MRI CHARACTERISTICS OF CEREBRAL MICROBLEEDS IN FOUR DOGS
There were no funding sources for this project. This paper has not been previously published or presented and there is no conflict of interest.
Abstract
Cerebral microbleeds in people are small foci of hemosiderin-containing macrophages in normal brain parenchyma. They are the remnant of previous hemorrhage and occur with greater frequency in older individuals. Our purpose was to describe the magnetic resonance (MR) appearance of cerebral microbleeds in four dogs. These lesions appeared as round, hypointense foci measuring ≤4 mm on T2*-gradient-recalled echo images. They were less conspicuous or absent on T2-weighting, being iso- or hypointense, and uniformly invisible on T1-weighted images. No contrast enhancement was seen in any of the cerebral microbleeds. Necropsy-derived histopathologic analysis of one brain confirmed these lesions to be chronic cerebrocortical infarcts containing hemosiderin. The MR changes seen in dogs were analogous to what has been described in people and will be helpful in distinguishing cerebral microbleeds from other brain lesions.
Introduction
The term intracerebral hemorrhage is a broad descriptor that refers to hemorrhage into the brain parenchyma or subarachnoid space. In people, large single areas of intraparenchymal brain hemorrhage typically result from either cardiovascular accidents or infarcts.1 Hemorrhagic cardiovascular accidents are usually secondary to hypertension or aneurysm rupture whereas hemorrhagic infarction occurs due to reperfusion of a blood vessel that has received endothelial damage from a thromboembolus.2, 3 Other causes of intracranial hemorrhage are less common.1, 3-7
One pattern of intracranial hemorrhage described in people is termed cerebral microbleeds, which are small foci of hemosiderin-containing macrophages in normal brain parenchyma.8-11 The hemosiderin deposits are from past hemorrhage, presumably due to leakage from damaged small vessels.8, 10-12 These characteristically appear as homogeneous, round foci of hypointense signal intensity on T2*-weighted gradient recalled-echo (T2*-GRE) magnetic resonance (MR) imaging.9, 10, 12, 13 In humans, cerebral microbleeds are seen with normal aging.14, 15 Cerebral microbleeds have a prevalence as low as 6.5% in people aged 45–50 years and as high as 35.7% in people 80 years and older.11, 16 The prevalence increases with intracranial hemorrhage or ischemic stroke, reportedly up to 70%.9, 11 One in five patients with Alzheimer's disease has cerebral microbleeds.15 Our goal was to describe the features of cerebral microbleeds in four dogs.
Materials and Methods
Medical records of all dogs that had brain MR imaging between March 2007 and November 2010 were identified. Inclusion criteria were: (1) presence of small (<5 mm), round, well-marginated signal voids within the brain parenchyma on T2*-GRE MR images; (2) absence of signal changes in brain parenchyma immediately surrounding T2*-GRE signal voids; (3) normal cerebrospinal fluid analysis; and (4) negative infectious disease testing including serology for Ehrlichia canis, Rickettsia rickettsia, Bartonella spp., Borrelia burgdorferi, Toxoplasma gondii, and Neospora caninum as well as blood or urine polymerase chain reaction for canine distemper virus. Additionally, dogs were required to have a necropsy confirming the presence of cerebral microbleeds or lack of neurologic progression over a minimum of 3 months. Signalment, time from onset of clinical signs to MR imaging (days), concurrent medical conditions, survival to discharge (yes/no), and final diagnosis were recorded. The onset of clinical signs was defined by the date of the first owner-reported intracranial neurologic sign.
Four dogs fit the inclusion criteria. Breeds included one each of the following: Beagle, Collie, Miniature Schnauzer, and Shetland Sheepdog. Median age was 13.25 years (range 11–15 years). Two of the dogs were neutered males, one was a neutered female, and one was an intact female.
MR imaging was performed using a 1.0T unit.1 Sequences were: pre- and postcontrast spin echo T1-weighted images (TI-W; repetition time [TR] 532–654 ms; echo time [TE] 15 ms), spin echo T2-weighted images (T2-W; TR 3500–4000 ms; TE 45 ms), T2-weighted fluid attenuated inversion recovery, and T2*-GRE (TR 760–920 ms; TE 26 ms).

