS5) as well as healthy looking trees, or reference strains AY1, BB and APh. About 50 μL PCR mixture contained 75 μM of dNTPs, 30 pmol of P1 and 40 pmol of P7 primers, 1× polymerase buffer, 1.5 mM MgCl2 and 1.5 U Taq DNA polymerase (Fermentas, Vilnius, Lithuania). The PCR parameters consisted of 25 cycles of denaturation at 94°C for 1 min (4 min for the first cycle), annealing at 48°C for 1 min and extension at 72°C for 2 min. The last cycle was extended for 5 min.
About 1 μL each of direct PCR products was re-amplified by nested PCR. The components of nested PCR mixtures were the same as described for the direct PCR except primers. The nested PCR was primed using the second set of primers R16mF2/R16mR1. A total of 30 thermal cycles were performed with denaturation at 94°C for 1 min (5 min for the first cycle), annealing at 50°C for 2 min, extension at 72°C for 3 min, which was extended for 5 min in the last cycle. An aliquot of 5 μL was analysed in 1% agarose gel and photographed.
RFLP analysis
R16 mF2/R16 mR1 primed PCR amplicons obtained each from SAS1 and SAS5 diseased trees, and the reference strains, namely AY1, BB and APh were purified separately on spin columns (Amersham Biosciences, Amersham, UK) and subjected to restriction digestion with 10 U of each restriction endonucleases AluI, BamHI, BfaI, EcoRI, DraI, HaeIII, HhaI, HindIII, HinfI, HpaI, HpaII, KpnI, MseI, RsaI, Sau3A1, SspI and TaqI following the manufacturer’s instructions (New England Biolabs, Ipswich, MA, USA). Digests were subjected to electrophoresis through 8% non-denaturing polyacrylamide gel (or as otherwise stated) using TBE (90 mM Tris–borate, 2 mM ethylenediaminetetraacetic acid) as running buffer. DNA bands were visualised with a UV transilluminator following staining with ethidium bromide. The RFLP patterns of SAS phytoplasma were compared with those of reference strains (AY1, BB and APh).
Cloning and sequencing of 16S rDNA
Nested PCR products obtained from infected leaf samples of SAS1 and SAS5 were cloned into the SmaI site of the vector pUC 19 using SureClone Ligation kit following the supplier’s instructions (Amersham Biosciences). Two representative clones derived from each of them were subjected to nucleotide sequence determination by an automated DNA sequencer.
Nucleotide sequence analysis and phylogenetic relationship
Sequences derived from the two independent 16S rDNA clones representing SAS1 and SAS5 were assembled, and the corresponding sequences of the reference strains AY1 and BB present between the primers R16mF2 and R16mR1, were retrieved from GenBank. Their putative restriction site maps were generated by using the pDRAW32 option of the AcaClone program sited at http://acaclone.com. The computer-generated putative endonuclease recognition sites of SAS phytoplasma and the reference strains were compared with those identified by actual enzymatic digestion.
Phylogenetic interrelationships among SAS phytoplasma, reference strains AY1 and BB and other phytoplasmas representing aster yellows group (16SrI) members were assessed based on 16S rDNA sequences. Acholeplasma laidlawii was used as an outgroup. The GenBank numbers are listed in Table 1. The nucleotide sequences were compiled, converted into FASTA format and aligned using the CLUSTALW version (Thompson et al., 1994). Additional sequences from other phytoplasma strains were trimmed and converted to MEGA format for phylogenetic analyses. Distance analyses were performed with the MEGA2 package (version 2.1; Kumar et al., 2001) using the Kimura–2 parameter model (Kimura, 1980). The reliability of each phylogenetic analysis was subjected to a bootstrap test with 1000 replicates for estimation of stability and support for clades. Phylogenetic tree was viewed using Tree Explorer.
| Disease Caused (Strain) | 16S rRNA Group/Subgroup | GenBank Accession Numbers | References |
|---|---|---|---|
| Aconitum proliferation (AcP) | 16SrI-A | AF510323 | Valiunas et al. (2001) |
| Sandal spike (SAS1) | This study | EF050071 | This study |
| Sandal spike (SAS5) | This study | EF198362 | This study |
| Michigan aster yellows (MIAY) | 16SrI-B | M30790 | Lim & Sears (1989) |
| Severe aster yellows (SAY) | 16SrI-B | M86340 | Kuske & Kirkpatrick (1992) |
| Maryland aster yellows (AY1) | 16SrI-B | AF322644 | Lee & Davis (1988), Lee et al. (1993) |
| Carrot yellows (Btsv2CarD3) | 16SrI-B | AY180945 | Unpublished |
| Carrot yellows (Btsv2CarD5) | 16SrI-A | AY180934 | Unpublished |
| Primose virescence (PRIVC) | 16SrI-B | AY265210 | Schneider et al. (1993) |
| Tomato big bud (BB) | 16SrI-A | AY180955 | Lee et al. (1993) |
| Hydrangea phyllody (HyPH) | 16SrI-B | AY265207 | Lee et al. (1998) |
| Clover phyllody (KVG) | 16SrI-C | AY265218 | Lee et al. (2004) |
| Acholeplasma laidlawii | Not applicable | M23932 | Weisberg et al. (1989) |
Results
Polymerase chain reaction
The nested PCR yielded DNA fragments of the same size (approximately 1.4 kb) from the samples taken from SAS diseased trees and reference strains AY1, BB and APh. Under the same conditions, no DNA fragments were obtained from asymptomatic leaves.
