MAGNETIC RESONANCE IMAGING FINDINGS IN 15 ACROMEGALIC CATS
Abstract
Feline acromegaly is characterized by chronic excessive growth hormone secretion, most commonly caused by a functional pituitary adenoma. In this study, acromegaly was diagnosed in 15 cats on the basis of compatible clinical signs, laboratory, and magnetic resonance imaging (MRI) findings. MRI findings were reviewed retrospectively. Enlargement of the pituitary gland with suprasellar extension was present in all cats. No characteristic signal patterns were identified on T1-weighted and T2-weighted sequences. Contrast enhancement was nonuniform in all cats, as was suspected involvement of the adjacent hypothalamus. A mass effect on the cavernous sinus and third ventricle was present in 13 cats. Mild peritumoral edema was present in four cats, and moderate edema in one cat. Transtentorial herniation was present in one cat. Histopathology confirmed the presence of a pituitary adenoma in two cases. MRI is a useful modality to establish the diagnosis of acromegaly.
Introduction
Chronic hypersecretion of growth hormone causes acromegaly, a disease characterized by insulin-resistant diabetes mellitus and progressive overgrowth of soft tissue, membranous bone, and viscera. In cats the most common cause is a functional pituitary adenoma. The most typical signalment is that of a middle-aged to older neutered male domestic short-haired cat.1–4 Acromegaly has been considered rare in cats but it may be more common than suspected previously.5–7
Clinical features result from a combined effect of growth hormone and insulin-like growth factor-1 (IGF-1), a hormone synthesised by the liver in response to growth hormone. Excessive growth hormone production usually results in insulin-resistant diabetes mellitus.2,8 High IGF-1 concentrations induce excessive growth of soft tissue, organomegaly, and bone remodelling.4 The anabolic effects of IGF-1 result in marked changes in the phenotype of acromegalic cats such as weight gain, broad facial features, and prognathia inferior. However, these features are not present consistently.9
Radiographic changes include increased oropharyngeal soft tissue, degenerative arthropathy, hepatomegaly, renomegaly, and cardiomegaly. Sonography may be unremarkable or there may be hepatomegaly, renomegaly, adrenomegaly, and pancreatic enlargement. Echocardiographic changes similar to hypertrophic cardiomyopathy may be found.2,4
Definitive diagnosis of feline acromegaly can be difficult because of the gradual onset and subtle clinical signs and unavailability of relevant laboratory tests, such as a feline growth hormone assay.1,4 A diagnosis of acromegaly is currently based upon a combination of clinical signs, measurement of growth hormone and/or IGF-1 and intracranial imaging.1,4,9
The usefulness of contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) in establishing the diagnosis of acromegaly in cats has been demonstrated in many reports.5–12 However, little information is available regarding MRI features of pituitary tumors in acromegalic cats. Our purposes were to evaluate if pituitary abnormalities were present on MRI in acromegalic cats, to describe MRI findings and to assess if specific morphologic criteria can be established.
Material and Methods
Medical records between January 2003 and August 2010 were searched for cats in which clinical signs and laboratory findings were compatible with acromegaly and in which an MRI was performed. Inclusion criteria were: elevated serum IGF-1 (>1000 ng/ml) as a surrogate marker of hypersomatotropism and insulin resistant diabetes mellitus. Insulin resistant diabetes mellitus was based on an exogenous insulin requirement >1.5 U/kg per injection, increased fructosamine, continued polydipsia, polyuria, and polyphagia despite insulin treatment. Other causes for insulin resistance had been excluded.
Fifteen cats met the inclusion criteria. Fourteen of the cats were included in a previous study where the response and outcome of cats with acromegaly and insulin-resistant diabetes mellitus to radiotherapy were described.13
The mean age was 10.5 years (range 6–14 years). Thirteen were domestic short haired and two were domestic long haired. Neutered male cats were over-represented (14/15); the other cat was a neutered female. The following were also present: broad facial features (12/15), prognathia inferior (2/15), widened interdental spaces (1/15), overgrowth of the soft tissues of head and neck (2/15), respiratory stridor (8/15), and enlargement of the extremities (9/15). All cats had signs of insulin-resistant diabetes mellitus at the time of presentation. Only one cat had neurologic signs; this was blindness characterized by bilateral mydriasis and a reduced pupillary light reflex, abnormal behavior, and circling. The blood pressure of the blind cat ranged between 140 and 160 mmHg.