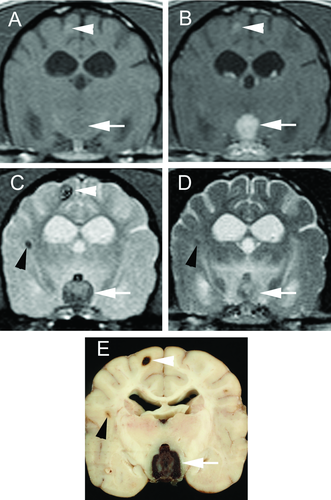
All images were reviewed independently in digital format by the primary author (C.V.F.), a board-certified radiologist (B.D.Y.), and a board-certified neurologist (J.M.L.). Any discrepancies between reviewers had a final resolution reached by group consensus. Lesions were defined as areas of signal void on the T2*-GRE sequences. The number and diameter of each lesion was measured on the GRE sequences. The following MR imaging characteristics were evaluated: mass effect, perilesional edema, signal intensity relative to surrounding tissue on T1-W and T2-W images, tissue predilection, and contrast enhancement.
Results
A total of 28 lesions were seen and multiple hemorrhagic lesions were present in all dogs (Figs. 1 and 2). On T2*-GRE images, cerebral microbleeds measured 1 mm (15 lesions), 2 mm (8 lesions), or 3 mm (5 lesions). No mass effect or perilesional T2-W hyperintensity was noted. All 28 lesions were T1-isointense to the surrounding tissue. Eight of the 28 lesions were T2-hypointense while the remaining 20 lesions were T2-isointense (Fig. 2). Lesions were in gray matter only in one dog (1) while the other three dogs had lesions in both gray and white matter (2–4) (Table 1). No cerebral microbleed had contrast enhancement.
| Age at Presentation (Years) | Breed | Number of Cerebral Microbleeds Lesions | Distribution Frequency | Concurrent Diseases | |
|---|---|---|---|---|---|
| 1 | 11 | Beagle | 3 | 3 GM, 0 WM | Hypothyroidism, lymphoma |
| 2 | 15 | Collie | 12 | 9 GM, 3 WM | Neuromuscular disease, hypertension |
| 3 | 14 | Miniature Schnauzer | 7 | 6 GM, 1 GM | Hypothyroidism, hyperadrenocorticism, vaginitis |
| 4 | 13 | Shetland Sheepdog | 6 | 4 GM, 2 GM | Thromboembolic disease |
- GM, gray matter; WM, white matter.
Two of the four dogs had additional abnormal findings. Dog 1 had mild forebrain cortical atrophy as evidenced by narrowing of the gyri and increased depth and width of the sulci. At time of MR imaging, dog 1 had a clinical diagnosis of cognitive dysfunction and hypothyroidism. Neurologic signs had been present for a year before MR imaging was performed. This patient was diagnosed 4 months subsequent to MR imaging with multicentric lymphoma; at the time of euthanasia, neurologic signs remained nonprogressive.
Dog 2 had no other brain lesions and was diagnosed with generalized neuromuscular disease and seizures. Concurrent medical conditions were osteoarthritis and hypertension. The time of onset of clinical signs to MR imaging was 2 days.
Dog 3 had no other brain lesions and was diagnosed with generalized weakness, severe muscle atrophy, and seizures. Concurrent medical conditions were hypothyroidism, hyperadrenocorticism, and vaginitis. Neurologic signs began 9 days before MR imaging with one isolated seizure 5 months prior to presentation.
Dog 4 had two additional MR brain lesions, and both were hemorrhagic as indicated by strong signal void on T2*-GRE. The largest was in the thalamus with broad contact to the sella turcica and had high perilesional T2 signal. The other lesion was in the gray matter of the right parietal lobe and was faintly T1- and T2-hyperintense with contrast enhancement. MR imaging of the spinal cord segments C1–C3 was unremarkable. This patient had cervical pain with a history of osteoarthritis. Time of onset of neurologic signs to MR imaging was 3 days.
Three of the four dogs survived until discharge. Dog 1 was euthanized due to complications from lymphoma unrelated to neurologic disease 4 months after MR imaging. Dog 2 was alive at publication with no progression of neurologic disease at 6 months post-MR imaging. Dog 3 was euthanized 3 months after the MR imaging due to multiple systemic diseases, including severe bacterial vaginitis, hyperadrenocorticism, hypothyroidism, severe muscle atrophy, and generalized weakness. Dog 4 was euthanized, and a necropsy was performed. Grossly, the cerebral microbleeds were multiple 1–2 mm areas of tan to dark red discoloration in the cerebral cortex along the gray-white matter junction. Histologically, these lesions ranged from discrete, cavitated areas containing extravasated erythrocytes (hematoma) to spongiotic and gliotic areas containing reactive astrocytes, a small amount of mineral, and numerous macrophages containing abundant hemosiderin and hematoidin. The larger lesion in the thalamus was composed largely of extravasated erythrocytes surrounded by fibrovascular tissue and was interpreted as an organizing hematoma. It was concluded that hemorrhage from this lesion likely resulted in the acute onset of neurologic signs. The right parietal lesion was consistent with a hematoma. Hemorrhage was also evident in the mesencephalic aqueduct and fourth ventricle as well as the pancreas and cecum. Nodular hyperplasia was found in the adrenal cortices.