RFLP and putative restriction sites analyses
The RFLP patterns observed for 16S rDNA of SAS1 and SAS5 of SAS phytoplasma following digestion by 17 different restriction enzymes were identical to reference strains AY1 and APh belonging to subgroup 16SrI-B. The restriction patterns for SAS1 and SAS5 were mutually indistinguishable. The endonucleases, namely AluI, DraI, EcoRI, HaeIII, HpaI, HpaII, HinfI, KpnI, Sau3AI, RsaI and TaqI, generated identical RFLP patterns for strains SAS1, SAS5, AY1, APh and BB (results not shown). Endonucleases BamHI, HindIII and SspI did not digest 16S rDNA fragment as confirmed by the absence of these restriction sites in the strains studied (results not shown). However, the endonucleases MseI, HhaI and BfaI could separate strain BB from that of SAS phytoplasma and reference strains (AY1 and APh).
MseI digests yielded a four-fragment RFLP pattern. While the three fragments (approximately 406, 266 and 131 bp) were identical in all the four strains, a 374-bp fragment was produced in strain BB instead of 386 bp present in strains of SAS phytoplasma, AY1 and APh (Fig. 1a).
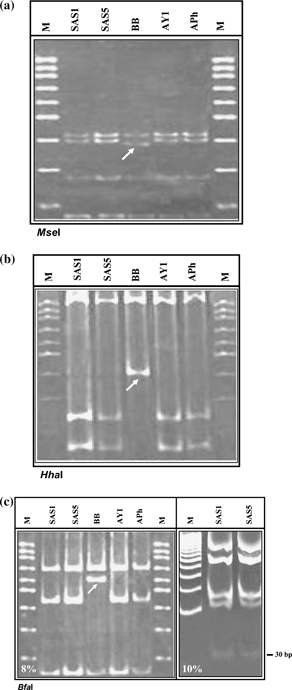
Restriction fragment length polymorphism analysis of nested polymerase chain reaction (PCR) – amplified 16S rDNA (1.4 kb) from sandal spike (SAS) phytoplasma (SAS1 and SAS5) and reference strains (BB, AY1 and APh) following digestion with endonucleases MseI (a), HhaI (b), BfaI (c). The arrows indicate restriction fragments that differentiate strain BB from SAS, AY1 and APh. The first PCR was primed by primer pair P1/P7 followed by re-amplification of target DNA in nested PCR by R16mF2/R16mR1. M, 100 bp DNA ladder, fragment sizes are in descending order (bp) 1000, 900, 800, 700, 600, 500, 400, 300, 200, 100.
HhaI-digested 16S rDNA yielded a two-fragment RFLP pattern (approximately 1034 and 392 bp) for strain BB and a three-fragment pattern (approximately 1034, 233 and 159 bp) was obtained for strains of SAS, AY1 and APh (Fig. 1b). This difference is reflected in the putative restriction site generated from the sequence data (Fig. 2). In strain AY1, HhaI restriction sites were present at positions 1034 and 1193 yielding three fragments of sizes 1034, 159 and 233 bp, respectively. Identical fragments were derived from SAS1 and SAS5 having restriction sites at the same locations as AY1, while in strain BB, HhaI is present at position 1034 (missing at position 1193), yielding two fragments of sizes 1042 and 392 bp, respectively. This difference is in line with the putative restriction sites based on nucleotide sequence data (Fig. 2).