MRI of the brain was performed using a 0.2 T scanner* with a dual-phased array coil. All cats were anesthetized and in sternal recumbency. T2-weighted (T2W) and pre- and postcontrast T1-weighted (T1W) images were acquired in transverse and sagittal planes using fast spin echo sequences. Slice thickness was 4 mm with an interslice gap of 0.4 mm, except in one cat where a slice thickness of 3 mm and an interslice gap of 0.3 mm were used. Contrast-enhanced T1W images were obtained following an intravenous bolus of 0.1 mmol/kg of gadobenate dimeglumine.† Fluid attenuation inversion recovery (FLAIR) images were acquired in transverse plane in four cats.
All MR images were reviewed by one board-certified radiologist (B.P.). The pituitary gland was measured on postcontrast T1W images using a published protocol.14 Signal intensity was described relative to the normal cerebral cortical gray matter. Suprasellar extension was assessed subjectively in both sagittal and transverse planes and was identified as none, mild, moderate, or marked. Compression/involvement of the hypothalamus was suspected where suprasellar extension and dorsal displacement of the third ventricle were noted. Cavernous sinus involvement was suspected where narrowing or lateral deviation of the luminal signal void of the cavernous sinus was seen. Dorsal displacement and/or lateral shift of the third and lateral ventricles were also noted. Loss of the normal low signal of the cortical bone of the sella turcica or replacement of the fat signal within the marrow space of the sphenoid bone were criteria for invasion of the base of the skull.
Findings of echocardiography and abdominal ultrasonography and radiographs were recorded, if available. All cats received radiotherapy, delivered in 10 fractions, three times a week to a total dose of 37 Gy.
Results
Thoracic radiographs were available in six cats. Mild cardiomegaly was present in four and thoracic spondylosis in four. Abdominal radiographs were available in three cats. There was mild hepatomegaly in one, mild renomegaly in one, and lumbar spondylosis in two. In two cats, radiographs of shoulder and elbows were available and degenerative changes in both joints were evident in both cats.
Echocardiography was performed in eight cats. The only abnormality was mild left ventricular muscular hypertrophy and left atrial enlargement in one cat.
Abdominal sonography was performed in eight cats. Findings were hepatic enlargement (6/8), renal enlargement (5/8), reduced renal corticomedullary definition (6/8), mild renal pelvic dilation (4/8), enlarged adrenal glands (4/8), and pancreatic enlargement (4/8).
The reference ranges for length, width, and height of the normal feline pituitary gland were exceeded in 14 of 15 cats. In the remaining cat, the length and height of the gland on sagittal images exceeded the normal range, but the width and height measured on transverse images were normal. However, no transverse images were obtained through the centre and therefore maximum dimension of the gland could not be assessed.
The mean pituitary length was 0.88 cm (0.6–1.8 cm), mean pituitary width was 0.78 cm (0.4–1.4 cm), and mean pituitary height on sagittal images was 0.74 cm (0.4–1.6 cm) and on transverse images 0.75 cm (0.3–1.4 cm). In 12/15 cats the pituitary gland was oval and in 3/15 cats it was more mushroom like due to lateral extension. In all but one cat, the margins were smooth.
In 13/15 cats the pituitary gland had a predominantly unilateral extension; in eight cats to the right and in five cats to the left. Suprasellar extension was present in all cats; this was mild in seven, moderate in five, and marked in three (Fig. 1).
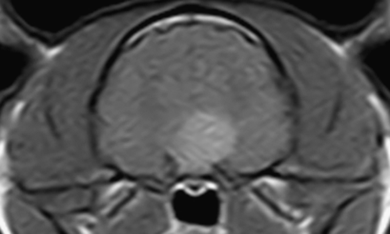
Transverse postcontrast T1-weighted (TR=800 ms, TE=18 ms) image of the pituitary gland. The strongly enhancing pituitary mass extends dorsally and to the left side.