Discussion
The cerebral microbleeds were characterized as multiple, small, round, homogenous lesions that measured ≤4 mm in diameter on GRE sequences. Hemorrhage can be diagnosed uniquely on MR imaging because blood breakdown products are paramagnetic.4 The transformation of a hematoma ends with iron storage as ferritin and hemosiderin within macrophages. This end stage can be present within days after onset and can last indefinitely. At MR field strengths that range between 1T and1.5T, ferritin and hemosiderin are T1-iso- to T1-hypointense and T2-hypointense.4
T2*-GRE sequences are the most sensitive for detecting brain hemorrhage due to the increased sensitivity to paramagnetic constituents.4 Hemorrhage will appear hypointense on T2*-GRE sequences at certain stages of breakdown, but many things can mimic hemorrhage including mineralization, gas, fibrous tissue, or iron deposits.5, 17 Mineralization, gas, and fibrous tissue would all be expected to be T1- and T2-hypointense, which was not evident in these patients.
Small (diameter ≤5 mm), homogeneous, round foci of low-signal intensity on T2*-GRE sequences that are unrelated to mass effect or contrast enhancement are referred to as cerebral microbleeds in humans.8-11, 13-16, 18 Cerebral microbleeds are a radiologic construct but are telling of specific microscopic pathological changes. Hemosiderin deposits from RBCs are often perivascular and are presumed to have leaked out of small vessels.8-11, 13-15 Cerebral microbleeds are found commonly in patients diagnosed with stroke (ischemic 34% and hemorrhagic 60%), are present in the general population at a prevalence of 5%, and have an incidence that increases with age.15, 16 Hypertension is the leading cause of intracranial hemorrhage.3, 19 Chronic hypertension leads to lipohyalinosis of the small intraparenchymal arteries, resulting in arterial rupture and intracranial hemorrhage.3, 19 There is an association between cerebral microbleeds and asymptomatic stages of age-associated small-vessel disease, such as hypertensive vasculopathy and cerebral amyloid angiopathy.8, 9 The etiopathogenesis of cerebral microbleeds seems to determine the location of lesions, with cerebral amyloid angiopathy resulting in lobar microbleeds and hypertension leading to nonlobar microbleeds.18 Cerebral amyloid angiopathy is deposition of amyloid in the walls of medium- and small-sized leptomeningeal and cortical arteries.20 Cerebral amyloid angiopathy increases with age and has been documented to cause intracranial hemorrhage in the elderly. Intracerebral hemorrhage is described infrequently in animals.
Intracranial hemorrhage in people refers to hemorrhage within the brain parenchyma or subarachnoid space resulting from rupture of a blood vessel. A hemorrhagic infarct results from blood vessel occlusion, typically due to an embolism.3 Several mechanisms are described that can lead to intracranial hemorrhage in humans, including hypertension, vascular malformations, tumors, bleeding disorders, cerebral amyloid angiopathy, vasculitis, sympathomimetic agents, hemorrhagic infarcts, and trauma.3, 4 Similar mechanisms for intracranial hemorrhage have been described in animals including congenital vascular abnormalities, neoplasia, cerebral amyloid angiopathy, vasculitis, impaired coagulation, and spontaneous rupture of vasculature (reported as rare in dogs).1, 5-7 Hemorrhage has been associated with ischemic infarcts in dogs.5, 7, 21 Hypertension is thought to be a cause of intracranial hemorrhage in dogs and is often related to underlying chronic renal disease and/or hyperadrenocorticism.7, 21 In a group of dogs with cerebellar infarction, one had multiple pinpoint foci of low signal on GRE images that were thought to be similar to the cerebral microbleeds seen in people.5 Cerebral amyloid angiopathy develops naturally as dogs age beyond 13 years, particularly affecting the leptomeningeal arteries, and this change has been positively associated with the incidence of intracranial hemorrhage.20
As cerebral microbleeds have been described poorly in animals, they may be under-recognized. The MR signal pattern in these four dogs was analogous to that reported for people.8-10, 13-15, 18 Recognition of cerebral microbleeds may prevent the erroneous diagnosis of other conditions.