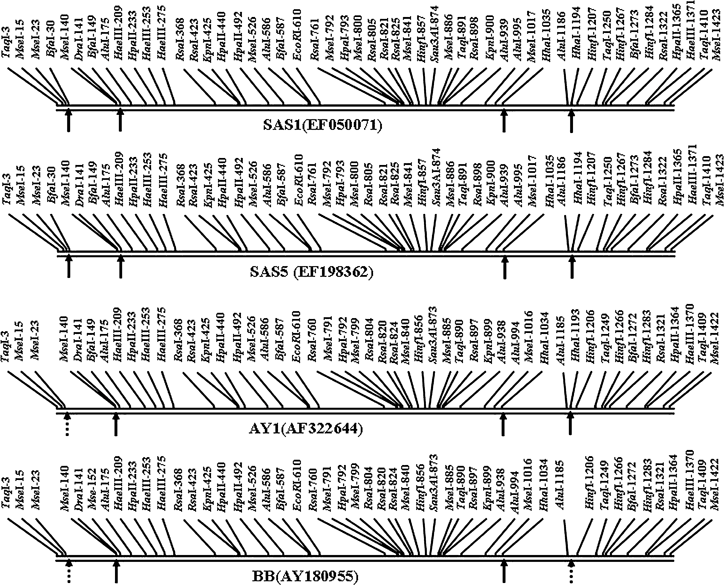
Putative restriction site analysis of 16S rDNA sequences from strains of sandal spike (SAS) phytoplasma (SAS1 and SAS5) and reference strains (BB, AY1 and APh) belonging to 16SrI group (Candidatus Phytoplasma asteris). Dashed arrows indicate restriction sites that differentiate SAS phytoplasma strains from the reference strains.
BfaI digests generated a three-fragment RFLP pattern for all the strains analysed in 8% gels (Fig. 1c). The positions of two fragments (approximately 685 and 154 bp) were similar in all the four strains; one fragment (approximately 585 bp) in the reference strain BB did not match the others. The nucleotide sequences of SAS phytoplasma and strain AY1 differed from that of strain BB by the absence of BfaI site at position 149 in BB (Fig. 2). In strain BB, two BfaI endonuclease recognition sites were located at positions 587 and 1272 generating three fragments of sizes 587, 685 and 154 bp, respectively. In contrast, three putative restriction sites were recognised in strain AY1 at positions 149, 587 and 1272 yielding four fragments of sizes 149, 438, 685 and 154 bp, respectively. Strain APh also yielded RFLP fragments identical to strain AY1 (Fig. 1c). However, in SAS phytoplasma, the BfaI restriction sites were present at positions 30, 149, 587 and 1273, in silico generating five fragments of 30, 119, 438, 686 and 154 bp, respectively (Fig. 2). In 10% gel with short-time runs, DNA fragment of 30 bp was also visible in addition to 686, 438, 154 and 119-bp fragments (Fig. 1c).
These data showed that the computer drawn as well as enzymatic digested restriction maps of 16S rDNA of SAS phytoplasma and the reference strains are identical (except the presence of BfaI site at position 30 in SAS), differing with that of strain BB.
Nucleotide sequence and phylogenetic analyses
Nucleotide sequences determined from the SAS phytoplasma (representing SAS1 and SAS5) were deposited in the GenBank database under accession numbers EF050071 and EF198362, respectively. They were compared with other related phytoplasmas already available in the database (Table 1). A comparison between R16mF2/R16mR1-primed 16S rDNA sequences from SAS phytoplasma (SAS1 and SAS5) yielded similarity value of 99.3% and 99.4% with strain AY1, and 98.8 and 98.9% with strain BB, respectively. Sequence similarities between SAS phytoplasma and 16SrI-B subgroup phytoplasmas ranged from 99.2% to 99.5%. The sequence identity between SAS1 and SAS5 was nearly identical (99.8%) confirming that both of them represent a strain of the same Candidatus species.
A phylogenetic distance tree was constructed from a data set, which included a total of 13 phytoplasma strains and A. laidlawii (Fig. 3, Table 1). The tree topology was supported by high bootstrap values. The tree patterns clearly showed that the SAS phytoplasma was evolutionary closest to subgroup 16SrI-B strains. The results are consistent with those obtained by RFLP analysis and nucleotide sequence identities of SAS phytoplasma with strains of 16SrI-B subgroup.
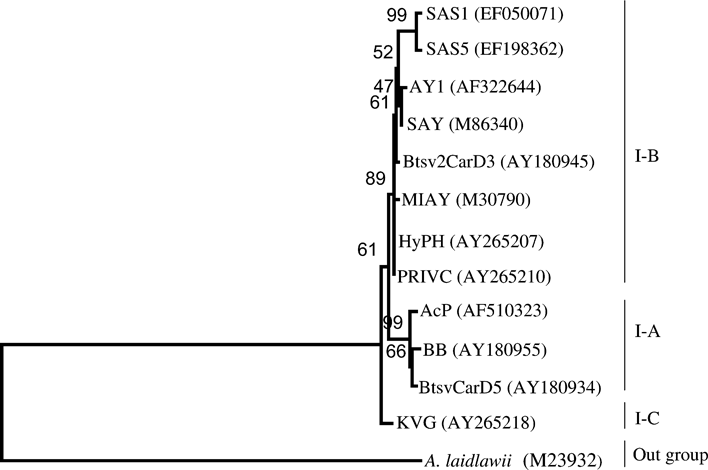
Phylogenetic tree drawn by neighbour-joining method based on the multiple sequence alignments of 16S rDNA (1.4 kb) from strains of sandal spike (SAS) phytoplasma (SAS1 and SAS5) and other phytoplasma strains representing 16SrI group. Numbers at nodes indicate percentage bootstrap support values. Acholeplasma laidlawii was used as an outgroup. Roman numerals denote 16S rDNA restriction fragment length polymorphism subgroups according to Lee et al. (1998). Designations given to strains and their GenBank accessions are given in Table 1.