The signal intensity of the pituitary gland on precontrast T1W images was variable, being mixed in 13/15 cats and either homogenously isointense or hyperintense to gray matter in the other two. In the 13 cats with a mixed T1W signal, nine had a predominately hypointense gland with a hyperintense rim. In two cats the caudal third of the gland was hyperintense with a hyperintense rim around the gland and in the other two the gland had a mixed hypo- and hyperintense signal.
On T2W images, only one cat had a homogenously hypointense pituitary gland. In all other cats the gland had mixed signal intensity. In 12/14 cats the gland was predominantly hypointense with a hyperintense rim (Fig. 2) and in the other two the gland had mixed hypo-and hyperintensity throughout.
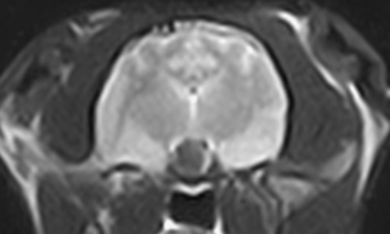
Transverse T2-weighted (TR=3000 ms, TE=80 ms) image of the pituitary gland. The hypointense pituitary mass is surrounded by a partial hyperintense ring.
Contrast enhancement of the pituitary gland was nonuniform in all cats. A ring-like enhancement was seen in four cats, a ring-like enhancement with a few additional areas of focal enhancement in five cats and a heterogeneous patchy pattern of enhancement in six cats (Fig. 3A and B). The degree of contrast enhancement was mild in seven cats, moderate in five cats, and strong in three cats, one of which had strong central enhancement.
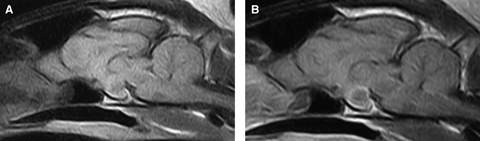
(A) Sagittal precontrast T1-weighted (TR=690 ms, TE=26 ms) image of the pituitary gland. Note the partial hyperintense rim ventrally. (B) Sagittal postcontrast T1-weighted (TR=690 ms, TE=26 ms) image of the pituitary gland. A ring-like enhancement with additional few small areas of focal enhancement is present.
On FLAIR images suppression of the hyperintense rim noted on T2W images was present in 2/4 cats.
Compression/involvement of the hypothalamus was suspected due to suprasellar extension in 15 cats, dorsal displacement of the floor of the third ventricle in 10 and a lateral shift of the third ventricle in 13 (Fig. 4). In two cats, involvement of the thalamus was suspected due to the marked dorsal extension of the pituitary mass and dorsolateral displacement of the lateral ventricles. In 13/15 cats, no mass-effect on the lateral ventricles was detected.
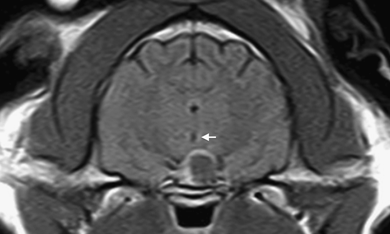
Transverse postcontrast T1-weighted (TR=690 ms, TE=26 ms) image of the pituitary gland. Note the peripheral enhancement. The third ventricle is shifted slightly to the right side (arrow).
Cavernous sinus involvement was suspected in 13/15 cats due to lateral extension of the pituitary gland (13/13), narrowing of the signal void of the cavernous sinus (5/13), and lateral deviation of the cavernous sinus (8/13) (Fig. 5).
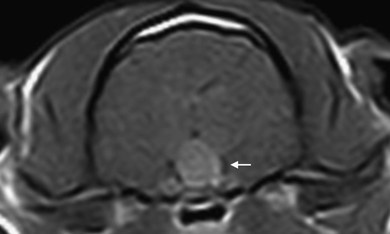
Transverse postcontrast T1-weighted (TR 690 ms, TE=18 ms) image of the pituitary gland. The contrast enhancement is heterogeneous (ring-like and small focal enhancement). A lateral deviation of the signal void of the left cavernous sinus is present (arrow).