Discussion
The present study characterises a phytoplasma associated with SAS disease in S. album. It was shown that the sandal plants, which exhibited symptoms characteristic of SAS phytoplasma disease described from India, were infected by a phytoplasma. On the basis of comparisons of the RFLP patterns, genetic relatedness of 16S rDNA from SAS phytoplasma with those of reference strains used in this study and our preliminary report, SAS phytoplasma should be classified as a member of 16S rRNA RFLP subgroup 16SrI-B.
The PCR amplicons representing 16S rDNA of SAS phytoplasma and the reference strains (AY1, BB and APh) allowed comparison of their RFLP patterns. The phytoplasma detected in SAS diseased plants (SAS1 and SAS5) exhibited the same collective RFLP patterns as that of reference strains AY1 and APh belonging to 16SrI-B subgroup (Lee et al., 1998; I.-M. Lee, Jana Fránová, personal communication). The restriction sites BfaI, HhaI and MseI present in 16S rDNA of SAS phytoplasma and reference strain AY1 and APh could clearly differentiate those from strain BB (subgroup 16SrI-A), although the SAS phytoplasma and the reference strain AY1 are closely related to strain BB showing 98.8–99.0% identity at the nucleotide level.
The 16S rRNA gene with well-conserved sequences among prokaryotes, including phytoplasmas, forms the basis to study genetic relatedness among micro-organisms (Gasparich et al., 2004). Strains in AY group share ≥97% similarity in their 16S rDNA sequences, which are relatively homogeneous, showing not more than 2.6% variability. Despite this low variation, this group could be subdivided into several distinct RFLP subgroups by extensive RFLP analysis of 16S rDNA employing a set of 17 restriction enzymes (Lee et al., 1998). The availability of good quality sequence information of a large number of phytoplasmas in database has allowed in silico digestion to generate computer-drawn RFLP patterns and gel plots, thus, significantly facilitating high throughput identification and classification of diverse arrays of phytoplasmas (Wei et al., 2007). With a view to confirm the RFLP patterns derived following digestion of 16S rDNA representing strains SAS1, SAS5 and AY1 by endonucleases BfaI, HhaI and MseI, computer-simulated RFLP analysis was performed with these three strains along with strains of subgroup 16SrI-B as deployed by Wei et al. (2007) to generate in silico gel plots. The in silico RFLP patterns and virtual gels representing strains SAS1, SAS5 and AY1 revealed identical patterns as developed by Wei et al. (2007) for their 16SrI-B strains (results not shown), thus, further confirming the placement of SAS phytoplasma strains SAS1, SAS5 in 16SrI-B subgroup.
In this communication, phylogenetic analysis based on 16S rDNA sequences from AY group clearly clustered SAS phytoplasma along with other strains of subgroup 16SrI-B. Although the SAS phytoplasma has already been placed with aster yellows group phytoplasma members (Schneider et al., 1993; Schneider & Seemüller, 1994), RFLP studies with 17 different restriction enzymes have been performed for the first time. It is evident from the combined analysis using RFLP patterns, putative restriction sites in 16S rDNA sequences and phylogenetic analysis, SAS phytoplasma belongs to subgroup B in group 16SrI. These findings provide a definitive classification of the phytoplasma associated with SAS disease in India.
To understand and manage the SAS disease, it is of utmost importance to clearly establish the identity of the phytoplasma involved, prior knowledge on the host range and correct identification of insect vectors. The available information on 16S rDNA of SAS phytoplasma, particularly its digestion by endonuclease BfaI, may help to search/confirm insect vector(s) and to gain an understanding of the spread of the phytoplasma within S. album and other plant species.
Acknowledgements
The authors are thankful to Dr Rakesh Tuli, Director, NBRI, for providing necessary research facilities; Dr I.-M. Lee (USA) and Dr Jana Fránová (Czech Republic) for the reference strains. Financial grant partially by Department of Biotechnology, New Delhi, Government of India, is gratefully acknowledged.