In 14 of 15 cats, compression of the optic chiasm was not present. There was a high suspicion of involvement of the optic chiasm in the other cat, which also had bilateral mydriasis, reduced pupillary light reflex, and other neurological signs.
Invasion of the sphenoid sinus was not found. However, in 4/15 cats there was a suspicion of invasion of the sphenoid bone due to loss of the normal low signal of the cortical bone (4/4) and suppression of the fat signal within the bone marrow (1/4) (Fig. 6).
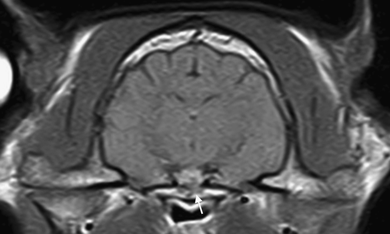
Transverse postcontrast T1-weighted (TR 690ms, TE=26 ms) image of the pituitary gland. Invasion of the sphenoid bone is suspected due to partial suppression of the fat signal within the bone marrow (arrow).
Mild edema around the pituitary gland was present in 4/15 cats and moderate edema was present in one cat. Transtentorial herniation was present in the cat that had neurologic signs. Mild cerebellar herniation through the foramen magnum was present in 6/15 cats.
Histopathological examination of the pituitary gland was performed in only 2/15 cats, which confirmed the presence of an acidophil pituitary adenoma.
Discussion
Because of the limited availability of a validated growth hormone assay in cats, measurement of IGF-1 is used as a screening test for acromegaly.5,7 Increased serum IGF-1 has been reported in diabetic cats without acromegaly. Therefore IGF-1 measurement alone does not permit a definitive diagnosis.7,9,13 This emphasises the importance of MRI or CT to allow identification of a pituitary mass.5–12 However, there are acromegalic cats without a pituitary mass.5,6 MRI is more sensitive than CT for detecting a pituitary mass but a negative study does not rule out a pituitary mass, especially if the mass is hyperplastic rather than neoplastic.5
All of our cats had enlargement of the pituitary gland and suprasellar extension. In one cat, the height and width of the pituitary gland were within normal limits on transverse images, but enlargement was evident on sagittal images. In this cat, the transverse images were obtained through the rostral and caudal aspect of the gland rather than through its center. Ideally, images will contain the largest cross section of the pituitary gland to prevent underestimation of its size.15 Slice thickness influences the identification of pituitary abnormalities, considering the small size of this structure.15,16 A slice thickness >3 mm may result in false-negative findings with small pituitary adenomas that do not lead to a change in size or shape of the gland.15,16 We used a slice thickness of 4 mm to maintain a high signal to noise ratio.
The pituitary gland had a variable appearance on precontrast T1W and T2W images. No specific signal characteristics or consistent pattern were identified. On T1W images, a hyperintense signal was present in the caudal one-third of the pituitary gland in two cats; this is normal in humans, dogs and cats due to the vasopressin secretory granules, or phospholipid membrane surrounding the neurosecretory granules, in the posterior lobe of the pituitary gland.14,17,18
There was a hyperintense rim surrounding the pituitary gland on T1W and T2W images in 12 cats. This is also described in the normal feline pituitary gland on T1W images.14 A curvilinear fat pad was mentioned as a possible explanation but there are no data to support this.14 This hyperintense rim might represent fatty infiltration due to chronic inflammation or compression. Late acute hemorrhage appears less likely. There was suppression of the hyperintense rim in FLAIR images in two of the four cats where this sequence was acquired. This suggests that the rim is due to fluid, but this is not consistent with the rim being hyperintense on T1W images.
Contrast medium administration improved assessment of the pituitary gland morphology and provided the best delineation. The normal feline pituitary gland tends to enhance uniformly.14 All of our cats had nonuniform contrast enhancement with a predominantly ring-like or heterogeneous pattern. This irregular enhancement could be related to areas of avascularity, such as necrosis, hemorrhage, or cyst formation. It was impossible to evaluate the cause of this heterogeneity, mainly due to the small size of the gland.
Contrast enhancement was mild in seven cats. Reduced contrast enhancement occurs in people with pituitary adenoma.19–21 In one acromegalic cat, a large pituitary mass did not enhance on MRI following contrast medium administration.11 Paramagnetic contrast agents improve the sensitivity and specificity to detect pituitary tumors in people, in particular microadenomas.21,22 Dynamic MRI imaging is the technique of choice for diagnosis of microadenomas in people.17 The normal dynamic MRI appearance of the normal feline pituitary gland is not known.
A mass effect on adjacent structures was present in all cats. Expanding pituitary lesions often compress the optic chiasm and hypothalamus. Optic chiasm compression by a pituitary adenoma is not uncommon in people.21 There was a suspicion of optic chiasm compression in only one cat in this study, which was the only cat with visual impairment and neurologic signs. In cats the optic chiasm is more rostral than in people, which likely explains the lower incidence of optic chiasm involvement in cats.
Suprasellar extension, dorsal displacement or lateral shift of the third ventricle were indirect signs for hypothalamic involvement. The bony sella turcica and incomplete diaphragm of the sella favors dorsal expansion of the gradually enlarging pituitary gland mass as the line of least resistance.23 This results in invagination of the mass into the infundibular cavity, dilation of the infundibular recess and the third ventricle.23 The resolution of our image system and the small size of the pituitary gland made it impossible to identify these structures. Furthermore, no clear distinction could be made between compression and invasion of the hypothalamus. Pituitary adenomas tend to grow noninvasively; however, invasive adenomas occur in people and animals.21,24
Infrasellar invasion into the sphenoid sinus can be seen in people.21 This was not seen in any cat, however, there was a suspicion of invasion of the sphenoid bone in four cats that seemed related to the size of the adenoma.
The sella turcica is bordered laterally by the dural bound space of the cavernous sinus.15 Cavernous sinus involvement causes lateral bowing or narrowing of the normal luminal signal void.15 Lateral deviation of the cavernous sinus was seen in eight cats in this study, narrowing of it in five cats.
True cavernous sinus invasion with vascular encasement is difficult to identify, even in people, and has to be confirmed by surgery or histopathology.21 Narrowing or deviation of the signal void of the cavernous sinus appeared to be caused by an asymmetric and predominately lateral extension of the gland. This asymmetry is described in humans and is related to somatotrophic cells residing in the lateral wings of the adenohypophysis.21 With pronounced dorsolateral expansion of the pituitary tumor a contralateral shift of the third ventricle could be observed.
In 10/15 cats there was no evidence of perilesional edema or, if present, it was mild. This is likely related to the slow-growing nature of this tumor.
Transtentorial herniation was noted in one cat with a large pituitary mass. This cat had unstable diabetes mellitus and 2 years later developed sudden onset of neurologic signs. Clinical signs due to functional pituitary adenomas are usually related to the endocrinopathy.15 Neurologic signs are uncommon but may develop as the tumor enlarges. Larger tumors are often nonfunctional and become apparent due to their mass effect.15 Nonfunctional pituitary neoplasms are uncommon.23 Although endocrinologically inactive, they can cause significant signs by compression of adjacent portions of the pituitary gland and dorsal extension.23
Mild cerebellar herniation was present in six cats. However, the significance of this finding is questionable because mild cerebellar extension through the foramen magnum can be observed occasionally in healthy cats.
In conclusion, pituitary abnormalities were present in 15 of 15 acromegalic cats but there was no consistent morphologic criterion. However, the pituitary gland in most cats had a heterogeneous appearance on both T1W and T2W images, with nonuniform contrast enhancement.
Footnotes
ACKNOWLEDGMENT
The authors would like to thank Nicolas Granger for his invaluable discussions on the neurological aspects of pituitary gland adenomas.